Abstract
Targeted Interleukin (IL)-10 therapy improves survival in preclinical models of critical illness, and intestine-specific IL-10 decreases inflammation in models of chronic inflammatory disease. We therefore sought to determine whether intestine-specific overexpression of IL-10 would improve survival in sepsis. Transgenic mice that overexpress IL-10 in their gut epithelium (Fabpi-IL-10 mice) and wild type (WT) littermates (n=127) were subjected to cecal ligation and puncture with a 27-gauge needle. Seven-day survival was 45% in transgenic animals and 30% in WT animals (p≤0.05). Systemic levels of IL-10 were undetectable in both groups of animals under basal conditions and were elevated to a similar degree in septic animals, regardless of whether they expressed the transgene. Local parameters of injury including gut epithelial apoptosis, intestinal permeability, peritoneal lavage cytokines and stimulated cytokines from intraepithelial lymphocytes were similar between transgenic and wildtype mice. However, in stimulated splenocytes, pro-inflammatory cytokines MCP-1 (189 ± 43 pg/ml vs. 40 ± 8 pg/ml) and IL-6 (116 ± 28 pg/ml vs. 34 ± 9 pg/ml) were lower in Fabpi-IL-10 mice than WT littermates despite the intestine-specific nature of the transgene (p<0.05). Cytokine levels were similar in blood and bronchoalveolar lavage fluid between the two groups as were circulating LPS levels. Transgenic mice also had lower white blood cell counts, associated with lower absolute neutrophil counts (0.5 ± 0.1 103/mm3 vs. 1.0 ± 0.2 103/mm3, p<0.05). These results indicate that gut-specific overexpression of IL-10 improves survival in a murine model of sepsis, and interactions between the intestinal epithelium and the systemic immune system may play a role in conferring this survival advantage.
Keywords: Gut, cecal ligation and puncture, cytokine, splenocyte, apoptosis, mortality, interleukin-6, MCP-1
INTRODUCTION
IL-10 is an anti-inflammatory cytokine that has been demonstrated to play a major role in the pathophysiology of critical illness. Plasma IL-10 levels in septic patients correlate with the magnitude of the systemic inflammatory response syndrome (SIRS) and elevated systemic levels are associated with poor outcome (1;2). In human volunteers, IL-10 administration prevents the fever and elevation of tumor necrosis factor alpha (TNFα), IL-6, and IL-18 levels induced by lipopolysaccharide (LPS) (3). The functional role of IL-10 in mediating mortality has been extensively studied in animal models with conflicting results. Manipulating the cytokine systemically by exogenous delivery, anti-IL10 antibodies, or using knockout animals improves, worsens or has no effect on survival in mice given LPS or in more authentic models of sepsis depending on timing of administration and model used (4-6).
Systemic levels of IL-10 do not necessarily correlate with local concentration or function as evidenced by the fact that IL-10 levels in bronchoalveolar lavage (BAL) fluid of patients with early onset acute respiratory distress syndrome are significantly lower in non-survivors than in patients who survive the disease (7). The disconnect between systemic levels and local levels and/or function has led investigators to pursue targeted administration of IL-10 in an attempt to focus therapeutic effects on a single tissue of interest while avoiding potential detrimental systemic effects (2). For example, Moldawer’s group introduced IL-10 into the lungs via an adenoviral vector administered intratracheally and then induced SIRS by zymosan injection. Lung-specific administration of IL-10 improved survival with a dose of 107 particles but increasing the dose to 109 particles worsened outcome when systemic IL-10 levels rose (8). The same group has also demonstrated that targeted administration of IL-10 to the thymus decreases apoptosis and improves mortality in sepsis (9).
In addition to its role in critical illness, IL-10 has been demonstrated to play a role in a number of other diseases. One important example that has been studied extensively is inflammatory bowel disease, where the balance between the gut epithelium and the immune systemic is chronically altered. IL-10 knockout mice develop spontaneous colitis (10) which is ameliorated by administration of IL-10 (11). The importance of intestine-specific IL-10 in the balance between the gut mucosa and immune system was demonstrated by the development of transgenic mice that overexpress this cytokine exclusively in their intestinal epithelium (12). Compared to WT animals, Fabpi-IL-10 mice have increased numbers of intraepithelial T-lymphocytes (IELs) and IgA producing B cells in their lamina propria. Activated IELs in transgenic mice have lower levels of Th1 cytokines TNFα and interferon gamma (IFNγ) but increased levels of the Th2 cytokine TGFβ. Importantly, Fabpi-IL-10 mice have decreased intestinal inflammation in two separate models of inducible colitis compared to WT mice, and introducing the transgene into IL-10-/- mice ameliorates the rectal prolapse seen in knockout mice.
Based upon a) the role systemic IL-10 has in sepsis, b) the role targeted IL-10 delivery has in improving mortality in critical illness, c) the role intestine-specific IL-10 has in ameliorating chronic inflammatory disease, and d) the role the gut has as the “motor” of the systemic inflammatory response (13;14), we examined Fabpi-IL-10 mice in sepsis to determine the role of intestine-specific delivery of IL-10 in a disease which has been estimated to kill 210,000 people annually (15).
METHODS
Sepsis Model
CLP was performed on Fabpi-IL-10 mice (12) (a generous gift from Hilde Cheroutre, La Jolla Institute) and WT C57/BL6 mice by the method of Baker et al. (16). Anesthesia was induced with 5% isoflurane and maintained with the same agent at a flow of 2.5%. Following a midline incision, the cecum was externalized, ligated with 4-0 silk approximately 5 mm from the ileocecal junction, and punctured once with a 27 gauge needle. After gently squeezing to extrude a small amount of stool, the cecum was then replaced into the abdomen, and the incision was closed in two layers. Septic mice were injected with 1mL of 0.9% saline subcutaneously immediately after wound closure to compensate for insensible fluid loss during the procedure. Sham operated mice were treated identically but without ligation or puncture of the cecum. All animals were maintained on 12 hour light-dark cycles in a specific pathogen-free environment with free access to food and water at all times. Experiments were conducted in accordance with the National Institute of Health guidelines for the use of laboratory animals and with approval of the Washington University Animal Studies Committee.
Survival Studies
Survival experiments comparing WT and transgenic mice subjected to CLP were conducted in age-matched mice by an investigator blinded to the presence or absence of the IL-10 transgene. Animal survival was recorded for 7 days after the onset of sepsis.
Intestinal Immunohistochemistry
All non-survival experiments were performed on different cohorts of animals sacrificed 24 hours following CLP unless otherwise specified. Following euthanasia, the small intestine was removed, opened along the longitudinal axis, washed in 10% formalin to remove luminal contents and fixed in the same solution. Apoptotic cells were identified by examination of hematoxylin-eosin (H&E) stained slides of the intestine by morphological identification of nuclear fragmentation (karyorrhexis) and cell shrinkage with condensed nuclei (pyknosis). Apoptosis was quantified by counting apoptotic cells in 100 well-oriented crypt-villus units as previously described (17;18). A well-oriented unit consisted of a villus sectioned parallel to the crypt-villus axis with an unbroken epithelial layer extending from Paneth cells at the crypt base to the villus tip.
To evaluate intestinal cytokine production, paraffin-embedded intestinal tissues were deparaffinized and rehydrated, endogenous peroxidase activity was blocked by incubating in 1% H2O2 in phosphate buffered saline for 15 minutes at 23° C. Antigen retrieval was performed by heating slides in citrate buffer (pH 6.0) for 45 minutes using a pressure cooker. To stain for IL-6, sections were incubated with polyclonal rat anti-IL-6 (1:30; BD Pharmingen, San Jose, CA) for one hour at 23° C, followed by secondary goat anti-rat antibody (1:200; Vector Laboratories, Burlingame, CA) for 30 minutes at 23° C. After rinsing, slides were incubated with a tertiary streptavidin-HRP complex (1:500; DAKO, Carpinteria, CA) for one hour at 23° C, developed with metal-enhanced diaminobinzidene (DAB), and counterstained with hematoxylin. To stain for MCP-1, sections were incubated with polyclonal goat anti-MCP-1 (1:20; R & D Systems, Minneapolis, MN) for one hour at 23° C, followed by secondary rabbit anti-goat antibody (1:200; Vector Laboratories, Burlingame, CA) for 30 minutes at 23° C. After rinsing, slides were incubated with Vectastain ABC Elite (Vector Laboratories, Burlingame, CA) for 30 minutes at 23° C, developed with DAB, and counterstained with hematoxylin.
Intestinal Permeability
Intestinal permeability was evaluated using an everted gut sac model (19;20) to the fluorescent tracer FITC dextran (FD4). Intestinal segments were prepared in Krebs-Henseleit bicarbonate buffer (KHBB), everted onto a rod, secured to a syringe containing KHBB and gently distended with 1.5 mL of KHBB. The everted gut sac was then suspended in a beaker with KHBB and FD4 (20ug/mL) for 30 minutes while being aerated continuously by a bubbling mixture of 95%O2 and 5% CO2 at 37°C. After incubation, the dimensions of the gut sac were measured and a sample from inside the sac was aspirated to establish serosal concentration. Mucosal and serosal samples were centrifuged and resulting supernatant diluted with PBS. Fluorescence was measured using a spectrophotometer at an excitation wavelength of 492 nm and an emission wavelength of 515 nm. Mucosal surface area, permeation rate and mucosal to serosal clearance of FD4 were calculated using published equations.
Peritoneal Lavage
Peritoneal fluid was collected by administering 5-6 mL of 0.9% saline into the peritoneum of a mouse with a 22g catheter taking care to avoid injury to internal organs or vessels. Fluid was withdrawn through the catheter after gently shaking the mouse.
Peripheral blood differential and cytokine analysis
Mice were anesthetized and blood was drawn from the inferior vena cava caudal to the splenic bifurcation using EDTA coated syringes immediately prior to sacrifice. Whole blood was either sent for complete blood count with manual differential (performed by Division of Comparative Medicine) or spun down for cytokine analysis according to manufacturer specifications using a cytometric bead array (BD Biosciences). Additionally, a single set of experiments was performed examining IL-10 levels by ELISA (DuoSet) according to manufacturer specifications, using blood taken 12 hours after CLP or sham laparotomy. The rationale for using ELISA on this single set of experiments was that they were performed prior to our having equipment to perform cytometric bead array, which was used on all subsequent cytokine experiments.
IEL Isolation
IELs were collected from the small intestine using a variation of a method described by Todd et al. (21). Following removal of the small intestine, fecal matter was removed by inserting a 14 gauge catheter into the duodenal opening and gently flushing the lumen with 10 mL of modified, cold RPMI (1L RPMI, 10% fetal calf serum, 1mL penicillin/streptomycin, 10mL glutamine, 0.1mM NEAA, 55uM beta-mercaptoethanol) over 3 minutes at low pressure to avoid perforation or damage to the mucus layer. The intestine was then flushed again at low pressure with 10 mL of modified, cold RPMI over several minutes and the eluate saved. This was followed by squeezing the intestine downward with thumb and forefinger from the duodenum to distal ileum without tearing, to extrude mucus and cellular contents. These two steps were repeated five times and all eluates and extrusions pooled together for each intestine. The eluate was then passed through a 70 micron nylon cell strainer. The suspension was then washed twice for 8 minutes at 1200 rpm at 4°C and the cells resuspended in modified RPMI and stimulated as described below.
Splenocyte Isolation
The spleen was removed from the abdomen, ground, and passed through a 70 micron nylon cell strainer with FACS buffer (+1mM EDTA). Cells were spun down and washed at 1200 rpm at 4°C for 10 minutes. After resuspension in 1mL of FACS buffer, red blood cells were removed by passing through Histopaque and washed again in FACS buffer. The cells were then resuspended in modified RPMI and stimulated as described below.
Lymphocyte Stimulation
Ten uL of cell suspension was used to determine cell count/mL by trypan blue exclusion. Percentage lymphocytes were assessed using flow cytometry (for IELs). Equal numbers of lymphocytes were incubated overnight with anti-CD3/CD28 or 10 μg/ml LPS (from E. coli 055: B5 cell-culture tested; Sigma, St. Louis, MO) at 37°C in a 5% CO2 cell incubator. Cell suspensions were spun down and the supernatant assayed for cytokines (22).
Endotoxin Determination
LAL Pyrochrome Chromogenic Test Kit (Associates of Cape Cod, Woods Hole, MA) was used according to manufacturer specifications to determine LPS concentration present in the bloodstream.
Bronchoalveolar Lavage
An incision was made along the midline of the neck exposing the trachea. The trachea was ligated proximally with 4-0 silk and a 22 gauge catheter inserted distal to the ligation. The lungs were then inflated with 1mL of 0.9% saline through the catheter and returning fluid was withdrawn slowly.
Statistical Analysis
Data comparing two groups was performed using the student’s T test. Differences in group survival were evaluated using log-rank analysis. All data was analyzed on the statistical program Prism 4.0 (GraphPad Software, San Diego, CA) and are presented as mean ± SEM. A p value ≤0.05 was considered to be significant.
RESULTS
Systemic Il-10 levels are similar in transgenic and WT mice
To confirm that Fabpi-IL-10 mice do not have elevated systemic IL-10 levels at baseline, IL-10 levels were assessed from unmanipulated WT and transgenic mice (Fig. 1). To examine whether the stress of surgery altered systemic IL-10 levels differently in transgenic and WT mice, both underwent sham laparotomy and had blood drawn 12 hours later. IL-10 levels were at or below the lower limits of detection in unmanipulated and sham operated mice (Fig. 1). Additionally, to determine whether gut-specific overexpression of IL-10 would change systemic levels of IL-10 following sepsis, Fabpi-IL-10 and WT were subjected to CLP and sacrificed 12 hours later. Both groups of animals had increased IL-10 levels compared to unmanipulated or sham animals, but there were no statistically significant differences between septic transgenic and septic WT mice (Fig. 1, p=0.43).
Fig. 1. Systemic IL-10 levels in WT and transgenic mice.
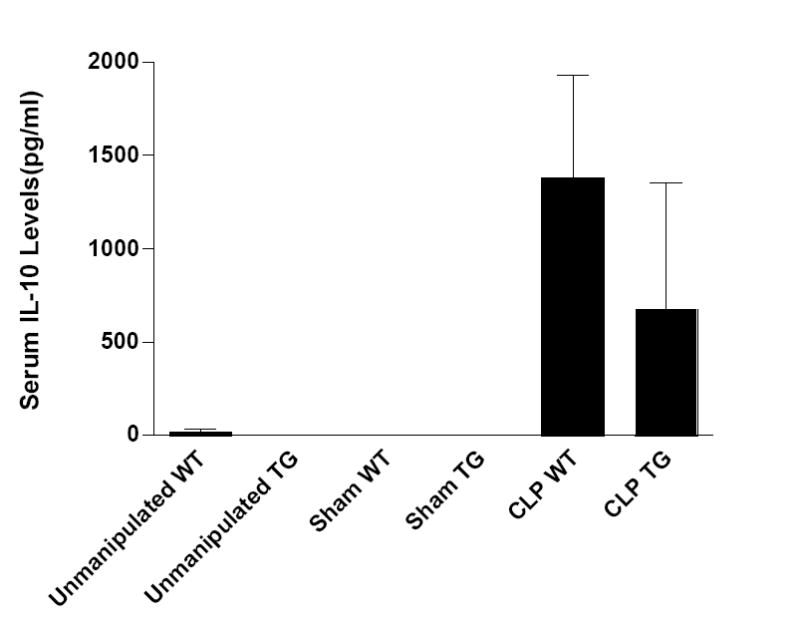
Serum IL-10 levels are either undetectable or at the lower limits of detection in both unmanipulated (n=4-5) WT and Fabpi-IL-10 mice as well as in those subjected to sham laparotomy (n=3-4). Serum IL-10 levels rise 12 hours after CLP but are statistically similar between WT and transgenic mice (n=7-9).
Intestine specific IL-10 overexpression improves survival following sepsis
WT and Fabpi-IL-10 mice (n=127 total) were subjected to a 1×27 CLP and followed seven days for survival. Transgenic animals had improved survival compared to WT littermates (p≤0.05, Fig. 2).
Fig. 2. Effect of intestine-specific IL-10 on mortality following CLP.
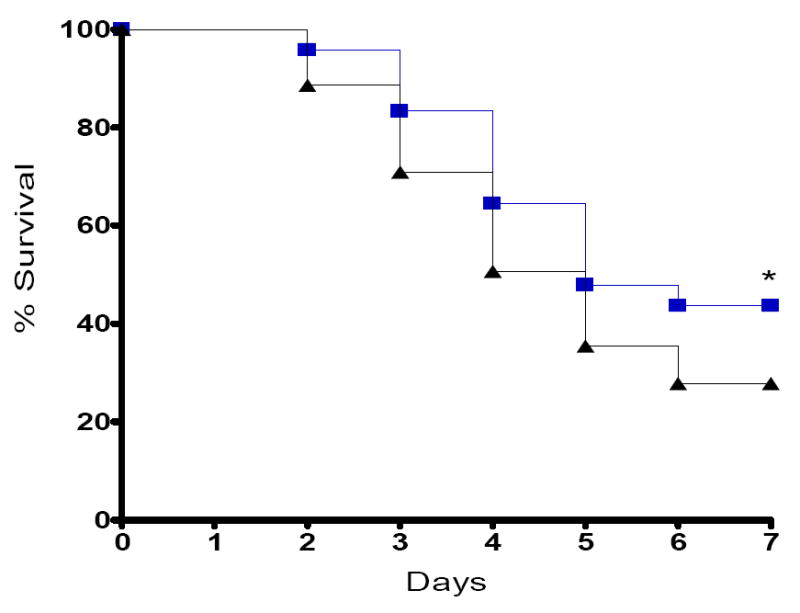
Seven-day survival is higher in Fabpi-IL-10 mice than WT mice following CLP. Survival curves begin separating two days following CLP and continue to be different one week following the onset of sepsis.
Effect of IL-10 overexpression on local physiologic parameters
Gut epithelial apoptosis was similar in WT and transgenic mice (Fig. 3). No differences in permeability to the fluorescent tracer FD-4 were noted between the two groups nor were there detectable differences when the intestine was stained for the chemokine monocyte chemoattractant protein-1 (MCP-1) or IL-6 (data not shown). Levels of TNFα, MCP-1 and IL-6 in peritoneal lavage fluid were elevated to a similar degree in both WT and transgenic animals (Fig. 4) while IFNγ and IL-12 were undetectable. To determine if IL-10 overexpression in the gut had secondary effects on immune cells within the intestine, IELs were also isolated and stimulated with anti-CD3/CD28. Stimulated IEL cytokine levels were similar between WT and transgenic animals (Fig. 5).
Fig. 3. Gut epithelial apoptosis 24 h following CLP.
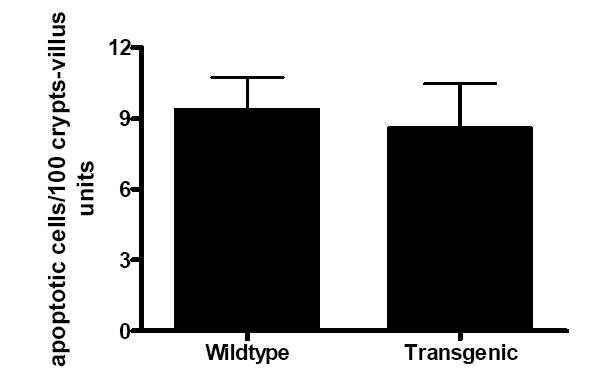
The number of apoptotic cells quantitated in H&E-stained sections is similar in both WT and transgenic mice (n=7-9 animals/group).
Fig. 4. Cytokine levels in peritoneal lavage fluid 24 h after CLP.
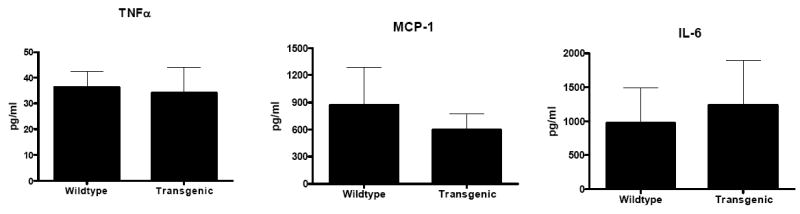
TNFα, MCP-1 and IL-6 levels are similar in both Fabpi-IL-10 and WT mice (n=7-9 animals/group).
Fig. 5. Cytokine levels in stimulated IELs 24 h after CLP.
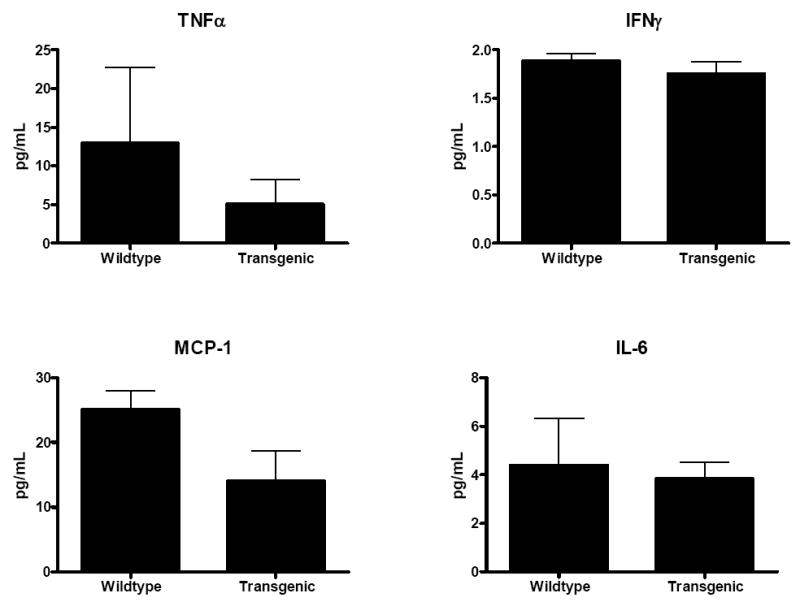
One day after sepsis was induced in WT and transgenic mice, IELs were isolated and stimulated with anti-CD3/CD28. Levels of TNFα, IFNγ, MCP-1 and IL-6 were similar in Fabpi-IL-10 and WT animals (n=7-8 animals/group).
Effect of IL-10 overexpression on systemic physiologic parameters
Analysis of pro- and anti-inflammatory serum cytokines TNFα, MCP-1, IL-10 and IL-6 24 hours after CLP demonstrates a trend towards higher levels in Fabpi-IL-10 mice compared to WT littermates but none of these reached statistically significance (Fig. 6). Circulating levels of LPS were similar between WT and transgenic mice (Fig. 7).
Fig. 6. Serum cytokines following CLP.
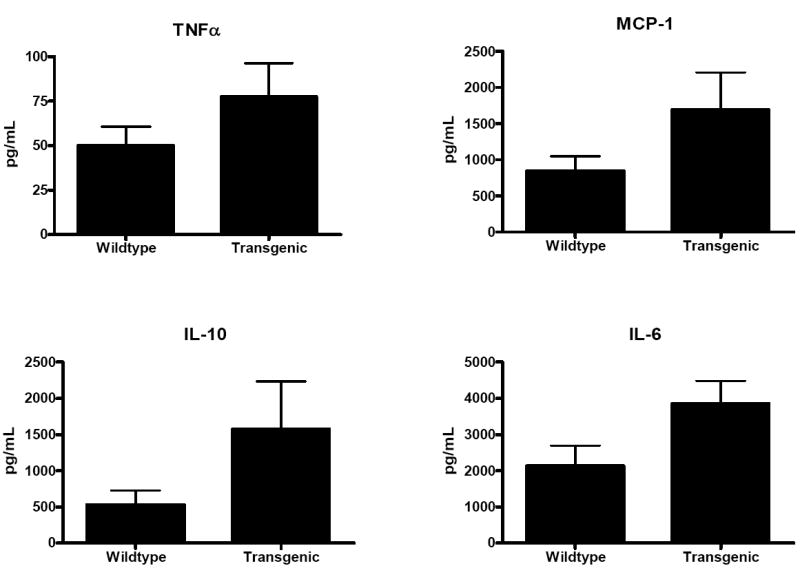
TNFα, MCP-1, IL-10 and IL-6 were statistically similar between Fabpi-IL-10 and WT mice 24 hours after CLP (n=8-10 animals/group).
Fig. 7. LPS levels 24 h following CLP.
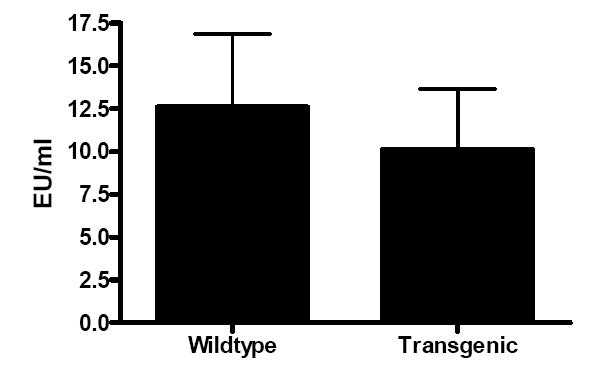
Circulating LPS levels were similar between Fabpi-IL-10 and WT mice (n= 7-8 animals/group). Intestinal IL-10 overexpression does not limit the release of this toxin from the gut in sepsis.
To determine if IL-10 overexpression in the gut had secondary effects on immune cells outside the intestine, splenocytes were isolated and stimulated with either anti-CD3/CD28 or LPS. With anti-CD3/CD28 stimulation, there was a significant decrease in stimulated levels of the pro-inflammatory cytokines MCP-1 and IL-6 24 hours after CLP without alterations in TNFα or IFNγ (Fig. 8). There were no differences in LPS reactivity between WT and transgenic mice in IL-6, TNFα or IFNγ ((Fig. 9). Of note, in contrast to anti-CD3/CD28 stimulation, MCP-1 levels were below the limit of detection following LPS stimulation. Since the gut has secondary effects on the lung (23) and BAL IL-10 levels have been demonstrated to be different in critical illness survivors (7), lung histology and BAL fluid were also evaluated. Pulmonary histology was similar in transgenic and WT mice and cytokine analysis of BAL fluid revealed no major increases in any cytokine measured in either group (data not shown).
Fig. 8. Cytokine levels in splenocytes stimulated with anti-CD3/CD28 24 h after CLP.
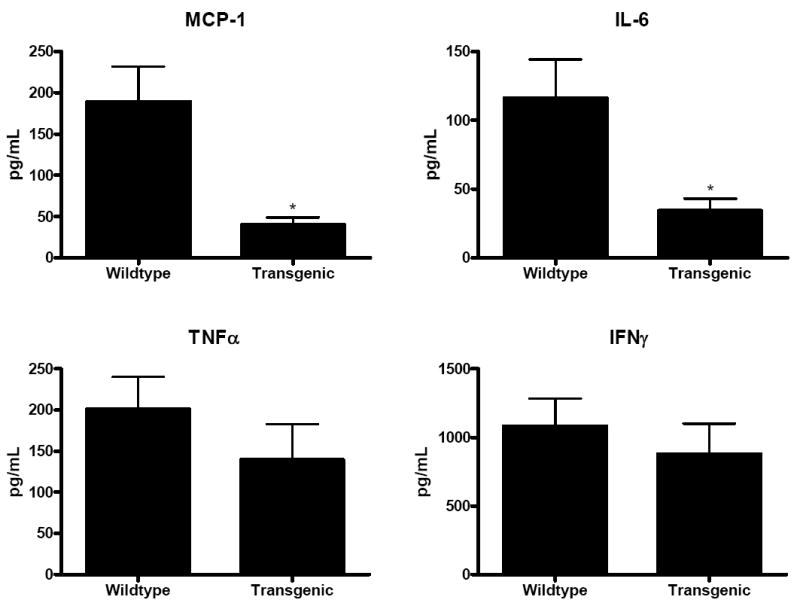
Levels of MCP-1 and IL-6 released from stimulated splenocytes were lower in Fabpi-IL-10 mice than in WT animals. Asterisks represents p values <0.05. In contrast, levels of TNFα and IFNγ levels produced by stimulated splenocytes was similar between transgenic and WT animals (n=8-10 animals/group).
Fig. 9. LPS reactivity of splenocytes 24 h after CLP.
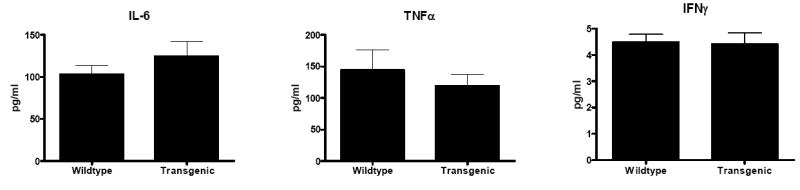
No statistically significant differences were identified between transgenic and WT mice in any cytokine evaluated (n= 7-8 animals/group).
An examination of peripheral blood demonstrated that white blood cell counts were lower in septic Fabpi-IL-10 mice than their WT littermates (Fig. 10). Manual differential revealed that this was due to a lower absolute neutrophil count, without differences in absolute lymphocyte count (Fig.10).
Fig. 10. White blood cell count and differential in transgenic and WT mice 24 hours after CLP.
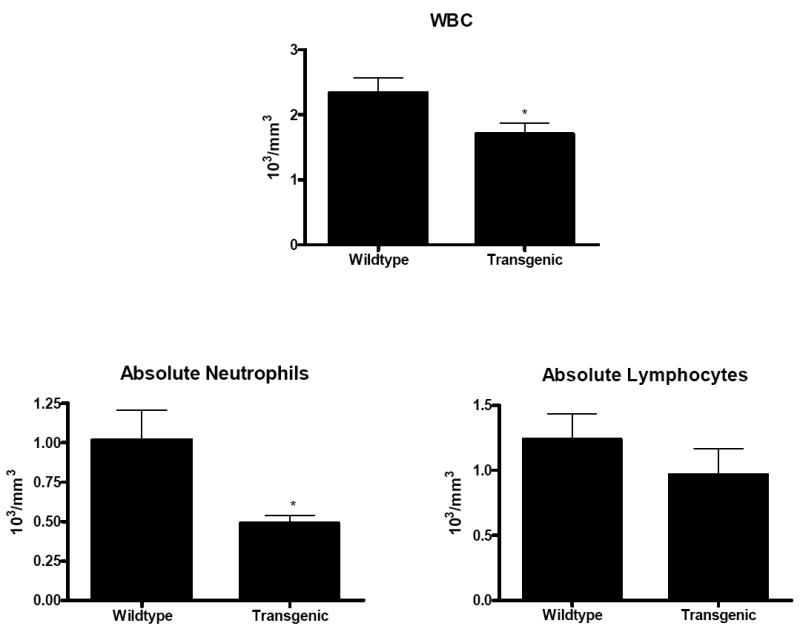
Total WBC count was lower in Fabpi-IL-10 mice. Manual differential demonstrates this is due to a decrease in absolute neutrophil count without alterations in absolute lymphocyte count (n=8-10 animals/group).
DISCUSSION
This study demonstrates that intestine-specific overexpression of IL-10 improves survival in a well-accepted model of murine sepsis. The survival advantage is associated with a decrease in pro-inflammatory cytokines released by activated splenocytes. However, it was not associated with differences in any local (intestinal) parameter measured, nor was it associated with any differences in serum or BAL cytokines or with circulating LPS levels or LPS reactivity. While intestinal IL-10 has long been understood to play a major role in the pathogenesis of chronic inflammatory bowel disease by interactions between the gut epithelium and the immune system, these results are the first to demonstrate the importance of gut-specific IL-10 in sepsis.
Theoretically, gut-specific overexpression of IL-10 could improve host survival in sepsis by affecting local or systemic parameters or impacting both intestinal and extra-intestinal physiology. Our results indicate that despite the gut-specific nature of the transgene, extraintestinal effects appear to predominate. Specifically, there appears to be cross-talk between the intestinal epithelium and the systemic immune system, wherein splenocytes generate a smaller pro-inflammatory response in Fabpi-IL-10 mice than in WT mice. This immune interaction appears to be compartmentalized since stimulated IL-6 and MCP-1 levels are lower in splenocytes but not in IELs. Further, this effect is independent of LPS since circulating endotoxim levels are similar in both transgenic and WT mice and LPS reactivity is similar in both. While alterations in stimulated cytokines are not associated with alterations in cytokine levels in serum, peritoneal lavage or bronchoalveolar lavage fluid, stimulated cytokines may actually be more reflective of what is happening in a host’s microenvironment than measurements obtained on a more global level. There are precedents in the literature where systemic and local cytokine cytokines vary widely. For instance, discrepancies between the significance of systemic IL-6 levels and local IL-6 levels in sepsis have previously been demonstrated (24). Multiple studies demonstrate that elevated serum IL-6 correlates with increased mortality (25-28) yet paradoxically this can simultaneously be associated with diminished local activity. It appears that both elevated and absent IL-6 are associated with increased mortality (29), and it is possible that the lower levels of IL-6 generated in septic Fabpi-IL-10 mice represent a more homeostatic middle ground than those seen in septic WT mice.
The fact that the survival advantage conferred in transgenic animals was independent of any intestinal parameter measured was a surprise. We assumed that alterations seen in IEL number and cytokine production in Fabpi-IL-10 mice under basal conditions (12) would have been exacerbated by the marked inflammatory state seen in sepsis. Similarly, since IL-10 has been shown to block T-cell induced apoptosis in intestinal epithelial cells in vitro (30) and preventing gut epithelial apoptosis is associated with improved survival in sepsis (31;32), it was reasonable to hypothesize that the survival benefit conferred with IL-10 overexpression would be associated with decreased intestinal cell death. However, this turned out not to be the case. Additionally, IL-10 knockout mice have increased intestinal permeability (33) while administration of IL-10 to septic mice has been shown to decrease intestinal permeability (34). However, despite the physiologic rationale for expecting differences between transgenic and WT mice, permeability, like other local parameters measured, was similar between the two groups. We therefore conclude that local parameters play a minor (if any) role in the saltatory effect of gut-specific IL-10 overexpression, while extraintestinal parameters resulting from gut-immune crosstalk predominate, with the gut functioning as the “motor” of the systemic inflammatory response syndrome primarily by affecting non-intestinal tissue.
The biological significance of the relative leukopenia seen in transgenic mice is unclear. While neutrophils play a key part of the innate response to local infection and injury, their continued presence results in collateral damage to host tissue and has been implicated in the pathology of the tissue injury associated with sepsis (35). However, we have no evidence to suggest that the small decreases in systemic neutrophil levels in transgenic mice correlate with altered neutrophil function at target organs or with changes in neutrophil demargination. It is possible that the static nature of obtaining only a single timepoint measurement (taken 24 hours before the earliest mortality of animals in either group) fails to account for a dynamic immune process that would have been apparent if white blood cell counts were measured at multiple timepoints. Alternatively, despite the fact that the neutrophil data comes from 18 animals that otherwise had similar hemoglobin and platelet levels on their complete blood counts, it is possible that this represents a difference that is statistically random or statistically significant but of limited biological significance.
This study has a number of limitations. At baseline, transgenic mice exhibit subtle differences locally in the intestine. Since we did not observe local differences in septic mice, this means either that a) the effects of sepsis overwhelm the basal differences between transgenic and WT mice or b) our interpretation that no meaningful local differences exist is incorrect and the apparent similarities actually represent a pseudonormalization phenomena. In light of the fact that Fabpi-IL-10 and WT mice appear grossly similar at baseline but have different mortalities in sepsis, we think it is more likely that the severe inflammatory changes induced by sepsis predominate over the basal local inflammatory changes; however, we cannot rule out that by overlaying a septic insult on two animals with subtly different local immune responses, we may be masking true differences in their inflammation-induced local immune response. Additionally, although the number of splenocytes in each sample was normalized at the outset and we have no evidence of differential lymphocyte apoptosis, we cannot rule out differential splenocyte death between samples during incubation as the cause of the observed differences in cytokine production. Also, although systemic IL-10 levels are statistically similar in transgenic and WT mice 12 and 24 hours following CLP, a comparison of figures 1 and 6 demonstrates a difference in the direction of the trend, with absolute IL-10 levels lower in transgenic mice at 12 hours and higher at 24 hours. While there is no evidence that transgenic mice are secreting IL-10 systemically in either this study or the initial description of Fabpi-IL-10 mice, it is possible that if we continued tracking levels at later timepoints, we may have seen statistically significant differences in serum IL-10 levels. Finally, transgenic studies have the inherent limitation of the lifelong presence of the transgene. Thus, IL-10 was overexpressed in Fabpi-IL-10 mice prior to the induction of sepsis, and we cannot conclude that similar results would be obtained if IL-10 was given in a tissue-specific manner in a post-treatment model. Finally, despite our novel finding that intestine-specific expression of IL-10 (as opposed to systemic IL-10 or targeted lung or thymic administration) is sufficient to decrease sepsis-induced mortality, the experiments presented herein fail to discover a definitive mechanism underlying the survival advantage in Fabpi-IL-10 mice.
Despite these limitations, our results demonstrate that intestine-specific overexpression of IL-10 confers a survival advantage in sepsis, and this is associated with differences in pro-inflammatory cytokine expression in activated splenocytes. Further investigation into crosstalk between the gut epithelium and the immune system should yield additional insights into why gut-specific IL-10 overexpression leads to improved survival from sepsis in the absence of any obvious differences in intestinal physiology.
Acknowledgments
We thank the Washington University Digestive Diseases Research Morphology Core for assistance with histologic studies. We thank Mitchell Fink, Runkuan Yang, Penny Sappington and Xiaonan Han for assistance with the permeability studies.
This study was supported by funding from National Institutes of Health (grant nos. GM072808, GM66202, T32 DK07130-32S, P30 DK52574).
Reference List
- 1.Marchant A, Alegre ML, Hakim A, Pierard G, Marecaux G, Friedman G, De GD, Kahn RJ, Vincent JL, Goldman M. Clinical and biological significance of interleukin-10 plasma levels in patients with septic shock. J Clin Immunol. 1995;15:266–273. doi: 10.1007/BF01540884. [DOI] [PubMed] [Google Scholar]
- 2.Scumpia PO, Moldawer LL. Biology of interleukin-10 and its regulatory roles in sepsis syndromes. Crit Care Med. 2005;33:S468–S471. doi: 10.1097/01.ccm.0000186268.53799.67. [DOI] [PubMed] [Google Scholar]
- 3.Pajkrt D, van der PT, Levi M, Cutler DL, Affrime MB, van den EA, ten Cate JW, van Deventer SJ. Interleukin-10 inhibits activation of coagulation and fibrinolysis during human endotoxemia. Blood. 1997;89:2701–2705. [PubMed] [Google Scholar]
- 4.van der PT, Marchant A, Buurman WA, Berman L, Keogh CV, Lazarus DD, Nguyen L, Goldman M, Moldawer LL, Lowry SF. Endogenous IL-10 protects mice from death during septic peritonitis. J Immunol. 1995;155:5397–5401. [PubMed] [Google Scholar]
- 5.Remick DG, Garg SJ, Newcomb DE, Wollenberg G, Huie TK, Bolgos GL. Exogenous interleukin-10 fails to decrease the mortality or morbidity of sepsis. Crit Care Med. 1998;26:895–904. doi: 10.1097/00003246-199805000-00025. [DOI] [PubMed] [Google Scholar]
- 6.Murphey ED, Sherwood ER. Bacterial clearance and mortality are not improved by a combination of IL-10 neutralization and IFN-gamma administration in a murine model of post-CLP immunosuppression. Shock. 2006;26:417–424. doi: 10.1097/01.shk.0000226343.70904.4f. [DOI] [PubMed] [Google Scholar]
- 7.Donnelly SC, Strieter RM, Reid PT, Kunkel SL, Burdick MD, Armstrong I, Mackenzie A, Haslett C. The association between mortality rates and decreased concentrations of interleukin-10 and interleukin-1 receptor antagonist in the lung fluids of patients with the adult respiratory distress syndrome. Ann Intern Med. 1996;125:191–196. doi: 10.7326/0003-4819-125-3-199608010-00005. [DOI] [PubMed] [Google Scholar]
- 8.McAuliffe PF, Murday ME, Efron PA, Scumpia PO, Ungaro R, Abouhamze A, Tannahill CL, Hutchins B, LaFace D, Moldawer LL. Dose-dependent improvements in outcome with adenoviral expression of interleukin-10 in a murine model of multisystem organ failure. Gene Ther. 2006;13:276–282. doi: 10.1038/sj.gt.3302600. [DOI] [PubMed] [Google Scholar]
- 9.Oberholzer C, Oberholzer A, Bahjat FR, Minter RM, Tannahill CL, Abouhamze A, LaFace D, Hutchins B, Clare-Salzler MJ, Moldawer LL. Targeted adenovirus-induced expression of IL-10 decreases thymic apoptosis and improves survival in murine sepsis. Proc Natl Acad Sci U S A. 2001;98:11503–11508. doi: 10.1073/pnas.181338198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- 11.Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De WH, Elewaut D, Turovskaya O, Huflejt M, Shimeld C, Hagenbaugh A, Binder S, Takahashi I, Kronenberg M, Cheroutre H. Regulation of mucosal immune responses by recombinant interleukin 10 produced by intestinal epithelial cells in mice. Gastroenterology. 2002;122:1829–1841. doi: 10.1053/gast.2002.33655. [DOI] [PubMed] [Google Scholar]
- 13.Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- 14.Carrico CJ, Meakins JL, Marshall JC, Fry D, Maier RV. Multiple-organ-failure syndrome. The gastrointestinal tract: the “motor” of MOF. Arch Surg. 1986;121:196–208. doi: 10.1001/archsurg.1986.01400020082010. [DOI] [PubMed] [Google Scholar]
- 15.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Baker CC, Chaudry IH, Gaines HO, Baue AE. Evaluation of factors affecting mortality rate after sepsis in a murine cecal ligation and puncture model. Surgery. 1983;94:331–335. [PubMed] [Google Scholar]
- 17.Husain KD, Stromberg PE, Woolsey CA, Turnbull IR, Dunne WM, Javadi P, Buchman TG, Karl IE, Hotchkiss RS, Coopersmith CM. Mechanisms of decreased intestinal epithelial proliferation and increased apoptosis in murine acute lung injury. Crit Care Med. 2005;33:2350–2357. doi: 10.1097/01.ccm.0000182797.89252.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turnbull IR, Buchman TG, Javadi P, Woolsey CA, Hotchkiss RS, Karl IE, Coopersmith CM. Age disproportionately increases sepsis-induced apoptosis in the spleen and gut epithelium. Shock. 2004;22:364–368. doi: 10.1097/01.shk.0000142552.77473.7d. [DOI] [PubMed] [Google Scholar]
- 19.Yang R, Han X, Uchiyama T, Watkins SK, Yaguchi A, Delude RL, Fink MP. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol. 2003;285:G621–G629. doi: 10.1152/ajpgi.00177.2003. [DOI] [PubMed] [Google Scholar]
- 20.Sims CA, Wattanasirichaigoon S, Menconi MJ, Ajami AM, Fink MP. Ringer’s ethyl pyruvate solution ameliorates ischemia/reperfusion- induced intestinal mucosal injury in rats. Crit Care Med. 2001;29:1513–1518. doi: 10.1097/00003246-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Todd D, Singh AJ, Greiner DL, Mordes JP, Rossini AA, Bortell R. A new isolation method for rat intraepithelial lymphocytes. J Immunol Methods. 1999;224:111–127. doi: 10.1016/S0022-1759(99)00015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 23.Deitch EA, Xu D, Kaise VL. Role of the gut in the development of injury- and shock induced SIRS and MODS: the gut-lymph hypothesis, a review. Front Biosci. 2006;11:520–528. doi: 10.2741/1816. [DOI] [PubMed] [Google Scholar]
- 24.Andrejko KM, Chen J, Deutschman CS. Intrahepatic STAT-3 activation and acute phase gene expression predict outcome after CLP sepsis in the rat. Am J Physiol. 1998;275:G1423–G1429. doi: 10.1152/ajpgi.1998.275.6.G1423. [DOI] [PubMed] [Google Scholar]
- 25.Remick DG, Bolgos GR, Siddiqui J, Shin J, Nemzek JA. Six at six: interleukin-6 measured 6 h after the initiation of sepsis predicts mortality over 3 days. Shock. 2002;17:463–467. doi: 10.1097/00024382-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Xiao H, Remick DG. Correction of perioperative hypothermia decreases experimental sepsis mortality by modulating the inflammatory response. Crit Care Med. 2005;33:161–167. doi: 10.1097/01.ccm.0000151049.19253.54. [DOI] [PubMed] [Google Scholar]
- 27.Vianna RC, Gomes RN, Bozza FA, Amancio RT, Bozza PT, David CM, Castro-Faria-Neto HC. Antibiotic treatment in a murine model of sepsis: impact on cytokines and endotoxin release. Shock. 2004;21:115–120. doi: 10.1097/01.shk.0000111828.07309.21. [DOI] [PubMed] [Google Scholar]
- 28.Vyas D, Javadi P, Dipasco PJ, Buchman TG, Hotchkiss RS, Coopersmith CM. Early antibiotic administration but not antibody therapy directed against IL-6 improves survival in septic mice predicted to die on basis of high IL-6 levels. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1048–R1053. doi: 10.1152/ajpregu.00312.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deutschman CS, Cereda M, Ochroch EA, Raj NR. Sepsis-induced cholestasis, steatosis, hepatocellular injury, and impaired hepatocellular regeneration are enhanced in interleukin-6 -/- mice. Crit Care Med. 2006;34:2613–2620. doi: 10.1097/01.CCM.0000240229.98275.07. [DOI] [PubMed] [Google Scholar]
- 30.Zhou P, Streutker C, Borojevic R, Wang Y, Croitoru K. IL-10 modulates intestinal damage and epithelial cell apoptosis in T cell-mediated enteropathy. Am J Physiol Gastrointest Liver Physiol. 2004;287:G599–G604. doi: 10.1152/ajpgi.00063.2004. [DOI] [PubMed] [Google Scholar]
- 31.Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM, Buchman TG, Karl IE, Hotchkiss RS. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002;287:1716–1721. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
- 32.Coopersmith CM, Chang KC, Swanson PE, Tinsley KW, Stromberg PE, Buchman TG, Karl IE, Hotchkiss RS. Overexpression of Bcl-2 in the intestinal epithelium improves survival in septic mice. Crit Care Med. 2002;30:195–201. doi: 10.1097/00003246-200201000-00028. [DOI] [PubMed] [Google Scholar]
- 33.Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis. 1999;5:262–270. doi: 10.1097/00054725-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Marshall JC. Neutrophils in the pathogenesis of sepsis. Crit Care Med. 2005;33:S502–S505. doi: 10.1097/01.ccm.0000186266.34541.5f. [DOI] [PubMed] [Google Scholar]


