Abstract
In recent years, a considerable emphasis has been focused on the importance of the naturally available botanicals that can be consumed in an individual’s everyday diet and that can also be useful as a chemopreventive or chemotherapeutic agent for certain diseases, including cancers. A wide variety of botanicals, mostly dietary flavonoids or polyphenolic substances, have been reported to possess substantial anticarcinogenic and antimutagenic activities because of their antioxidant and anti-inflammatory properties. Proanthocyanidins are considered as one of them, and are abundantly available in various parts of the plants, such as fruits, berries, bark and seeds. Their modes of action were evaluated through a number of in vitro and in vivo studies which showed their potential role as anti-carcinogenic agent. We summarize and highlight the latest developments on anti-carcinogenic activities of proanthocyanidins from different sources, specifically from grape seeds, and their molecular targets, such as NF-κB, mitogen-activated protein kinases, PI3K/Akt, caspases, cytokines, angiogenesis and cell cycle regulatory proteins and other check points, etc. Although the bioavailability and metabolism data on proanthocyanidins is still largely unavailable, certain reports indicate that at least monomers and smaller oligomeric procyanidins are absorbed in the gut. The modulation of various molecular targets by proanthocyanidins in vitro and in vivo tumor models suggests their importance, contribution and mechanism of action to the prevention of cancers of different organs.
1. Introduction
Proanthocyanidins are naturally occurring compounds that are widely found in fruits, vegetables, nuts, seeds, flowers and bark. They are a class of phenolic compounds that take the form of oligomers or polymers of polyhydroxy flavan-3-ol units, such as (+)-catechin and (−)-epicatechin [1]. These compounds are mostly found in pine bark, grape seed and red wines. However, bilberry, cranberry, black currant, green tea, black tea, and other plants also contain these flavonoids. The seeds of the grape (Vitis vinifera) are particularly rich source of proanthocyanidins. The grape seed proanthocyanidins (GSPs) are mainly dimers, trimers and highly polymerized oligomers of monomeric catechins [2,3]. GSPs have been shown to be potent antioxidants and free radical scavengers, being more effective than either ascorbic acid or vitamin E [4,5]. In addition to have anti-oxidant activity, GSPs have been shown to have anti-carcinogenic activity in different tumor models [6,7,8]. GSPs were subjected to a limited toxicity testing which included acute and subchronic toxicity in rats, and genotoxicity testing, comprising test for induction of gene mutation in bacteria, test for induction of chromosomal aberrations in mammalian cell in vitro, and mouse micronucleus test in vivo, the results of which indicate that these compounds are of a low toxicity and have no genotoxic potential [1]. As there has been considerable interest in the use of botanicals for the prevention of various diseases, phytochemicals might be of interest as protective agents for various cancers. Research on proanthocyanidins is however limited and many questions still remain to be answered. The present review highlights the latest developments and knowledge on the cancer chemopreventive and/or chemotherapeutic effects of proanthocyanidins including molecular targets, in vitro cell culture and in vivo animal studies, clinical trials and bioavailability and metabolism.
2. Chemistry of proanthocyanidins
Proanthocyanidins are synonymous with condensed tannins, and also known as oligomeric proanthocyanidins, pycno-genols or leukocyanidins, oligomers or polymers of flavan-3-ols and these units are linked mainly through C4→C8 bond, but the C4→C6 linkage also exists (Fig. 1). These linkages are called B-type linkages. An additional ether bond between C2→C7 resulting in doubly linkage of the flavan-3-ol units is called an A-type linkage. The most common types of compounds and linkages are shown in Fig. 1. The proanthocyanidins that exclusively consist of epicatechin units are designated procyanidins, the most abundant type of proanthocyanidins in plants. The less common proanthocyanidins containing epigallocatechin subunits are called prodelphinidin. The flavan-3-ol subunits may carry acyl subtituents like gallic acid or glycosyl substituents like the sugars both of which may be linked at the C3 or C5 position of the oligomers [9]. The knowledge about the distribution and nature of proanthocyanidins in foods has until recently very limited; however the reported content of proanthocyanidins in various food items varies due to different analytical methods or to the nature of the samples analyzed, variety, stage of ripeness, part of the food, level of processing, etc. [9]. Most of the plant-based foods, like fruits and berries, but also nuts, beans, some cereals foods, such as barley and sorghum, spices curry and cinnamon, wine and beers were found to contain exclusively the homogeneous B-type procyanidins. A-type proanthocyanidins was only determined in curry, cinnamon, cranberry, peanut and plums etc. [10].
Figure 1.
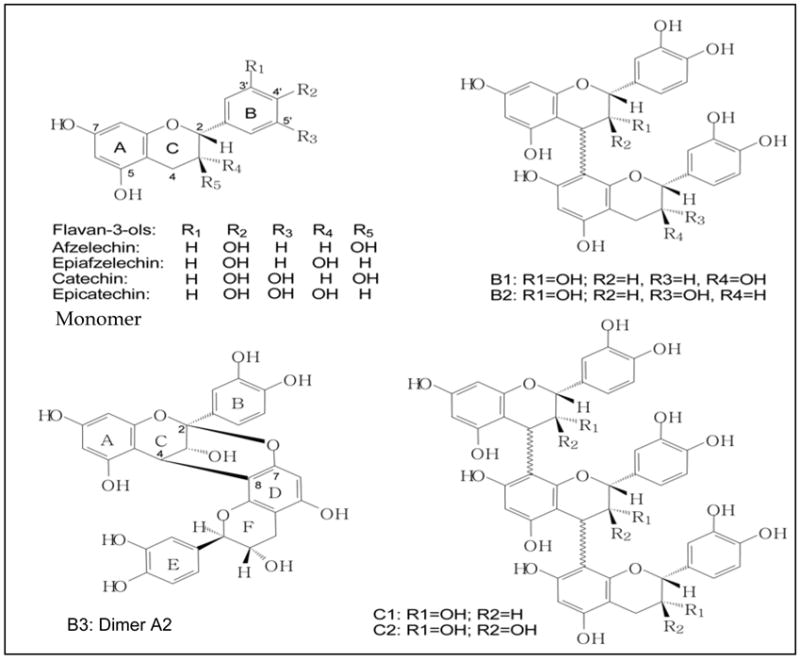
Chemical structures of the monomer (flavan-3-ols), dimers (B1, B2, B3) and trimers (C1 and C2) are shown. An example of the A-type double linkage is shown as dimer A2.
3. Molecular targets of proanthocyanidins
The extensive investigations with the proanthocyanidins have identified various molecular targets that can potentially be used for the prevention or treatment of cancers of various organs. Here, we will summarize the latest developments on chemopreventive and/or chemotherapeutic effects of proanthocyanidins in general and with particular emphasis on grape seed proanthocyanidins (GSPs) which were extensively investigated against the risk of cancers in vitro and in vivo models. Moreover, the GSPs that have been used in the author’s laboratory was obtained from Kikkoman Corporation (Noda, Japan), and commercially known as ‘Gravinol’. Its chemical composition has been described elsewhere [6,8 ].
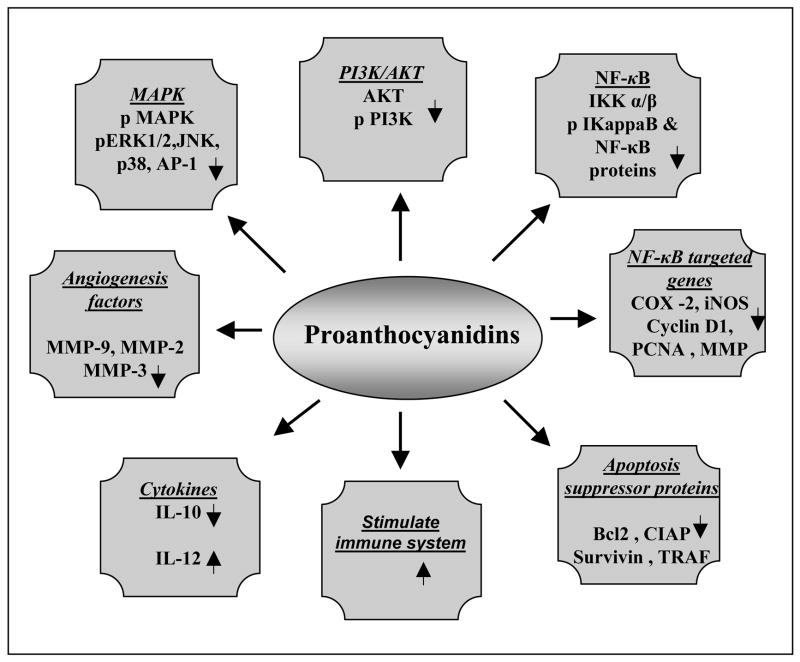
3.1. NF-κB and its target proteins
The activation of NF-κB has been involved in inflammation, cell proliferation and oncogenic processes and its activation depends on the phosphorylation and subsequent degradation of IkappaB proteins [11]. A number of studies have shown that GSPs exert their anti-cancer effects through the suppression of NF-κB. In vitro treatment of human epidermoid carcinoma A431 cells with GSPs down-regulates the constitutive expression or basal level of NF-κB/p65 and IKKα in these cells and simultaneously inhibits the degradation of IκBα protein, a regulator of NF-κB [12]. Irradiation of normal human epidermal keratinocytes to ultraviolet (UV) radiation results in activation of NF-κB and IKKα. Pretreatment of these cells with GSPs inhibits UV-induced activation of NF-κB/p65 and IKKα, and also inhibits the degradation of IκBα which indicates the attenuation of UV-induced adverse effects.
Cyclooxygenase -2 (COX-2) is a rate limiting enzyme and is constitutively overexpressed in practically every premalignant and malignant condition involving the colon, liver, pancreas, breast, lung, bladder, skin, stomach, head and neck, and esophagus [13] in response to various mitogens, tumor promoters, cytokines, growth factors and exposure to solar UV radiation. Nitric oxide synthase is responsible for the release of the gaseous free radical nitric oxide whose excessive and prolonged generation has been linked with inflammation and tumorogenesis [13]. Inhibitory effects of GSPs on the constitutive expression of various NF-κB- responsive genes/proteins, such as COX-2, inducible nitric oxide synthase, proliferating cell nuclear antigen, cyclin D1 and MMP-9, were observed in the human epidermoid carcinoma A431 cells [12].
3.2. Mitogen-activated protein kinases (MAPK)
MAPK signaling pathway is an important upstream regulator of transcriptional factor activities and their signaling affects a wide variety of extracellular stimuli into intracellular events and thus control the activities of downstream transcription factors implicated in carcinogenesis [14]. UV-induced oxidative stress has been implicated in the activation of MAPK proteins. Treatment of human epidermal keratinocytes with GSPs inhibits UV-induced oxidative stress-mediated activation of ERK1/2, JNK and p38 proteins of MAPK family [15]. Similar effects were also observed in UV-exposed mouse skin when GSPs were given in diet to the animals [16]. Treatment of human epidermoid carcinoma A431 cells with GSPs also resulted in inhibition of constitutive activation of MAPK proteins [12] which plays a major role in cell growth and proliferation [17], and thus GSPs induce anticarcinogenic effects.
3.3. PI3K/AKT
The PI3K/Akt pathway plays critical role in mammalian cell survival signaling and has been shown to be activated in various cancers [18,19]. A key downstream effector of PI3K is the serine-threonine kinase Akt, which in response to PI3K activation phosphorylates and regulates the activity of number of molecular targets. GSPs has been shown to decrease the phosphorylation of Akt in A431 cells which otherwise gets activated on PI3K activation and promotes cancer cell growth [12].
3.4. Apoptosis
Apoptosis plays a major role in establishing a natural balance between cell death and cell renewal in mature animals by destroying excess, damaged or abnormal cells. The activation of NF-κB promotes cell survival and proliferation, and down-regulation of NF-κB sensitizes the cells to apoptosis induction. Expression of several NF-κB-regulated genes such as Bcl-2, cIAP, survivin, TRAF have been reported to function by blocking the apoptosis pathway [13]. Apoptosis is governed by a complex network of anti-apoptotic and pro-apoptotic effector molecules, like Bcl-2 and Bax [20,21]. The higher ratio of Bax/Bcl-2 leads to the cleavage of caspases and that stimulates the induction of apoptosis [22]. Both in vitro and in vivo studies showed the downregulation of the expression of Bcl-2 while increasing the expression of Bax by GSPs in the mouse mammary cancer cells (4T1) and human epidermoid carcinoma A431 cells followed by increase in the levels of caspase-3 in comparison to the expression of these proteins in the tumors grown in athymic nude mice that were not fed GSPs in diet [6, 22].
3.5. Cytokines
Cytokines, such as TNF-α [23] and interleukin (IL)-10 [24], and other soluble factors produced in UV-induced skin carcinogenesis model [25] have been implicated as mediators of systemic immune suppression. IL-10 has been implicated in immunosuppressive effects, while IL-12 has been demonstrated to play an important role in the induction and elicitation of immune reactions [26] and to augment antitumor cell-mediated immune responses [27]. Dietary GSPs reduced UVB radiation-induced increases in the levels of IL-10 in the skin while enhanced the production of IL-12 in the skin and lymphatic system of mice [28], thus suggesting that GSPs inhibit UV-induced suppression of immune reactions.
3.6. Angiogenesis
Matrix metalloproteinases (MMP) play a crucial role in tumor development and metastatic spread of cancer. One of the earliest events in the metastatic spread of cancer is the invasion through the basement membrane and proteolytic degradation of the extracellular matrix proteins. MMPs are the important regulators of tumor growth, both at the primary site and in distant metastases [29]. Given the clear implications of MMPs in many human cancers, MMPs remain important targets of cancer therapy. Metastatic spread of cancer continues to be the greatest barrier in prevention or cure of cancer. The recognition that MMPs facilitate tumor cell growth, invasion and metastasis of cancer has led to the development of MMP inhibitors as cancer therapeutic agents [30]. The procyanidin extract from Japanese quince fruit proved to be an effective inhibitor of the enzymes activities MMP-2 and MMP-9 [31]. A flavonoid-rich extract of highbush blueberry (V. angustifolium) inhibited expression of MMP-2 and MMP-9 in the DU-145 prostate tumor model [32]. Treatment with blueberry fractions also increased expression of tissue inhibitors of metalloproteinase expression (TIMP-1) [33]. GSPs inhibit fibroblast conditioned medium-induced expression of MMP-2 and MMP-9 in androgen-insensitive (DU145) as well as androgen-sensitive (LNCaP) human prostate cancer cell lines [34]. The treatment of inhibitors of MEK and p38 to DU145 cells inhibited the phosphorylation of proteins of MAPK family and simultaneous inhibition of MMPs synthesis or their extracellular secretion. The reduction in secretion of MMP-2 and MMP-9 by GSPs may be associated with the inhibition of phosphorylation of proteins of MAPK family and activation of NFκB. Agarwal et al. [35] demonstrated that treatment of grape seed extract to human umbilical vein endothelial cells in culture inhibited capillary tube formation on Matrigel and MMP-2 secretion which suggested its anti-angiogenic potential.
3.7. Cell cycle
Disruption of the normal regulation of cell-cycle progression and division are important events in the development of cancer. Several proteins are known to regulate the timing of the events in the cell cycle. Major control switches of the cell cycle are the cyclins and the cyclin-dependent kinases (CDK) [Reviewed in 36]. It was observed that treatment of A431 cells with GSPs resulted in a marked reduction in the expression levels of CDK2, CDK4 and CDK6. Similarly, a marked reduction in the expression levels of cyclins D1, D2 and E was observed after GSPs treatment. The Cip1/p21 and Kip1/p27 regulate the progression of cells in the Go/G1 phase of the cell cycle and induction of these proteins causes a blockade of the G1 to S transition, thereby resulting in a Go/G1 phase arrest of the cell cycle. The in vitro observations indicate that the GSPs-induced enhancement of the levels of CDK-inhibitors may have an important role in the GSP-induced G1-phase arrest of cell cycle progression in A431 cells, possibly through their inhibition of CDK kinase activity. This event may lead to the apoptotic cell death of cancer cells. Apoptosis plays a crucial role in eliminating the mutated neoplastic and hyperproliferating neoplastic cells from the system and therefore is considered as a protective mechanism against the development of cancer [36]. In an in vivo study, it was observed that administration of GSPs by oral gavage inhibits the growth of A431 tumor-xenografts in athymic nude mice. The mechanism of inhibition of the growth of tumor xenografts by GSPs was associated with the inhibition of proliferating cell nuclear antigen and cyclin D1, markers of tumor cell proliferation, and induction of apoptotic cell death of tumor cells. Similar observation were noted when the prostate cancer cells, DU145 and LNCaP, were treated with grape seed extract. These studies indicate that treatment of prostate cancer cells with GSPs results in inhibition of proliferation, induction of apoptosis, G1 phase arrest, increases in Cip1/p21 and decreases in CDK4, CDK2 and cyclin E [36].
4. Anti-cancer properties of proanthocyanidins: in vitro and in vivo studies
The anti-carcinogenic properties of the proanthocyanidins and its associated molecular mechanisms are illustrated through the following in vitro, in vivo experimental studies and clinical trials.
4.1. In vitro studies
The treatment of JB6 C141 cells, a well-developed cell culture model for studying tumor promotion in keratinocytes, with GSPs resulted in the induction of apoptosis [37]. The induction of apoptosis by GSPs was primarily p53-dependent because it occurred mainly in cells expressing wild type p53 to a much greater extent than in p53-deficient cells [37]. This study also suggested the involvement of Bax/Bcl-2 proteins and caspase-3 activation in the induction of apoptosis by GSPs treatment. Treatment of normal human epidermal keratinocytes with GSPs decreased UV-induced oxidative stress and oxidative stress-mediated phosphorylation of the proteins of the MAPK family and activation of the transcription factor, NF-κB and its related genes [38]. The treatment of GSPs resulted in inhibition of cellular proliferation and the expression of MMP-2 and -9 in androgen-insensitive human prostate cancer DU145 cells, as well as in androgen-sensitive human prostate carcinoma LNCaP cells [34]. The dose-dependent inhibition of cell viability and proliferation together with induction of apoptosis by GSPs was also observed on the mouse mammary carcinoma 4T1 cells [6], and human colorectal cancerous HT29 and LoVo cells [6,39]. GSPs induced G0/G1 phase cell cycle arrest along with a marked increase in Cip1/p21 protein levels and a decrease in G0/G1 phase associated cyclins and cyclin-dependent kinases. Concentration and time-dependent effects of GSPs were also observed on MCF-7 breast cancer cells, A-427 lung cancer and gastric adenocarcinoma cells [40].
Procyanidin-rich fractions from grapes and pine bark extract showing different mean degrees of polymerization, percentage of galloylation (percentage of gallate esters) and reactive oxygen species-scavenging capacity were tested on HT29 human colon cancer cells at varying concentrations of the polyphenolic mixtures. It was observed that the most efficient fractions inhibiting cell proliferation, arresting the cell cycle in G2 phase and inducing apoptosis were the grape fractions with the highest percentage of galloylation and mean degree of polymerization [41].
The berry extracts from various sources of blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry containing a considerable proportion of proanthocyanidins were evaluated for their ability to inhibit growth and stimulate apoptosis of human oral (KB, CAL-27), breast (MCF-7), colon (HT-29, HCT116), and prostate (LNCaP) tumor cells at concentrations ranging from 25–200μg/ml in vitro [42]. In vitro studies employing breast tumor models treated with cranberry extract have reported dose-dependent induction of apoptosis coupled with cell cycle arrest in both G1 and G2 phase [43]. Treatment of LNCaP cells with 20 μg/ml of a wild blueberry proanthocyanidin fraction inhibited the growth of the cells. Two similar proanthocyanidin-rich fractions from cultivated blueberries at the same concentration significantly inhibited the growth of LNCaP cells while less effect was observed in DU145 cells. Differences in cell growth inhibition of human prostate cancer LNCaP and DU145 cells by blueberry fractions rich in proanthocyanidins indicate that blueberry proanthocyanidins have an effect primarily on androgen-sensitive prostate cancer cells [44].
4.2. In vivo studies
The first evidence for the prevention of UV-induced skin cancer by GSPs came from our laboratory [8,45]. We showed that dietary feeding of GSPs inhibits UVB-induced photocarcinogenesis in SKH-1 hairless mice in terms of tumor incidence, tumor multiplicity and tumor growth/size. Dietary GSPs also inhibited the malignant transformation of UVB-induced papillomas to carcinomas in mice in terms of carcinoma incidence and carcinoma multiplicity compared with non-GSPs treated mice following photocarcinogenesis protocol [8].
UV-induced immunosuppression is considered as a risk factor for the development of melanoma and nonmelanoma skin cancers [46]; therefore, the prevention of UV-induced immunosuppression represents a potential strategy for the prevention of skin cancers. UVB irradiation of mice results in a significantly lower response to contact sensitizer, 2,4-dinitrofluorobenzene, that indicates the suppression of the immune system [28]. Dietary administration of GSPs exhibited a significant reduction in UVB-induced suppression of the contact hypersensitivity response to contact sensitizer, and this prevention was associated with the enhanced production of IL-12 and reduced expression of IL-10 in the UV-irradiated skin as well as in the draining lymph nodes of UV exposed mice [28]. There are studies that implicate the immunoregulatory cytokine, IL-12, in the induction and elicitation of the immune system. On the other hand, IL-10 possesses immunosuppressive activity and inhibits antigen presentation in in vitro and in vivo systems. Dietary administration of GSPs to mice resulted in a reduced level of IL-10 in the UV-irradiated skin, as well as in the draining lymph nodes compared to the control mice. These in vivo effects of GSPs suggest a possible mechanism by which dietary GSPs decrease UVB-induced immune suppression in mice [28].
IL-12 regulates the growth and functions of T-cells and especially augments the development of Th1 type cells by stimulating the production of IFN-γ [47,48]. Intraperitoneal injection of recombinant IL-12 in mice prevents UV-induced immune suppression. The provision of dietary GSPs resulted in higher levels of IL-12 in the skin and draining lymph nodes of UVB-exposed C3H/HeN mice than those observed in UVB-exposed mice that did not receive GSPs [28]. The higher levels of IL-12 may contribute to stimulation of the immune responses. In GSPs-treated mice, the i.p. injection of the anti-IL-12 antibody significantly reversed or blocked the preventive effect of GSPs on UV-induced immune suppression [28]. These studies provide convincing evidence that prevention of UV-induced suppression of immune system by GSPs is mediated, at least in part, through IL-12 induction, and that the protection from UVB-induced immunosuppression afforded by dietary GSPs may be associated with the protection from UVB-induced photocarcinogenesis in mice.
An in vivo study conducted in immunocompetent Balb/c mice indicated the inhibition of tumor growth formed on subcutaneous inoculation of viable 4T1 murine mammary cancer cells by dietary GSPs accompanied with an increase in the survival period of the tumor-bearing mice by several days [6]. This study also showed the inhibition of metastasis of 4T1 cells from the primary tumor site to the lungs by GSPs, and that may be the reason that the mice fed GSPs survived longer period of time than those mice which were not given GSPs [6]. Dietary supplementation with GSPs was found to inhibit the incidence of dimethylbenz[a] anthracene-induced mammary tumors in the Sprague-Dawley rats [49]. Similar inhibitory effects were also observed in the azoxymethane-induced distal colon-crypt foci in female rats fed with dietary GSPs [50]. The in vivo effect of oral GSPs was examined on HT29 tumor xenograft growth in athymic nude mice. In this experiment, GSPs feeding to mice resulted in inhibition of tumor growth without any apparent toxicity in mice. GSPs inhibited cell proliferation and increased apoptotic cell death in tumors [39]. Recently, Raina et al. demonstrated that administration of grape seed extract by oral gavage inhibits the spontaneous development of prostate cancer in male TRAMP mice [51]. The inhibition of prostate cancer growth by grape seed extract was associated with the reduction in the weight of genitourinary tract organs, the inhibition of prostate intraepithelial neoplasia, proliferating cell nuclear antigen and the levels of cyclins in prostate tissues of male TRAMP mice.
5. Present status of clinical trials
At present limited data are available on the clinical trials on proanthocyanidins. Tissue hardness (induration), pain and tenderness are common late adverse effects of curative radiotherapy for early breast cancer. Sixty-six eligible research volunteers with moderate or marked breast induration at a mean 10.8 years since radiotherapy for early breast cancer were randomised to active drug (n=44) or placebo (n=22). All patients were given IH636 grape seed proanthocyanidin extract (100 mg) three times a day orally, or corresponding placebo capsules, for 6 months. The primary endpoint was percentage change in surface area (cm2) of palpable breast induration measured at the skin surface 12 months after randomisation. Secondary endpoints included change in photographic breast appearance and patient self-assessment of breast hardness, pain and tenderness. At 12 months post-randomisation, ≥50% reduction in surface area (cm2) of breast induration was recorded in 13/44 (29.5%) of grape seed proanthocyanidins extract treated patients [52]. The effect of chocolate containing high procyanidins content was assessed on fecal free radical production and antioxidant activity in 18 volunteers. The volunteers were given chocolate for 2 to 4-wk periods separated by a 4-wk washout period. Free radical production in the fecal water was lowered from 122 ±10 μM/h to 94± 9 μM/h when the high procyanidin chocolate diet was consumed [53].
6. Bioavailability and metabolism of proanthocyanidins
The study of bioavailability and metabolism of any medicinal or edible phytochemical is an important part of all investigations. Some studies have been performed to examine the bioavailability and metabolism of proanthocyanidins. One-half of 88 tested foods derived from plants were found to be dietary sources of proanthocyanidins, which suggests that these are among the most abundant polyphenols in our diet [10]. Polymeric proanthocyanidins are not absorbed as such in the gut [54]. The detection of proanthocyanidin dimers B1 and B2 in human plasma was reported in 2 studies [55,56]. The absorption of these dimers was ~100-fold lower than that of the monomeric flavanols [55]. However, these compounds were found to have direct effects on the intestinal mucosa and protect it against oxidative stress or the actions of carcinogens. In addition, the consumption of proanthocyanidin-rich foods, such as cocoa, red wine, or grape seed extracts, has been shown to increase the plasma antioxidant capacity, to have positive effects on vascular function, and to reduce platelet activity in humans [57]. These procyanidin-rich sources always contain 5–25% monomers or other polyphenols with which the proanthocyanidins may have effects through interactions with other components, such as lipids or iron, in the gut [54]. Rios et al. demonstrated the prevention of the polymer-degradation during the stomach transit due to the buffering effect exhibited by the food bolus, making the acidic conditions milder than required for proanthocyanidin hydrolysis [57].
The incubation of purified, 14C-labeled, proanthocyanidin oligomers with human colonic microflora led to the formation of m-hydroxyphenylpropionic acid, m-hydroxyphenylacetic acid, and their p-hydroxy isomers, m-hydroxyphenylvaleric acid, phenylpropionic acid, phenylacetic acid, and benzoic acid [58]. Some of these compounds, namely, m-hydroxyphenylpropionic acid and m-hydroxyphenylacetic acid, as well as m-hydroxybenzoic acid, were shown to increase in human urine after consumption of procyanidin-rich chocolate [59]. Gonthier at al. showed that the extent of degradation into aromatic acids decreased by 21-times in the polymer feed as compared to the catechin monomers fed to rats, probably because of the antimicrobial properties and protein-binding capacity frequently described for proanthocyanidins [60]. But the degradation of proanthocyanidins into microbial metabolites needs more investigations.
7. Conclusion
The in vitro and in vivo experimental data supports the concept that proanthocyanidins, specifically grape seed proanthocyanidins, can act as anti-carcinogenic agents. Potential cancer chemopreventive activities include reduced proliferation, increased apoptosis, cell cycle arrest in tumor cells and the modulation of expression and activity of NF-κB and NF-κB-targeted genes including the invasion and metastasis-specific molecular targets. The available data from in vitro tests and in vivo animal toxicity studies indicate that GSPs are not genotoxic in nature. The efficacy of proanthocyanidins in general, and GSPs in particular, against tumor development is largely depend on its bioavailability to various tissues, which could be considered as a key area of future study since it is the metabolites that may be capable of producing the biological effects in various tissues. Conclusively, proanthocyanidins hold promise for better chemopreventive and/or chemotherapeutic agents against cancers of all organs.
Figure 2.
Molecular targets of proanthocyanidins in prevention or therapy of cancers.
Acknowledgments
The work reported from the author’s laboratory was supported by the funds from National Cancer Institute/NIH (CA104428) and Veterans Affairs Merit Review Award (S.K.K.). The content of this article does not necessarily reflect the views or policies of the funding sources. The authors apologize for not discussing and citing several valuable publications because of the limitations of space and the number of references.
Abbreviations used
- GSPs
grape seed proanthocyanidins
- IL
interleukin
- MAPK
mitogen-activated protein kinases
- MMP
matrix metalloproteinases
- NF-κB
nuclear factor-kappaB
- UV
ultraviolet
References
- 1.Yamakoshi J, Saito M, Kataoka S, Kikuchi M. Safety evaluation of proanthocyanidins-rich extract from grape seeds. Food Chemical Toxicol. 2002;40:599–607. doi: 10.1016/s0278-6915(02)00006-6. [DOI] [PubMed] [Google Scholar]
- 2.Silva RC, Rigaud J, Cheynier V, Chemina A. Procyanidin dimers and trimers from grape seeds. Phytochemistry. 1991;30:1259–1264. [Google Scholar]
- 3.Prieur C, Rigaud J, Cheynier V, Moutounet M. Oligomeric and polymeric procyanidins from grape seeds. Phytochemistry. 1994;36:781–789. [Google Scholar]
- 4.Joshi SS, Kuszynski CA, Bagchi D. The cellular and molecular basis of health benefits of grape seed proanthocyanidin extract. Curr Pharm Biotechnol. 2001;2:187–200. doi: 10.2174/1389201013378725. [DOI] [PubMed] [Google Scholar]
- 5.Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidin extract in vitro. Res Commun Mol Pathol Pharmacol. 1997;95:179–189. [PubMed] [Google Scholar]
- 6.Mantena SK, Baliga MS, Katiyar SK. Grape seed proanthocyanidins induce apoptosis and inhibit metastasis of highly metastatic breast carcinoma cells. Carcinogenesis. 2006;27:1682–1691. doi: 10.1093/carcin/bgl030. [DOI] [PubMed] [Google Scholar]
- 7.Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer. 2004;108:733–740. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- 8.Mittal A, Elmets CA, Katiyar SK. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: Relationship to decreased fat and lipid peroxidation. Carcinogenesis. 2003;24:1379–1388. doi: 10.1093/carcin/bgg095. [DOI] [PubMed] [Google Scholar]
- 9.Santos-Buelga C, Scalbert A. Proanthocyanidins and tannin-like compounds – nature, occurrence, dietary intake and effects on nutrition and health. J Sci Food Agric. 2000;80:1094–1117. [Google Scholar]
- 10.Gu L, Kelm MA, Hammerstone JF. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J Agric Food Chem. 2003;51:7513–7521. doi: 10.1021/jf034815d. [DOI] [PubMed] [Google Scholar]
- 11.Thanos D, Maniatis T. NF-kappaB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 12.Meeran SM, Katiyar SK. Proanthocyanidins inhibit mitogenic and survival-signalling in vitro and tumor growth in vivo. Front Biosci. 2008;13:887–897. doi: 10.2741/2729. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;14:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 14.McCarty MF. Polyphenol-mediated inhibition of AP-1 transactivating activity may slow cancer growth by impeding angiogenesis and tunor invasiveness. Med Hypotheses. 1998;50:511–514. doi: 10.1016/s0306-9877(98)90273-0. [DOI] [PubMed] [Google Scholar]
- 15.Mantena SK, Katiyar SK. Grape seed proanthocyanidins inhibit UV radiation-induced oxidative stress and activation of MAPK and NF-κB signaling in human epidermal keratinocytes. Free Rad Biol Med. 2006;40:1603–1614. doi: 10.1016/j.freeradbiomed.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 16.Sharma SD, Meeran SM, Katiyar SK. Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative stress and activation of mitogen-activated protein kinases and nuclear factor- B signaling in in vivo SKH-1 hairless mice. Mol Cancer Ther. 2007;6:995–1005. doi: 10.1158/1535-7163.MCT-06-0661. [DOI] [PubMed] [Google Scholar]
- 17.Bode AM, Dong Z. Mitogen-activated protein kinase activation in UV-induced signal transduction. Sci STKE. 2003:RE2. doi: 10.1126/stke.2003.167.re2. [DOI] [PubMed] [Google Scholar]
- 18.Clarke RB. p27KIP1 phosphorylation by PKB/Akt leads to poor breast cancer prognosis. Breast Cancer Res. 2003;5:162–163. doi: 10.1186/bcr596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang F, Lee JT, Navolonic PM, Steelman LS, Shelton Jg, Blalock WL, Franklin RA, McCubrey JA. Involvement ok PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 20.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 21.Sedlak TW, Oltvai ZN, Yang E, Wang K, Boise LH, Thompson CB, Korsmeyer SJ. Multiple Bcl-2 family members demonstrate selective dimerizations with Bax. Proc Natl Acad Sci USA. 1995;92:7834–7838. doi: 10.1073/pnas.92.17.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meeran SM, Katiyar SK. Grape seed proanthocyanidins promote apoptosis in human epidermoid carcinoma A431 cells through alterations in Cdki-Cdk-cyclin cascade, and caspase-3 activation via loss of mitochondrial membrane potential. Exp Dermatol. 2007;16:405–415. doi: 10.1111/j.1600-0625.2007.00542.x. [DOI] [PubMed] [Google Scholar]
- 23.Yoshikawa T, Streilein JW. Tumor necrosis factor-alpha and ultraviolet B light have similar effects on contact hypersensitivity in mice. Reg Immunol. 1991;3:139–144. [PubMed] [Google Scholar]
- 24.Rivas JM, Ullrich SE. Systemic suppression of delayed- type hypersensitivity by supernatants from UV-irradiated keratinocytes. An essential role for keratinocyte-derived IL-10. J Immunol. 1992;149:3865–3871. [PubMed] [Google Scholar]
- 25.Luger TA, Schwarz T. Evidence for an epidermal cytokine network. J Invest Dermatol. 1990;95:100S–104S. doi: 10.1111/1523-1747.ep12874944. [DOI] [PubMed] [Google Scholar]
- 26.Muller G, Saloga J, Germann T, Schuler G, Knop J, Enk AH. IL-12 as mediator and adjuvant for the induction of contact sensitivity in vivo. J Immunol. 1995;155:4661–4668. [PubMed] [Google Scholar]
- 27.Nastala CL, Edington HD, McKinney TG, Tahara H, Nalesnik MA, Brunda MJ, Gately MK, Wolf SF, Schreiber RD, Storkus WJ. Recombinant IL-12 administration induces tumor-regression in association with IFN-gamma production. J Immunol. 1994;153:1697–1706. [PubMed] [Google Scholar]
- 28.Sharma SD, Katiyar SK. Ditary grape-seed proanthocyanidin inhibition of ultraviolet B-induced immune suppression is associated with induction of IL-12. Carcinogenesis. 2006;1:95–102. doi: 10.1093/carcin/bgi169. [DOI] [PubMed] [Google Scholar]
- 29.Katiyar SK. Matrix metalloproteinases in cancer metastasis: Molecular targets for prostate cancer prevention by green tea polyphenols and grape seed proanthocyanidins. Endocrine, Metabolic & Immune Disorders-Drug Targets. 2006;6:17–24. doi: 10.2174/187153006776056648. [DOI] [PubMed] [Google Scholar]
- 30.Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis, and metastasis. Trends Cell Biol. 2001;11:37–43. doi: 10.1016/s0962-8924(01)02122-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strek M, Gorlach S, Podsedek A, Sosnowska D, Koziolkiewicz M, Hrabec Z, Hrabec E. Procyanidin oligomers from Japanese quince (Chaenomeles japonica) fruit inhibit activity of MMP-2 and MMP-9 metalloproteinases. J Agric Food Chem. 2007;16:6447–6452. doi: 10.1021/jf070621c. [DOI] [PubMed] [Google Scholar]
- 32.Matchett MD, MacKinnon SL, Sweeney ML, Gottschall-Pass KT, Hurta AR. Blueberry flavonoids inhibit matrix metalloproteinase activity in DU145 human prostate cancer cells. Biochem Cell Biol. 2005;83:637–643. doi: 10.1139/o05-063. [DOI] [PubMed] [Google Scholar]
- 33.Neto CC. Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases. Mol Nutr Food Res. 2007;51:652–664. doi: 10.1002/mnfr.200600279. [DOI] [PubMed] [Google Scholar]
- 34.Vayalil PK, Mittal A, Katiyar SK. Proanthocyanidins from grape seeds inhibit expression of matrix metalloproteinases in human prostate carcinoma cells, which is associated with the inhibition of activation of MAPK and NFκB. Carcinogenesis. 2004;25:987–995. doi: 10.1093/carcin/bgh095. [DOI] [PubMed] [Google Scholar]
- 35.Agarwal C, Singh RP, Dhanalakshmi S, Agarwal R. Anti-angiogenic efficacy of grape seed extract in endothelial cells. Oncol Rep. 2004;11:681–685. [PubMed] [Google Scholar]
- 36.Meeran SM, Katiyar SK. Cell cycle control as a basis for cancer chemoprevention through dietary agents. Front Biosci. 2008;13:2191–2202. doi: 10.2741/2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roy AM, Baliga MS, Elmets CA, Katiyar SK. Grape seed proanthocyanidins induce apoptosis through p53, Bax, and Caspase 3 pathways. Neoplasia. 2005;7:24–36. doi: 10.1593/neo.04412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mantena SK, Katiyar SK. Grape seed proanthocyanidins inhibit UV-radiation-induced oxidative stress and activation of MAPK and NF-kappaB signaling in human epidermal keratinocytes. Free Radic Biol Med. 2006;40:1603–1614. doi: 10.1016/j.freeradbiomed.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 39.Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin Cancer Res. 2006;12:6194–6202. doi: 10.1158/1078-0432.CCR-06-1465. [DOI] [PubMed] [Google Scholar]
- 40.Ye X, Krohn RL, Liu W, Joshi SS, Kuszynski CA, McGinn TR, Bagchi M, Preuss HG, Stohs SJ, Bagchi D. The cytotoxic effects of a novel IH636 grape seed proanthocyanidin extract on cultured human cancer cells. Mol Cell Biochem. 1999;196:99–108. [PubMed] [Google Scholar]
- 41.Lizarraga D, Lozano C, Briedé JJ, van Delft JH, Touriño S, Centelles JJ, Torres JL, Cascante M. The importance of polymerization and galloylation for the antiproliferative properties of procyanidin-rich natural extracts. FEBS J. 2007;274:4802–4811. doi: 10.1111/j.1742-4658.2007.06010.x. [DOI] [PubMed] [Google Scholar]
- 42.Seeram NP, Adams LS, Zhang Y, Lee R, Sand D, Scheuller HS, Heber D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J Agric Food Chem. 2006;54:9329–9339. doi: 10.1021/jf061750g. [DOI] [PubMed] [Google Scholar]
- 43.Neto CC. Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases. Mol Nutr Food Res. 2007;51:652–664. doi: 10.1002/mnfr.200600279. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt BM, Erdman JW, Jr, Lila MA. Differential effects of blueberry proanthocyanidins on androgen sensitive and insensitive human prostate cancer cell lines. Cancer Lett. 2006;231:240–246. doi: 10.1016/j.canlet.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 45.Baliga MS, Katiyar SK. Chemoprevention of photocarcinogenesis by selected dietary botanicals. Photochem Photobiol Sci. 2006;11:243–253. doi: 10.1039/b505311k. [DOI] [PubMed] [Google Scholar]
- 46.Yoshikawa T, Rae V, Bruins-Slot W, vand-den-Berg JW, Taylor JR, Streilein JW. Susceptibility to effects of UVB radiation on induction of contact hypersensitivity as a risk factor for skin cancer in humans. J Invest Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 47.Manetti R, Parronchi P, Giudizi MG, Piccinni P, Maggi E, Trinchieri G, Romagnani S. Natural killer cell stimulatory factor (interleukin-12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J Exp Med. 1993;177:1199–1204. doi: 10.1084/jem.177.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsieh C, Macatonia SE, Tripp CS, Wolf SE, O’Garra A, Murphy KM. Development of Th1 CD4+T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 49.Davis CD, Hord NG. Nutritional “omics” technologies for elucidating the role(s) of bioactive food components in colon cancer prevention. J Nutr. 2005;11:2694–2697. doi: 10.1093/jn/135.11.2694. [DOI] [PubMed] [Google Scholar]
- 50.Singletary KW, Meline B. Effect of grape seed proanthocyanidins on colon aberrant crypts and breast tumors in a rat dual-organ tumor model. Nutr Cancer. 2001;39:252–258. doi: 10.1207/S15327914nc392_15. [DOI] [PubMed] [Google Scholar]
- 51.Raina K, Singh RP, Agarwal R, Agarwal C. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer Res. 2007;67:5976–5982. doi: 10.1158/0008-5472.CAN-07-0295. [DOI] [PubMed] [Google Scholar]
- 52.Brooker S, Martin S, Pearson A, Bagchi D, Earl J, Gothard L, Hall E, Porter L, Yarnold J. Double-blind, placebo-controlled, randomised phase II trial of IH636 grape seed proanthocyanidin extract (GSPE) in patients with radiation-induced breast induration. Radiother Oncol. 2006;79:45–51. doi: 10.1016/j.radonc.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Record IR, McInerney JK, Noakes M, Bird AR. Chocolate consumption, fecal water antioxidant activity, and hydroxyl radical production. Nutr Cancer. 2003;47:131–135. doi: 10.1207/s15327914nc4702_4. [DOI] [PubMed] [Google Scholar]
- 54.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 55.Holt RR, Lazarus SA, Sullards MC, Zhu QY, Schramm DD, Hammerstone JF, Fraga CG, Schmitz HH, Keen CL. Procyanidin dimer B2 [epicatechin-(4β-8)-epicatechin] in human plasma after the consumption of a flavanol-rich cocoa. Am J Clin Nutr. 2002;76:798–804. doi: 10.1093/ajcn/76.4.798. [DOI] [PubMed] [Google Scholar]
- 56.Sano A, Yamakoshi J, Tokutake S, Tobe K, Kubota Y, Kikuchi M. Procyanidin B1 is detected in human serum after intake of proanthocyanidin-rich grape seed extract. Biosci Biotechnol Biochem. 2003;67:1140–1143. doi: 10.1271/bbb.67.1140. [DOI] [PubMed] [Google Scholar]
- 57.Rios LY, Bennett RN, Lazarus SA, Remesy C, Scalbert A, Williamson G. Cocoa procyanidins are stable during gastric transit in humans. Am J Clin Nutr. 2002;76:1106–1110. doi: 10.1093/ajcn/76.5.1106. [DOI] [PubMed] [Google Scholar]
- 58.Déprez S, Brézillon C, Rabot S, Philippe C, Mila I, Lapierre C, Scalbert A. Polymeric proanthocyanidins are catabolized by a human colonic microflora into low molecular weight phenolic acids. J Nutr. 2000;130:2733–2738. doi: 10.1093/jn/130.11.2733. [DOI] [PubMed] [Google Scholar]
- 59.Rios LY, Gonthier MP, Remesy C, Mila I, Lapierre C, Lazarus SA, Williamson G, Scalbert A. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am J Clin Nutr. 2003;77:912–918. doi: 10.1093/ajcn/77.4.912. [DOI] [PubMed] [Google Scholar]
- 60.Gonthier MP, Donovan JL, Texier O, Felgines C, Remesy C, Scalbert A. Metabolism of dietary procyanidins in rats. Free Radic Biol Med. 2003;35:837–844. doi: 10.1016/s0891-5849(03)00394-0. [DOI] [PubMed] [Google Scholar]