Abstract
Cytochrome P450 2D6 (CYP2D6), an important CYP isoform with regard to drug-drug interactions, accounts for the metabolism of ∼30% of all medications. To date, few studies have assessed the effects of botanical supplementation on human CYP2D6 activity in vivo. Six botanical extracts were evaluated in three separate studies (2 extracts per study), each incorporating 18 healthy volunteers (9 females). Subjects were randomized to receive a standardized botanical extract for 14 days on separate occasions. A 30-day washout period was interposed between each supplementation phase. In study 1, subjects received milk thistle (Silybum marianum) and black cohosh (Cimicifuga racemosa). In study 2, kava kava (Piper methysticum), and goldenseal (Hydrastis canadensis) extracts were administered, and in study 3 subjects received St. John's wort (Hypericum perforatum) and Echinacea (Echinacea purpurea). The CYP2D6 substrate, debrisoquine (5 mg), was administered before and at the end of supplementation. Pre- and post-supplementation phenotypic trait measurements were determined for CYP2D6 using 8-hour debrisoquine urinary recovery ratios (DURR). Comparisons of pre- and post-supplementation DURR revealed significant inhibition (∼50%) of CYP2D6 activity for goldenseal, but not for the other extracts. Accordingly, adverse herb-drug interactions may result with concomitant ingestion of goldenseal supplements and drugs that are CYP2D6 substrates.
Keywords: Botanical supplements, cytochrome P450 2D6, herb-drug interactions, goldenseal, debrisoquine
INTRODUCTION
Over the past decade, an upsurge in botanical supplement usage, particularly within the United States, has engendered concerns among health care professionals regarding herb-drug interactions [1-3]. Such concerns may be well founded given that recent surveys indicate 21-31% of prescription drug users take medications concomitantly with herbal dietary supplements, oftentimes without notifying their physician [4-6]. One mechanism that appears to underlie many herb-drug interactions is the modulation of human cytochrome P450 drug metabolizing enzyme (CYP450) activity by unique phytochemicals found in botanical extracts [2,3]. CYP450s are heme-containing monooxygenases located in the small intestine, liver, and other tissues that play pivotal roles in the detoxification and bioactivation of diverse xenobiotic substances. Phytochemical-mediated changes in the activity of specific CYP450 isoforms (e.g. CYP1A2, CYP2D6, CYP3A4, etc.) may significantly alter the efficacy or toxicity of conventional medications whose biotransformation pathways are dependent on these same isoforms.
To date, the most recognized botanical supplement associated with CYP450-mediated herb-drug interactions is St. John's wort (Hypericum perforatum) [1-3,7-10]. When taken concomitantly, St. John's wort can render many medications less effective; the clinical consequences of which may be severe [11,12]. The phytochemical culprit underlying St. John's wort's drug interaction potential appears to be hyperforin, a phloroglucinol component that has a high affinity for the human steroid xenobiotic receptor (SXR) [13]. SXR is an orphan nuclear receptor that, when activated by various ligands, functions as a transcription factor for the human CYP3A4 gene resulting in over expression or induction of the CYP3A4 enzyme [13]. This interaction has significant clinical consequences since CYP3A4 facilitates the metabolism of almost 50% of all prescription medications, making it one of the most important drug metabolizing enzymes in humans.
Another important CYP450 with regard to herb-drug interactions is CYP2D6. CYP2D6 ranks second only to CYP3A4 in terms of the number of drugs whose biotransformation it facilitates. Drugs whose pharmacokinetic profiles are dictated by CYP2D6 include many antidepressants, antipsychotics, beta-receptor antagonists, analgesics, and antiarrhythmic agents. Many of these agents exhibit narrow therapeutic indices, and when coupled with the polymorphic nature of the CYP2D6 gene, individuals taking these drugs are especially susceptible to drug interactions [14]. While a host of clinical studies have evaluated the interaction potential of botanicals on CYP3A4 substrates [1-3, 7-10, 15-40], fewer have evaluated the effect of botanical supplementation on human CYP2D6 activity in vivo [29-40], and most of these have focused only on St. John's wort [29-31, 34, 39]. Interestingly, CYP2D6 does not appear to be readily inducible; [41-43] therefore, herb-mediated effects on this enzyme are likely to be limited to either inhibition or no effect at all. In this study we describe the effects of six top-selling botanical supplements (black cohosh, Echinacea, goldenseal, kava kava, milk thistle, and St. John's wort) on the activity of human CYP2D6 in vivo using a validated phenotyping technique, the debrisoquine urinary recovery ratio (DURR) [32, 44-47].
MATERIALS AND METHODS
Study subjects
This report summarizes the results of 3 separate studies each incorporating 2 botanical extract preparations. Each study protocol was approved by the University of Arkansas for Medical Sciences Human Research Advisory Committee (Little Rock, AR) and all participants provided written informed consent before commencing the study. Three separate groups of 16 young adults (8 females) (age, mean ± SD = 27 ± 6.0 years; weight, 75.8 ± 15.6 kg) were recruited for each study. All subjects were in good health as indicated by medical history, routine physical examination, and clinical laboratory testing. All subjects were extensive metabolizers of CYP2D6 as confirmed by debrisoquine urinary recovery screenings [45]. All subjects were nonsmokers, ate a normal diet, and were not users of botanical dietary supplements, nor were they taking any prescription medications. All female subjects had a negative pregnancy test at baseline and all subjects were instructed to use a barrier method of contraception during the study. Participants were instructed to abstain from alcohol, caffeine, fruit juices, cruciferous vegetables, and charbroiled meat throughout the study. Adherence to these restrictions was further emphasized five days before probe drug administration. Participants were also asked to refrain from taking prescription and nonprescription medications during supplementation periods. Documentation of compliance to these restrictions was achieved through the use of a food/medication diary.
Due to reports of possible hepatotoxicity associated with prolonged kava kava use [48, 49], blood chemistry profiles, including assessment of various liver function enzymes (AST, ALT, and GGT), were evaluated in each subject before and at the end of each supplementation period.
Supplementation and phenotyping procedure
Each of the 3 studies utilized an open-label design randomized for supplementation sequence. In study 1, the ability of black cohosh and milk thistle extracts to modulate human CYP2D6 activity was evaluated individually on separate occasions. Study 2 evaluated the effects of goldenseal and kava on CYP2D6, while subjects in study 3 received St. John's wort and Echinacea purpurea. Each supplementation period lasted 14 days and was followed by a 30-day washout period. This randomly assigned sequence of supplementation followed by washout was repeated until each subject, in their respective study, had received both botanical supplements. Product labels were followed regarding the administration of milk thistle extract (Enzymatic Therapy, Inc. Green Bay, WI, lot #41678, 300 mg, three times daily, standardized to contain 80% silymarin per capsule); black cohosh extract (Enzymatic Therapy, Inc., Green Bay, WI, lot #41924, 40 mg, twice daily, standardized to 2.5% triterpene glycosides per tablet); goldenseal root extract (Nature's Resource Products, Mission Hills, CA, lot #OI10184, 1,070 mg, three times daily, standardized to contain 24.1 mg isoquinoline alkaloids per capsule); kava kava rhizome extract (Nature's Resource Products, Mission Hills, CA, lot #A10062504, 136.3 mg, three times daily, standardized to contain 75 mg kava lactones per capsule); St. John's wort extract (Nature's Way, Springville, UT, lot #530812, 300 mg, three times daily, standardized to contain 3% hyperforin) and Echinacea purpurea extract (Gaia Herbs, Inc., Brevard, NC, lot #41924, 267 mg, three times daily, standardized to contain 2.2 mg isobutylamides per capsule). Telephone and electronic mail reminders were used to facilitate compliance, while pill counts and supplementation usage records, were used to verify compliance.
CYP2D6 phenotypes were assessed before (Day 0) and at the end of each supplementation phase (Day 14). The day before supplementation (Day 0), subjects emptied their bladder prior to ingesting a 5mg oral dose of debrisoquine. Urine was then collected for 8 hours, at which time the volume was recorded and a 10-milliliter aliquot stored for analysis. All samples were stored frozen at −70°C until analyzed. CYP2D6 phenotypes were again assessed on supplementation Day 14. The CYP2D6 modulatory capability of each botanical supplement was evaluated by comparing individual differences in phenotype before and at the end of 14 days of supplementation.
Phenotype Assessment
CYP2D6 activity was assessed using 8-hour debrisoquine urinary recovery ratios (DURR): [4-hydroxydebrisoquine/(debrisoquine + 4-hydroxydebrisoquine)]. Justification of DURR as a phenotypic probe for CYP2D6 has been previously addressed [32, 39, 44-47].
Analytical methods
The HPLC method described by Frye and Branch employing fluorescence detection was utilized for the quantitation of debrisoquine and 4-hydroxydebrisoquine in urine [50].
The phytochemical content of each supplement was independently analyzed for specific “marker compounds” by various HPLC, capillary electrophoresis, and mass spectrometric methods. The isoquinoline alkaloid content (hydrastine and berberine) of goldenseal was performed via the method of Abourashed and Khan [51]. Quantitation of kava lactones (kavain, dihydrokavain, methysticin, dihydromethysticin, yangonin, and desmethoxyyangonin) was performed per the method of Ganzera and Khan [52]. Black cohosh was analyzed for triterpene glycosides (cimiracemosides, cimicifugoside, 27-deoxyactein, and actein) using reversed phase HPLC with evaporative light scattering detection as described by Ganzera et al [53]. Flavanolignan (taxifolin, silychristin, silydianin, silibinin A, and silibinin B) content of milk thistle was quantitated using a previously published gradient HPLC method [54]. Quantitative determination of hyperforin, hypericin, and various flavonoids in St. John's wort was achieved by HPLC using photodiode array detection with confirmation via mass spectrometry according to the method of Liu et al [55]. E. purpurea was analyzed for various phenolic acids (e.g. chicoric acid, echinacoside, and caftaric acid) and isobutylamide content using a proprietary gradient HPLC method similar to that described by Molgaard et al. [56.]
Disintegration tests
An absence of botanical-mediated changes in CYP phenotype could stem from products exhibiting poor disintegration and/or dissolution characteristics. To address this concern, each product was subjected to disintegration testing as outlined in the United States Pharmacopeia 27 [57]. The disintegration apparatus consisted of a basket-rack assembly operated at 29-32 cycles per minute with 0.1 N HCl (37°C) as the immersion solution. One dosage unit of each supplement was placed into each of the six basket assembly tubes. The time required for the complete disintegration of six dosage forms was determined. This process was repeated with an additional six dosage units to assure accuracy. Since there are no specifications for the disintegration time of the botanical supplements used in this study, the mean of six individual dosage forms was taken as the disintegration time for that particular product. A product (e.g. hard gelatin capsule, soft gelatin capsule, uncoated tablet) was considered completely disintegrated if the entire residue passed through the mesh screen of the test apparatus, except for capsule shell fragments, or if the remaining soft mass exhibited no palpably firm core.
Statistics
A repeated measures ANOVA model was fit for each phenotype response using SAS Proc Mixed software (SAS Institute, Inc. Cary, N.C.). Since pre- and post-supplementation phenotypic ratios were determined in each subject for all four supplements, a covariance structure existed for measurements within subjects. Sex, supplement, and supplement-by-sex terms were estimated for each phenotype using a Huynh-Feldt covariance structure fit. If supplement-by-sex interaction terms for a specific phenotypic measure were significant at the 5% level, the focus of the post-supplementation minus pre-supplementation response was assessed according to sex. If the supplement-by-sex interaction was not statistically significant, responses for both sexes were combined. Additionally, a power analysis was performed to estimate the ability to detect significant post- minus pre-supplementation effects. At least 80% power at the 5% level of significance to detect a Cohen effect size of 1.32 to 1.71 standard deviation units was obtainable with this design. [58]
RESULTS
General Experimental Observations
No serious adverse events occurred during the course of the study. Participants in the 3 studies did note a few minor side effects during supplementation. They included an increase in headaches reported by 3 subjects taking milk thistle and 2 subjects taking kava kava. One subject associated black cohosh with the onset of “vivid dreams,” while drowsiness was noted by 2 subjects taking St. John's wort. Those taking Echinacea or goldenseal reported no significant side effects. Nor were any side effects associated with the ingestion of debrisoquine. No significant changes were noted in serum concentrations of AST (25.2 ± 4.6 vs. 25.0 ± 9.2 IU/L), ALT (26.0 ± 12.0 vs. 25.9 ± 14.1 IU/L), or GGT (22.2 ± 12.6 vs. 22.3 ± 13.0 IU/L) following kava kava supplementation.
Effect of Supplementation on CYP2D6 Phenotype
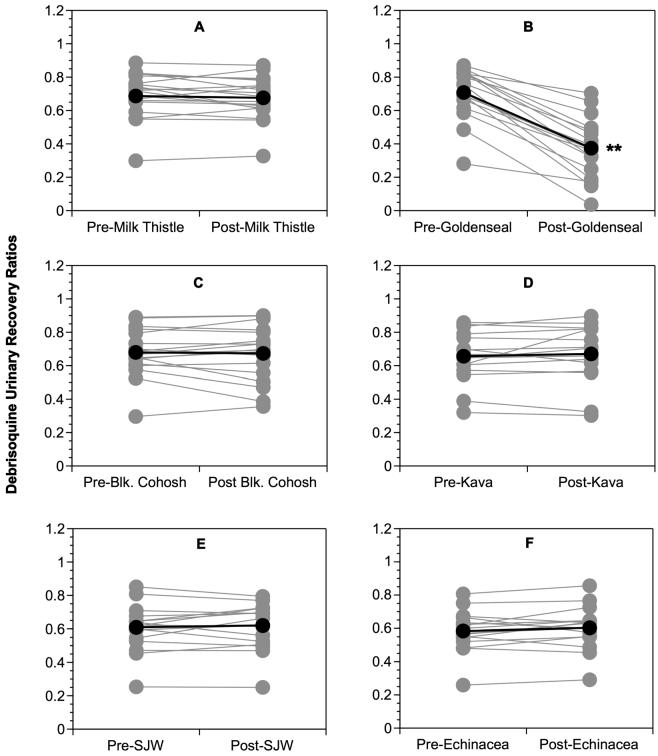
The effects of 14 days of milk thistle, goldenseal, black cohosh, kava kava, St. John's wort, and Echinacea extract supplementation on DURR are shown in Figure 1 and Table 1. For each of the 3 studies, no significant differences were observed among mean baseline DURR (Figure 1, Table 1). Of the six botanicals tested, only goldenseal produced significant reductions in DURR (p < 0.05, Figure 1B, Table 1), however, this effect was not sex-related.
Figure 1.
Comparison of pre- and post-supplementation phenotypic ratios (8-hour debrisoquine urinary recovery ratios) for CYP2D6. (A) Milk thistle, (B) Goldenseal, (C) Black cohosh, (D) Kava kava, (E) St. John's wort, and (F) Echinacea. Gray circles, Individual values; Black circles, Group means. Asterisks = statistically significant difference from baseline.
Table 1.
Pre- and Postsupplementation Debrisoquine Urinary Recovery Ratios
| Supplement | Presupplementation | Postsupplementation. | Difference | Post/Pre Ratios† |
|---|---|---|---|---|
| (mean and 95% CI) | (mean and 95% CI) | (mean and 95% CI) | (geo. mean and 90% CI) | |
| Goldenseal | 0.708 (0.632 to 0.785) | 0.374 (0.285 to 0.463) | −0.334 (−0.270 to −0.398)* | 0.53 (0.44 to 0.64)* |
| Kava kava | 0.654 (0.568 to 0.739) | 0.669 (0.573 to 0.765) | 0.015 (0.021 to −0.052) | 1.01 (0.96 to 1.06) |
| Black cohosh | 0.684 (0.610 to 0.759) | 0.679 (0.593 to 0.765) | 0.005 (0.041 to −0.030) | 0.99 (0.94 to 1.04) |
| Milk thistle | 0.694 (0.621 to 0.767) | 0.672 (0.602 to 0.743) | −0.022 (0.051 to −0.007) | 0.97 (0.93 to 1.10) |
| St. John's wort | 0.606 (0.522 to 0.691) | 0.620 (0.537 to 0.703) | 0.014 (0.022 to −0.049) | 1.02 (0.97 to 1.07) |
| Echinacea | 0.583 (0.514 to 0.653) | 0.601 (0.530 to 0.673) | 0.018 (0.013 to −0.048) | 1.03 (0.98 to 1.07) |
CI = confidence interval, HDEB = 6-hydroxydebrisoquine, DEB = debrisoquine
= geometric mean of postsupplementation/presupplementation ratios and 90% CI
= p < 0.05
Phytochemical Content and Disintegration Testing
Analytical determinations of various “marker” phytochemicals and the daily amount ingested by each subject are represented in Table 2. Intra- and interday relative standard deviations for each assay were less than 10%. Table 2 also depicts the mean disintegration time for each supplement dosage form. Disintegration times for dosage forms containing goldenseal, kava kava, black cohosh, milk thistle and Echinacea extracts were less than 13 minutes, while coated tablets containing St. John's wort required almost 45 minutes to disintegrate.
Table 2.
Phytochemical analysis and disintegration times for botanical dosage forms.
|
Supplement (dosage form) |
Compound |
Content (mg/capsule) |
Daily Dose) (mg) |
Disintegration Time (minutes) |
|---|---|---|---|---|
| Goldenseal (hard gelatin capsule) |
Isoquinoline alkaloids | 3.5 | ||
| Hydrastine | 22.0 | 132.0 | ||
| Berberine | 12.8 | 76.8 | ||
| Total | 34.8 | 208.8 | ||
| Kava kava (softgel capsule) |
Kava lactones | 12.0 | ||
| Kavain | 23.1 | 69.3 | ||
| Dihydrokavain | 19.8 | 59.4 | ||
| Methysticin | 9.9 | 29.7 | ||
| Dihydromethysticin | 13.1 | 39.3 | ||
| Yangonin | 11.4 | 34.2 | ||
| Desmethoxyyangonin | 7.2 | 21.6 | ||
| Total | 84.5 | 253.5 | ||
| Milk Thistle (softgel capsule) |
Silymarin | 12.6 | ||
| Silibinin A | 17.3 | 103.8 | ||
| Silibinin B | 30.5 | 183.0 | ||
| Silichristin | 18.6 | 111.6 | ||
| Silidianin | 5.4 | 32.4 | ||
| Taxifolin | 1.6 | 9.6 | ||
| Total | 73.4 | 440.4 | ||
| Black Cohosh (uncoated tablet) |
Triterpene glycosides | 3.5 | ||
| Actein | 0.17 | 0.68 | ||
| 27-deoxyactein | 0.13 | 0.52 | ||
| Cimiracemosides A,C,E,F | 0.14 | 0.56 | ||
| Cimicifugoside | 0.21 | 0.84 | ||
| Triterpene A | 0.05 | 0.20 | ||
| Triterpene B | 0.03 | 0.12 | ||
| Total | 0.73 | 2.92 | ||
| St. John's wort (coated tablet) |
Hyperforin | 8.0 | 24.0 | 44.8 |
| Hypericin | 0.1 | 0.3 | ||
| Pseudohypericin | 0.3 | 0.9 | ||
| Flavonoids | ||||
| Rutin | 5.6 | 16.8 | ||
| Hyperoside | 3.6 | 10.8 | ||
| Isoquercetin | 2.1 | 6.3 | ||
| Quercetrin | 0.8 | 2.4 | ||
| Quercetin | 0.8 | 2.4 | ||
| Total | 21.3 | 63.9 | ||
| Echinacea (softgel capsule) |
Phenolic acids | 6.1 | ||
| Chicoric acid | 3.3 | 29.7 | ||
| Echinacoside | 1.3 | 11.7 | ||
| Caftaric acid | 1.4 | 12.6 | ||
| Cynarin | 0.3 | 2.7 | ||
| Chlorogenic acid | 0.2 | 1.8 | ||
| Isobutylamides | 1.3 | 11.7 | ||
| Total | 7.8 | 70.2 | ||
DISCUSSION
Considerable evidence from both in vitro and animal studies suggests that CYP2D6 activity can be inhibited by goldenseal [59, 60], milk thistle [61, 62], kava kava [59, 63-65], Echinacea [59], and St. John's wort [59, 66-68]. Currently, no in vitro results are available for black cohosh's effect on CYP2D6, although certain triterpene glycosides isolated from black cohosh modestly inhibited human CYP3A4 in vitro [69]. However, evidence of in vitro CYP450 inhibition, or that observed in animal models, may not accurately predict in vivo effects observed in humans [70]. Our purpose in this series of clinical studies was to assess whether botanical supplement formulations containing either goldenseal, milk thistle, kava kava, Echinacea, St. John's wort, or black cohosh could modulate human CYP2D6 activity using standard phenotyping techniques.
Five of the botanicals evaluated in these 3 studies exerted no significant effects on CYP2D6 activity as determined by DURR. These included milk thistle, kava kava, Echinacea, black cohosh and St. John's wort. Our clinical findings run counter to many of the in vitro predictions of CYP2D6 inhibition by these supplements [59-68]. Significant divergence between in vitro predictions and in vivo realities are not uncommon. Reasons for such in vivo/in vitro discrepancies have been discussed in detail by von Moltke [70], but certain other basic pharmaceutics issues relevant to dietary supplements may also contribute; these include poor dissolution characteristics of botanical formulations and significant inter-product variability in phytochemical content [52, 71-74]. Still other considerations include extensive pre-systemic in vivo conjugation of phytochemicals via Phase II enzymes (e.g. glucuronidation, sulfation, glycination), a process that may preclude CYP inhibition by these compounds [75]. Nevertheless, our results confirm previous clinical findings that milk thistle [39], kava kava [76], Echinacea [37, 39], black cohosh [39], and St. John's wort [29-32, 34, 38, 40] are not potent modulators of human CYP2D6 in vivo. Several of the clinical studies involving St. John's wort, however, utilized dextromethorphan/dextrorphan urinary ratios as a phenotypic measure of CYP2D6 activity [29-31, 34, 38], and this particular approach has been recently called into question [46, 77, 78]. This uncertainty stems from the fact that normal physiological changes in urine pH can alter the dextromethorphan/dextrorphan ratio by as much as 20-fold [77]. DURR, however, is not affected by urine pH and thus appears to be a more reliable phenotypic measure of CYP2D6 activity [46, 47]. Collectively, these studies, particularly those using DURR, strongly suggest that these 5 botanicals pose little to no significant concerns regarding pharmacokinetic herb-drug interactions with drugs metabolized by CYP2D6.
Of the 6 botanical extracts evaluated in this series of clinical studies, only goldenseal appeared to have significant inhibitory effects on human CYP2D6 in vivo. The almost 50% reduction in mean post-supplementation DURR signifies that goldenseal is a potent inhibitor of human CYP2D6 in vivo. Moreover, these results corroborate an earlier report by our group, which utilized a different goldenseal formulation [76]. Our findings also bolster recent in vitro investigations demonstrating inhibition of CYP2D6-mediated biotransformations by goldenseal extracts [59, 60]. During an evaluation of a series of single-entity herbal tea extracts, Foster et al observed that Hydrastis canadensis (goldenseal) produced the greatest percent inhibition of cDNA expressed human CYP2D6 [59]. In addition, Chatterjee and Franklin observed that goldenseal extract and its principal isoquinoline alkaloids, berberine and hydrastine, inhibited CYP2D6-mediated bufuralol 1'-hydroxylation in human hepatic microsomes [60]. Of the two alkaloids, berberine exhibited a greater effect on bufuralol 1'-hydroxylation (IC50 = 45 μM) than hydrastine (IC50 = 350 μM), signifying a greater contribution of this phytochemical to CYP2D6 inhibition.
At present, little is known about the pharmacokinetics of goldenseal alkaloids in humans, but animal studies indicate that berberine bioavailablity is relatively low [79, 80]. Although the daily dose of isoquinoline alkaloids ingested in the present study was 209 mg (Table II), plasma concentrations of berberine and hydrastine were not determined; nevertheless, the significant effect observed on CYP2D6 phenotype indicates that phytochemicals present in goldenseal can cross the intestinal mucosa. Taken together, both in vitro and in vivo findings imply that goldenseal, if taken concomitantly with CYP2D6 substrates, may give rise to clinically significant herb-drug interactions.
In summary, the data presented herein supports previous clinical investigations that milk thistle, kava kava, black cohosh, Echinacea, and St. John's wort are not potent modulators of human CYP2D6 in vivo. Thus, concomitant ingestion of these specific botanicals with drugs that are CYP2D6 substrates is not likely to result in clinically relevant herb-drug interactions. On the other hand, goldenseal significantly inhibits human CYP2D6 in vivo and may give rise to significant pharmacokinetic herb-drug interactions. Accordingly, patients should be strongly discouraged from taking goldenseal-containing supplements concomitantly with prescription medications, especially those extensively metabolized by CYP2D6.
Acknowledgements
This work was supported by the NIH/NIGMS under grant RO1 GM71322 and by the NIH/NCRR to the General Clinical Research Center of the University of Arkansas for Medical Sciences under grant M01 RR14288.
Abbreviations
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- CYP
cytochrome P450
- CYP2D6
cytochrome P450 2D6
- DEB
debrisoquine
- DURR
debrisoquine urinary recovery ratio
- GGT
gamma-glutamyl transferase
- HDEB
4-hydroxydebrisoquine
REFERENCES
- 1.Brazier NC, Levine MAH. Drug-herb interaction among commonly used conventional medicines: a compendium for health care professionals. Am. J. Ther. 2003;10:163–169. doi: 10.1097/00045391-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Hu Z, Yang X, Ho PCL, Chan SY, et al. Herb-drug interactions: a literature review. Drugs. 2005;65:1239–1282. doi: 10.2165/00003495-200565090-00005. [DOI] [PubMed] [Google Scholar]
- 3.Johne A, Roots I. Clinical drug interactions with medicinal herbs. Evid. Based Integrative Med. 2005;2:207–228. [Google Scholar]
- 4.Wold RS, Lopez ST, Yau L, Butler LM, et al. Increasing trends in elderly persons' use of nonvitamin, nonmineral dietary supplements and concurrent use of medications. J. Am. Diet. Assoc. 2005;105:54–63. doi: 10.1016/j.jada.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Shahrokh LE, Lukaszuk JM, Prawitz AD. Elderly herbal supplement users less satisfied with medical care than nonusers. J. Am. Diet. Assoc. 2005;105:1138–1140. doi: 10.1016/j.jada.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Gardiner P, Graham RE, Legedza ATR, Eisenberg DM, Phillips RS. Factors associated with dietary supplement use among prescription medication users. Arch. Intern. Med. 2006;166:1968–1974. doi: 10.1001/archinte.166.18.1968. [DOI] [PubMed] [Google Scholar]
- 7.Delgoda R, Westlake ACG. Herbal interactions involving cytochrome P450 enzymes. Toxicol. Rev. 2004;23:239–249. doi: 10.2165/00139709-200423040-00004. [DOI] [PubMed] [Google Scholar]
- 8.Zhou S, Chan E, Pan S-Q, Huang M, Lee EJD. Pharmacokinetic interactions of drugs with St John's wort. J. Psychopharmacol. 2004;18:262–276. doi: 10.1177/0269881104042632. [DOI] [PubMed] [Google Scholar]
- 9.Tirona RG, Bailey DG. Herbal product–drug interactions mediated by induction. Br. J. Clin. Pharmacol. 2006;61:677–681. doi: 10.1111/j.1365-2125.2006.02684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitten DL, Myers SP, Hawrelak JA, Wohlmuth H. The effect of St John's wort extracts on CYP3A4: a systematic review of prospective clinical trials. Br. J. Clin. Pharmacol. 2006;62:512–526. doi: 10.1111/j.1365-2125.2006.02755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barone GW, Gurley BJ, Ketel BL, Lightfoot ML, Abul-Ezz SR. Drug interaction between St. John's wort and cyclosporine. Ann. Pharmacother. 2000;34:1013–1016. doi: 10.1345/aph.10088. [DOI] [PubMed] [Google Scholar]
- 12.Barone GW, Gurley BJ, Ketel BL, Abul-Ezz SR. Herbal dietary supplements: a source for drug interactions in transplant recipients. Transplantation. 2001;71:239–241. doi: 10.1097/00007890-200101270-00012. [DOI] [PubMed] [Google Scholar]
- 13.Wentworth JM, Agostini M, Love J, Schwabe JW, Chatterjee VKK. St. John's wort, a herbal antidepressant, activates the steroid X receptor. J. Endocrinol. 2000;166:R11–R16. doi: 10.1677/joe.0.166r011. [DOI] [PubMed] [Google Scholar]
- 14.Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2005;5:6–13. doi: 10.1038/sj.tpj.6500285. [DOI] [PubMed] [Google Scholar]
- 15.Sugimoto K, Ohmori M, Tsuruoka S, Nishiki K, et al. Different effects of St. John's wort on the pharmacokinetics of simvastatin and pravastatin. Clin. Pharmacol. Ther. 2001;70:518–524. doi: 10.1067/mcp.2001.120025. [DOI] [PubMed] [Google Scholar]
- 16.Piscitelli SC, Burstein AH, Welden N, Gallicano KD, Falloon J. The effect of garlic supplements on the pharmacokinetics of saquinavir. Clin. Infect. Dis. 2002;34:234–238. doi: 10.1086/324351. [DOI] [PubMed] [Google Scholar]
- 17.Gallicano K, Foster B, Choudhri S. Effect of short-term administration of garlic supplements on single-dose ritonavir pharmacokinetics in healthy volunteers. Br. J. Clin. Pharmacol. 2003;55:199–202. doi: 10.1046/j.1365-2125.2003.01736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sandhu RS, Prescilla RP, Simonelli TM, Edwards DJ. Influence of goldenseal root on the pharmacokinetics of indinavir. J. Clin. Pharmacol. 2003;43:1283–1288. doi: 10.1177/0091270003258660. [DOI] [PubMed] [Google Scholar]
- 19.Hall SD, Wang Z, Huang S-M, Hamman MA, et al. The interaction between St John's wort and an oral contraceptive. Clin. Pharmacol. Ther. 2003;74:525–535. doi: 10.1016/j.clpt.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Anderson GD, Rosito G, Mohustsy MA, Elmer GW. Drug interaction potential of soy extract and panax ginseng. J. Clin. Pharmacol. 2003;43:643–648. [PubMed] [Google Scholar]
- 21.DiCenzo R, Shelton M, Jordan K, Koval C, et al. Coadministration of milk thistle and indinavir in healthy subjects. Pharmacotherapy. 2003;23:866–870. doi: 10.1592/phco.23.7.866.32723. [DOI] [PubMed] [Google Scholar]
- 22.Rajnarayana K, Reddy MS, Vidyasagar J, Krishna DR. Study on the influence of silymarin pretreatment on metabolism and disposition of metronidazole. Arzneim. Forsch./Drug Res. 2004;54:109–113. doi: 10.1055/s-0031-1296944. [DOI] [PubMed] [Google Scholar]
- 23.van Erp NPH, Baker SD, Zhao M, Rudek MA, et al. Effect of milk thistle (Silybum marianum) on the pharmacokinetics of irinotecan. Clin. Cancer Res. 2005;11:7800–7806. doi: 10.1158/1078-0432.CCR-05-1288. [DOI] [PubMed] [Google Scholar]
- 24.Mills E, Wilson K, Clarke M, Foster B, et al. Milk thistle and indinavir: a randomized controlled pharmacokinetics study and meta analysis. Eur. J. Clin. Pharmacol. 2005;61:1–7. doi: 10.1007/s00228-004-0843-z. [DOI] [PubMed] [Google Scholar]
- 25.Gurley B, Hubbard MA, Williams DK, Thaden J, et al. Assessing the clinical significance of botanical supplementation on human cytochrome P450 3A activity: comparison of a milk thistle and black cohosh product to rifampin and clarithromycin. J. Clin. Pharmacol. 2006;46:201–213. doi: 10.1177/0091270005284854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox MC, Low J, Lee J, Walshe J, et al. Influence of garlic (Allium sativum) on the pharmacokinetics of docetaxel. Clin. Cancer Res. 2006;12:4636–4640. doi: 10.1158/1078-0432.CCR-06-0388. [DOI] [PubMed] [Google Scholar]
- 27.Uchida S, Yamada H, Li XD, Maruyama S, et al. Effects of ginkgo biloba extract on pharmacokinetics and pharmacodynamics of tolbutamide and midazolam in healthy volunteers. J. Clin. Pharmacol. 2006;46:1290–1298. doi: 10.1177/0091270006292628. [DOI] [PubMed] [Google Scholar]
- 28.Portolés A, Terleira A, Calvo A, Martínez I, et al. Effects of Hypericum perforatum on ivabradine pharmacokinetics in healthy volunteers: an open-label, pharmacokinetic interaction clinical trial. J. Clin. Pharmacol. 2006;46:1188–1194. doi: 10.1177/0091270006291623. [DOI] [PubMed] [Google Scholar]
- 29.Markowitz JS, DeVane CL, Boulton DW, Carson SW. Effect of St. John's wort (Hypericum perforatum) on cytochrome P-450 2D6 and 3A4 activity in healthy volunteers. Pharmacol. Lett. 2000;66:133–139. doi: 10.1016/s0024-3205(99)00659-1. [DOI] [PubMed] [Google Scholar]
- 30.Roby CA, Dryer DA, Burstein AH. St. John's wort: effect on CYP2D6 activity using dextromethorphan-dextrorphan ratios. J. Clin. Psychopharmacol. 2001;21:530–532. doi: 10.1097/00004714-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Gorski JC, Hamman MA, Huang S-M, et al. The effects of St. John's wort (Hypericum perforatum) on human cytochrome P450 activity. Clin. Pharmacol. Ther. 2001;70:317–326. [PubMed] [Google Scholar]
- 32.Gurley BJ, Gardner SF, Hubbard MA, Williams DK, et al. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin. Pharmacol. Ther. 2002;72:276–282. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- 33.Markowitz JS, DeVane CL, Chavin KD, Taylor RM, et al. Effects of garlic (Allium sativum L.) supplementation on cytochrome P450 2D6 and 3A4 activity in healthy volunteers. Clin. Pharmacol. Ther. 2003;74:170–177. doi: 10.1016/S0009-9236(03)00148-6. [DOI] [PubMed] [Google Scholar]
- 34.Markowitz JS, Donovan JL, DeVane CL, Taylor RM, et al. Effect of St John's wort on drug metabolism by induction of cytochrome P450 3A4 enzyme. JAMA. 2003;290:1500–1504. doi: 10.1001/jama.290.11.1500. [DOI] [PubMed] [Google Scholar]
- 35.Markowitz JS, Donovan JL, DeVane CL, Taylor RM, et al. Multiple doses of saw palmetto (Serenoa repens) did not alter cytochrome P450 2D6 and 3A4 activity in normal volunteers. Clin. Pharmacol. Ther. 2003;74:536–542. doi: 10.1016/j.clpt.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 36.Markowitz JS, Donovan JL, Devane CL, Sipkes L, Chavin KD. Multiple-dose administration of Ginkgo biloba did not affect cytochrome P-450 2D6 or 3A4 activity in normal volunteers. J. Clin. Psychopharmacol. 2003;23:576–581. doi: 10.1097/01.jcp.0000095340.32154.c6. [DOI] [PubMed] [Google Scholar]
- 37.Gorski JC, Huang S-M, Pinto A, Hamman MA. The effect of Echinacea (Echinacea purpurea root) on cytochrome P450 activity in vivo. Clin. Pharmacol. Ther. 2004;75:89–100. doi: 10.1016/j.clpt.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 38.Wenk M, Todesco L, Krähenbühl S. Effect of St. John's wort on the activities of CYP1A2, CYP3A4, CYP2D6, N-acetyltransferase 2, and xanthine oxidase in healthy males and females. Br. J. Clin. Pharmacol. 2004;57:495–499. doi: 10.1111/j.1365-2125.2003.02049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gurley BJ, Gardner SF, Hubbard MA, Williams DK, et al. In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, Echinacea purpurea, milk thistle, and saw palmetto. Clin. Pharmacol. Ther. 2004;76:428–440. doi: 10.1016/j.clpt.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Gurley BJ, Gardner SF, Hubbard MA, Williams DK, et al. Clinical assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly: St. John's wort, garlic oil, panax ginseng, and ginkgo biloba. Drugs Aging. 2005;22:525–539. doi: 10.2165/00002512-200522060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Branch RA, Adedoyin A, Frye RF, Wilson JW, Romkes M. In vivo modulation of CYP enzymes by quinidine and rifampin. Clin. Pharmacol. Ther. 2000;68:401–411. doi: 10.1067/mcp.2000.110561. [DOI] [PubMed] [Google Scholar]
- 42.Edwards RJ, Price RJ, Watts PS, Renwick AB, et al. Induction of cytochrome P450 enzymes in cultured precision-cut human liver slices. Drug Metab. Dispos. 2003;31:282–288. doi: 10.1124/dmd.31.3.282. [DOI] [PubMed] [Google Scholar]
- 43.Madan A, Graham RA, Carroll KM, Mudra DR, et al. Effects of prototypical microsomal enzyme inducers on cytochrome P450 expression in cultured human hepatocytes. Drug Metab. Dispos. 2003;31:421–431. doi: 10.1124/dmd.31.4.421. [DOI] [PubMed] [Google Scholar]
- 44.Streetman DS, Bertino JS, Nafziger AN. Phenotyping of drug metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics. 2000;10:187–216. doi: 10.1097/00008571-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Frye RF, Matzke GR, Adedoyin A, Porter JA, Branch RA. Validation of the five-drug “Pittsburg cocktail” approach for assessment of selective regulation of drug-metabolizing enzymes. Clin. Pharmacol. Ther. 1997;62:365–376. doi: 10.1016/S0009-9236(97)90114-4. [DOI] [PubMed] [Google Scholar]
- 46.Özdemir M, Crewe KH, Tucker GT, Rostami-Hodjean A. Assessment of in vivo CYP2D6 activity: differential sensitivity of commonly used probes to urine pH. J. Clin. Pharmacol. 2004;44:1398–1404. doi: 10.1177/0091270004269582. [DOI] [PubMed] [Google Scholar]
- 47.Frank D, Jaehde U, Fuhr U. Evaluation of probe drugs and pharmacokinetic metric for CYP2D6 phenotyping. Eur. J. Clin. Pharmacol. 2007;63:321–333. doi: 10.1007/s00228-006-0250-8. [DOI] [PubMed] [Google Scholar]
- 48.Russman S, Helbing A. Kava hepatoxicity. Ann. Int. Med. 2001;135:68–69. doi: 10.7326/0003-4819-135-1-200107030-00036. [DOI] [PubMed] [Google Scholar]
- 49.Clough AR, Bailie RS, Currie B. Liver function test abnormalities in users of aqueous kava extracts. J. Toxicol. Clin. Toxicol. 2003;41:821–829. doi: 10.1081/clt-120025347. [DOI] [PubMed] [Google Scholar]
- 50.Frye RF, Branch RA. Improved high-performance liquid chromatographic determination of debrisoquine and 4-hydroxydebrisoquin in human urine following direct injection. J. Chromatogr. B Biomed. Appl. 1996;677:178–182. doi: 10.1016/0378-4347(95)00380-0. [DOI] [PubMed] [Google Scholar]
- 51.Abourashed EA, Khan IA. High-performance liquid chromatography determination of hydrastine and berberine in dietary supplements containing goldenseal. J. Pharm. Sci. 2001;90:817–822. doi: 10.1002/jps.1035. [DOI] [PubMed] [Google Scholar]
- 52.Ganzera M, Khan IA. Analytical techniques for the determination of lactones in Piper methysticum Forst. Chromatographia. 1999;50:649–653. [Google Scholar]
- 53.Ganzera M, Bedir E, Khan IA. Separation of Cimicifuga racemosa triterpene glycosides by evaporative light scattering detection. Chromatographia. 2000;52:301–304. [Google Scholar]
- 54.Gurley BJ, Barone GW, Williams DK, Carrier JC, et al. Effect of milk thistle (Silybum marianum) and black cohosh (Cimicifuga racemosa) supplementation on digoxin pharmacokinetics in humans. Drug Metab. Dispos. 2006;34:69–74. doi: 10.1124/dmd.105.006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu FF, Ang CYW, Heinze TM, Rankin JD, et al. Evaluation of major active components in St. John's wort dietary supplements by high-performance liquid chromatography with photodiode array detection and electrospray mass spectrometric confirmation. J. Chromatogr. A. 2000;888:85–92. doi: 10.1016/s0021-9673(00)00555-0. [DOI] [PubMed] [Google Scholar]
- 56.Molgaard P, Johnsen S, Christensen P, Cornett C. HPLC method validated for the simultaneous analysis of cichoric acid and alkamides in Echinacea purpurea planta and products. J. Agric. Food Chem. 2003;51:6922–6933. doi: 10.1021/jf026158f. [DOI] [PubMed] [Google Scholar]
- 57.Anonymous . United States Pharmacopeia and National Formulary, 27th revision. 22nd ed. United States Pharmacopeial Convention, Inc.; Rockville: 2004. Disintegration and dissolution of dietary supplements; pp. 2645–2646. [Google Scholar]
- 58.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Lawrence Erlbaum Associates; Hillsdale, NJ: 1988. [Google Scholar]
- 59.Foster BC, Vandenhoek S, Hana J, Krantis A, et al. In vitro inhibition of human cytochrome P450-mediated metabolism of marker substrates by natural products. Phytomedicine. 2003;10:334–342. doi: 10.1078/094471103322004839. [DOI] [PubMed] [Google Scholar]
- 60.Chatterjee P, Franklin MR. Human cytochrome P450 inhibition and metabolic-intermediate complex formation by goldenseal extract and its methylenedioxyphenyl components. Drug Metab. Dispos. 2003;31:1391–1397. doi: 10.1124/dmd.31.11.1391. [DOI] [PubMed] [Google Scholar]
- 61.Beckmann-Knopp S, Rietbrock S, Weyhenmeyer R, Böcker RH, et al. Inhibitory effects of silibinin on cytochromes P-450 enzymes in human liver microsomes. Pharmacol. Toxicol. 2000;86:250–256. doi: 10.1111/j.0901-9928.2000.860602.x. [DOI] [PubMed] [Google Scholar]
- 62.Zuber R, Modriansky M, Dvorák Z, Rohovsky P, et al. Effect of silybin and its congeners on human liver microsomal cytochrome P450 activities. Phytother. Res. 2002;16:632–638. doi: 10.1002/ptr.1000. [DOI] [PubMed] [Google Scholar]
- 63.Matthews JM, Etheridge AS, Black SR, et al. Inhibition of human cytochrome P450 activities by kava extract and kavalactones. Drug Metab. Dispos. 2002;30:1153–1157. doi: 10.1124/dmd.30.11.1153. [DOI] [PubMed] [Google Scholar]
- 64.Unger M, Frank A. Simultaneous determination of the inhibitory potency of herbal extracts on the activity of six major cytochrome P450 enzymes using liquid chromatography/mass spectrometry and automated online extraction. Rapid Commun. Mass Spectrom. 2004;18:2273–2281. doi: 10.1002/rcm.1621. [DOI] [PubMed] [Google Scholar]
- 65.Clayton NP, Yoshizawa K, Kissling GE, Burka LT, et al. Immunohistochemical analysis of expression of hepatic cytochrome P450 in F344 rats following treatment with kava extract. Exp. Toxicol. Path. 2007;58:223–236. doi: 10.1016/j.etp.2006.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Obach RS. Inhibition of human cytochrome P450 enzymes by constituents of St. John's wort, an herbal preparation used in the treatment of depression. J. Pharmacol. Exp. Ther. 2000;294:88–95. [PubMed] [Google Scholar]
- 67.Zou L, Harkey MR, Henderson GL. Effects of herbal components on cDNA-expressed cytochrome P450 enzyme catalytic activity. Life Sci. 2002;71:1579–1589. doi: 10.1016/s0024-3205(02)01913-6. [DOI] [PubMed] [Google Scholar]
- 68.Hodek P, Trefil P, Stiborová M. Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chemico-Biol. Int. 2002;139:1–21. doi: 10.1016/s0009-2797(01)00285-x. [DOI] [PubMed] [Google Scholar]
- 69.Tsukamoto S, Aburatani M, Ohta T. Isolation of CYP3A4 inhibitors from the black cohosh (Cimicifuga racemosa) eCAM. 2005;2:223–226. doi: 10.1093/ecam/neh086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.von Moltke LL, Greenblatt DJ, Schmider J, Wright CE, et al. In vitro approaches to predicting drug interactions in vivo. Biochem. Pharmacol. 1998;55:113–122. doi: 10.1016/s0006-2952(97)00239-6. [DOI] [PubMed] [Google Scholar]
- 71.Wallace S, Carrier DJ, Clausen EC. Extraction of nutraceuticals from milk thistle. Appl. Biochem. Biotech. 2003;105-108:891–903. doi: 10.1385/abab:108:1-3:891. [DOI] [PubMed] [Google Scholar]
- 72.Gilroy CM, Steiner JF, Byers T, Shapiro H, Georgian W. Echinacea and truth in labeling. Arch. Intern. Med. 2003;163:699–704. doi: 10.1001/archinte.163.6.699. [DOI] [PubMed] [Google Scholar]
- 73.Jiang B, Kronenberg F, Nuntanakorn P, Qiu M-H, Kennelly EJ. Evaluation of the botanical authenticity and phytochemical profile of black cohosh products by high performance liquid chromatography with selected ion monitoring liquid chromatography-mass spectrometry. J. Agric. Food Chem. 2006;54:3242–3253. doi: 10.1021/jf0606149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.De Los Reyes GC, Koda RT. Determining hyperforin and hypericin content in eight brands of St.John's wort. Am. J. Health-Syst. Pharm. 2002;59:545–547. doi: 10.1093/ajhp/59.6.545. [DOI] [PubMed] [Google Scholar]
- 75.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 76.Gurley BJ, Gardner SF, Hubbard MA, Williams DK, et al. In vivo effects of goldenseal, kava kava, black cohosh, and valerian on human cytochrome P450 1A2, 2D6, 2E1, and 3A4/5 phenotypes. Clin. Pharmacol. Ther. 2005;77:415–426. doi: 10.1016/j.clpt.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Labbé L, Sirois C, Pilote S, Arseneault M, et al. Effect of gender, sex hormones, time tables and physiological urinary pH on apparent CYP2D6 activity as assessed by metabolic ratios of marker substrates. Pharmacogenetics. 2000;10:425–438. doi: 10.1097/00008571-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 78.Borges S, Li L, Hamman MA, Jones DR. Dextromethorphan to dextrorphan urinary metabolic ratio does not reflect dextromethorphan oral clearance. Drug Metab Dispos. 2005;33:1052–1055. doi: 10.1124/dmd.104.003459. [DOI] [PubMed] [Google Scholar]
- 79.Bhide MB, Chavan SR, Dutta NK. Absorption, distribution and excretion of berberine. Ind J Med Res. 1969;57:2128–2131. [PubMed] [Google Scholar]
- 80.Pan G, Wang G-J, Liu X-D, Fawcett JP, Xie Y-Y. The involvement of p-glycoprotein in berberine absorption. Pharmacol. Toxicol. 2002;91:193–197. doi: 10.1034/j.1600-0773.2002.t01-1-910403.x. [DOI] [PubMed] [Google Scholar]