Abstract
Introduction
Epidemiological studies report a male predominance in lone atrial fibrillation (LAF). Phenotypic differences between sporadic and familial LAF could aid in deciding which cases should undergo family screening. We sought to determine gender distribution in sporadic and familial LAF, gender-based differences, and phenotypic differences between sporadic and familial LAF.
Methods
Since November 2000, 192 unrelated LAF probands were recruited. Sporadic LAF was defined as the absence of a family history of LAF. Familial LAF was classified as possible if one first- or second-degree relative had LAF, or confirmed if ≥ 2 relatives had LAF. Affected relatives (n = 87) of 34 confirmed familial probands were also evaluated. For unrelated LAF probands, differences in proportions and means were tested using χ2 and ANOVA, respectively. Difference in gender ratio among the family history groups was tested using mixed models.
Results
Male proportion was greater among sporadic (82%) and possible familial probands (84%) than confirmed familial probands (62%), and affected relatives (54%), P < 0.001. Sporadic LAF was more common in men (62%) than women (51%), P = 0.03. More women were affected by palpitation and nocturnal symptoms than men. More patients had permanent AF in the confirmed familial group (27%), compared with the possible familial (7%) and the sporadic LAF group (8%), P = 0.05, but no other phenotypic discriminators were identified.
Conclusions
Male predilection for LAF is attenuated as the likelihood of dominant Mendelian inheritance increases. Increased frequency of “sporadic” LAF among men could be partially due to X-linked recessive inheritance. Finally, sporadic and familial LAF are clinically indistinguishable.
Keywords: lone atrial fibrillation, familial atrial fibrillation, gender
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia, affecting more than 2.2 million Americans.1 Many studies report a male preponderance in AF: a population-based cohort estimated a 1.5-fold greater risk in men of developing AF, compared with women.2 Additionally, in a prospective cohort analysis within the Framingham Heart Study, men were at higher risk than women of developing AF irrespective of whether they had hypertension, diabetes, clinically overt heart disease, or parental AF.3 In a subset of younger patients (<60 years of age), AF develops in the absence of known risk factors, a condition classified as lone AF (LAF).4 Similar to the common acquired form of AF, LAF appears to exhibit male predominance. This is supported by several large epidemiological studies 5-7 in which the male to female ratios ranged from 3:1 to 4:1. Although compelling, these studies do not provide an explanation for male predilection. There are also limited data on gender-based clinical differences in LAF. In one study, men were less likely than women to have urination as a trigger for AF, nausea preceding AF, and palpitations and dyspnea during AF.8
Familial forms of LAF with autosomal dominant inheritance have been described.9-15 In a referral population, we reported that at least 15% of LAF cases have a positive family history of LAF.10 Recent evidence has shown that LAF is not entirely benign; advancing age or development of hypertension increases the risk of thromboembolic complications in LAF patients, compared with the general population.16 Given its adverse clinical impact and potential familial aggregation, selective clinical screening of family members of index cases should be considered. However, family history may be an insensitive or unreliable discriminator. Phenotypic differences between familial and sporadic LAF, if present, could aid in deciding which index cases should undergo family screening. Presently, there are limited data on phenotypic differences between familial and sporadic LAF. In one study that included 180 patients with LAF, patients with a family history of AF were more likely to have permanent AF, undergone cardioversion, or experienced syncope, compared with sporadic LAF patients.8
In a large cohort of unrelated LAF probands recruited for genetic studies, we sought to determine whether there is a gender predilection in sporadic and familial LAF as well as define any gender-based differences in LAF phenotype. Additionally, we sought to define phenotypic differences between sporadic and familial LAF.
Methods
Study Population
Since November 2000, patients referred to the Mayo Clinic Heart Rhythm Center with LAF were identified and invited to participate in our study following informed, written consent. AF was defined as replacement of sinus P waves by rapid oscillations or fibrillatory waves that varied in size, shape, and timing and were associated with an irregular ventricular response when atrioventricular conduction was intact. Documentation of AF on an ECG, rhythm strip, event monitor, or Holter monitor recording was required. LAF was defined as AF in individuals < 60 years of age without hypertension or overt structural heart disease by clinical examination, ECG, and echocardiography.4 Patients were excluded if they had history of congestive heart failure, primary cardiomyopathy, myocardial infarction, valvular heart disease, or preexisting hypertension.
Clinical Evaluation
Family history information was obtained from the medical record and a structured questionnaire. The questionnaire pertained to past medical history, family history, clinical symptoms, and treatment history. Based on this information, a study coordinator (K.H.) subsequently obtained a more detailed family history. First- and second-degree relatives with LAF by history were contacted to obtain medical records and asked to fill out the questionnaire. Screening ECG and transthoracic echocardiography were performed, if these tests had not been previously done for clinical indications. The research protocol and questionnaire were approved by the Institutional Review Board of the Mayo Clinic.
“Paroxysmal AF” was defined as AF lasting more than 30 seconds that terminated spontaneously. AF was classified as “persistent” when it lasted more than 7 days and required either pharmacologic therapy or electrical cardioversion for termination. AF that was completely refractory to cardioversion or was allowed to continue was classified as “permanent.”17
Two classifications for familial AF were used: possible familial AF, one first- or second-degree relative with documented LAF; and confirmed familial AF, at least two first-or second-degree relatives with documented LAF. In the absence of at least one first- or second-degree relative with documented LAF, AF was classified as sporadic.
Statistical Analysis
Baseline phenotypic characteristics were compared between genders and among the following family history groups: sporadic LAF probands, possible familial AF probands, and confirmed familial AF probands. Continuous variables were compared using ANOVA; categorical variables were compared using χ2 or Fisher’s exact test.
We included “affected relatives” as the fourth comparison group in the univariate analysis for gender ratio. The affected relatives were first- or second-degree relatives of the confirmed familial AF probands. Since the “affected relatives” group did not include any probands, it presented an opportunity to evaluate gender distribution that might be influenced by gender-based referral or ascertainment bias. To account for clustering effects introduced by affected relatives of familial AF probands, differences in gender distribution were tested using PROC MIXED (SAS version 9.1.3, SAS Institute, Cary, NC, USA). All P values reported were two-sided, and statistical significance was evaluated at the 5% level.
The authors have full access to and take responsibility for the integrity of the data. All authors have read and agree to the article as written.
Results
Since November 2000, 192 patients (79% male) referred to the Mayo Clinic Heart Rhythm Center had documented LAF and consented to enrollment in the study. Of the 192 probands, 114 (59%) had no documented family history of LAF (sporadic), and 78 (41%) had at least one first- or second-degree relative with documented LAF (familial). Thirty-four (18%) of the familial probands had at least two first- or second-degree relatives with documented LAF, that is, confirmed familial AF, whereas the remaining 44 (23%) probands had possible familial AF.
Table 1 summarizes the baseline characteristics of the sporadic LAF probands, possible familial AF probands, confirmed familial AF probands, and affected relatives of the confirmed familial AF probands. More patients had permanent AF in the confirmed familial AF group (27%), as compared with the possible familial AF (7%) and the sporadic LAF (8%) groups. Conversely, there were slightly fewer paroxysmal AF cases among the confirmed familial AF probands (57%) than the possible familial AF (68%) and the sporadic LAF probands (69%), P = 0.05.
TABLE 1.
Baseline Clinical Characteristics by Family History
| Characteristic | Sporadic Probands (n = 114) | Possible Familial Probands (n = 44) | Confirmed Familial Probands (n = 34) | Affected Relatives (n = 87) | *P-Value |
|---|---|---|---|---|---|
| Age at diagnosis (years) | 45.3 ± 10.9 | 42.8 ± 11.5 | 42.2 ± 13.6 | 46.1 ± 14.2 | 0.29 |
| Male gender | 93 (81.6) | 37 (84.1) | 21 (61.8) | 47 (54.0) | 0.03 |
| Ejection fraction (%) | 58.4 ± 7.9 | 57.8 ± 10.1 | 60.4 ± 8.5 | 60.4 ± 9.9 | 0.44 |
| ECG characteristics in sinus rhythm | |||||
| PR interval (ms) | 177.4 ± 31.8 | 169.5 ± 30.9 | 170.8 ± 22.7 | 166.0 ± 33.8 | 0.36 |
| QRS duration (ms) | 101.2 ± 20.8 | 101.5 ± 20.7 | 100.3 ± 20.0 | 96.2 ± 31.7 | 0.97 |
| QTc interval (ms) | 426.2 ± 31.5 | 428.8 ± 28.4 | 431.1 ± 27.7 | 427.1 ± 36.5 | 0.70 |
| QRS axis (°) | 19.3 ± 45.3 | 29.9 ± 38.3 | 35.3 ± 60.6 | 22.6 ± 56.7 | 0.17 |
| Ventricular rate (bpm) | 72.5 ± 24.1 | 72.1 ± 21.5 | 73.4 ± 15.9 | 73.3 ± 16.8 | 0.97 |
| Type of AF | 0.05 | ||||
| Paroxysmal | 76 (69.1) | 30 (68.2) | 17 (56.7) | 13 (28.9) | |
| Persistent | 25 (22.7) | 11 (25.0) | 5 (16.7) | 24 (53.3) | |
| Permanent | 9 (8.2) | 3 (6.8) | 8 (26.6) | 8 (17.8) | |
| Symptoms | |||||
| Palpitations | 47 (68.1) | 10 (76.9) | 14 (73.7) | 44 (73.3) | 0.77 |
| Awakened by palpitations at night | 25 (36.2) | 8 (61.5) | 7 (36.8) | 35 (53.0) | 0.22 |
| Dyspnea | 53 (74.6) | 10 (76.9) | 17 (89.5) | 25 (65.8) | 0.39 |
P-value is for comparison among sporadic, possible familial, and confirmed familial probands, excluding affected relatives.
Continuous variables are presented as mean ± SD; categorical variables are presented as no. (%).
The proportion of men versus women was less among the confirmed familial AF probands (62%), as compared with the possible familial AF (84%) and the sporadic LAF probands (82%), P = 0.03. When affected relatives (54% men) were included in the analysis, two patterns of gender distribution emerged: a distinct male predominance among sporadic LAF or possible familial AF probands, and a less evident male predominance among confirmed familial AF probands or their affected relatives (Fig. 1). After accounting for clustering effects introduced by familial AF probands and their affected relatives, there was a significant difference in the male proportion among the four groups: sporadic LAF, possible familial AF, confirmed familial AF, and the affected relatives, P < 0.001.
Figure 1.
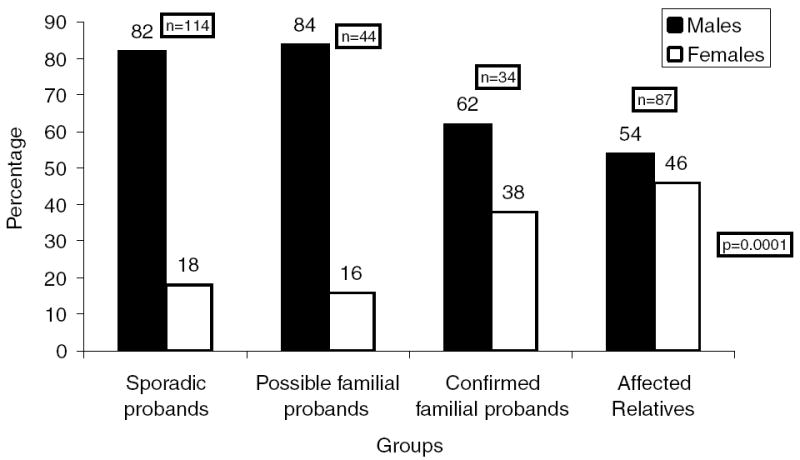
Gender proportion based on family history.
Table 2 shows the distribution of baseline characteristics with respect to gender. Women were more likely to report palpitation and nocturnal symptoms than men. Additionally, there were gender-based differences in echocardiographic and ECG parameters. Men had lower left ventricular ejection fraction (LVEF) than women. In terms of ECG characteristics during sinus rhythm, women had significantly higher ventricular rate and shorter conduction times—PR interval and QRS duration—–than men.
TABLE 2.
Baseline Clinical Characteristics by Gender
| Characteristic | Female Probands (n = 41) | Male Probands (n = 151) | P-Value |
|---|---|---|---|
| Age at diagnosis (years) | 45.3 ± 14.0 | 43.9 ± 10.9 | 0.49 |
| Confirmed familial AF | 13 (31.7) | 21 (13.9%) | 0.03 |
| Ejection fraction (%) | 61.1 ± 5.9 | 58.0 ± 9.0 | 0.02 |
| ECG characteristics in sinus rhythm | |||
| PR interval (ms) | 156.2 ± 30.1 | 179.3 ± 28.8 | < 0.001 |
| QRS duration (ms) | 93.6 ± 19.0 | 103.2 ± 20.5 | < 0.01 |
| QTc interval (ms) | 433.8 ± 29.1 | 425.9 ± 30.2 | 0.15 |
| QRS axis (°) | 25.2 ± 56.5 | 24.3 ± 44.1 | 0.91 |
| Ventricular rate (bpm) | 83.2 ± 33.9 | 69.6 ± 16.7 | < 0.001 |
| Type of AF | 0.15 | ||
| Paroxysmal | 29 (76.3) | 94 (64.4) | |
| Persistent | 4 (10.5) | 37 (25.3) | |
| Permanent | 5 (13.2) | 15 (10.3) | |
| Symptoms | |||
| Palpitations | 23 (88.5) | 48 (64.0) | 0.02 |
| Awakened by palpitations at night | 16 (61.5%) | 24 (32.0) | 0.01 |
| Dyspnea | 22 (84.6) | 58 (75.3) | 0.33 |
Continuous variables are presented as mean ± SD; categorical variables are presented as no. (%).
Figure 2 shows family history status with respect to gender. Sporadic LAF was more common in male probands (62%) than in female probands (51%); conversely, possible or confirmed familial AF was more common in female probands (49%) than in male probands (38%), P = 0.03.
Figure 2.
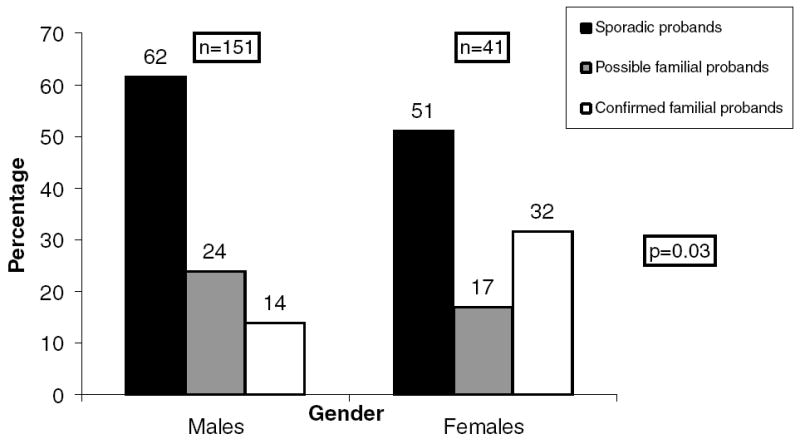
Family history status based on gender.
Discussion
The novel findings from this study of a large cohort of 192 unrelated LAF probands are as follows: first, male predominance exists in sporadic and possible familial LAF probands, but to a significantly lesser extent in confirmed familial LAF probands or their affected relatives; second, more men have sporadic LAF than women. Apart from a greater proportion of permanent AF in the confirmed familial LAF probands, as compared to possible familial and sporadic LAF probands, we did not find any other phenotypic differences among the three groups. Our data indicate that the male predominance of LAF is attenuated as the likelihood of Mendelian inheritance increases, attributable to a dominant effect of a single gene. In addition, the greater proportion of “sporadic” LAF among men compared with women may be partially explained by unrecognized X-linked recessive inheritance. Finally, sporadic and familial LAF are clinically indistinguishable.
Gender Imbalance in LAF
Population-based cohort studies have indicated a male predominance in common acquired AF.2,3 Similarly, several epidemiological studies have attested to a male predominance in LAF.5,6 In 30 years of follow-up in the Framingham study (n = 5,209), LAF occurred in 32 men and 11 women.5 In another population-based study spanning three decades from Olmsted County, Minnesota,6 97 patients developed LAF, of whom 78 were men and 19 were women. In a cohort with follow-up of 30 years from Trieste in Italy, 118 of 145 patients who developed LAF were men.7 In yet another prospective cohort of 180 LAF patients enrolled for genetic studies, 82% were men.8 The ratio of men to women in these cohorts ranged from 3:1 to 4:1.
These data collectively implicate an intrinsic male predilection for LAF, a finding we also observed in the LAF probands we recruited. We cannot, however, exclude the possibility of gender-based referral or ascertainment bias in our cohort. To address this issue, we examined the gender distribution in different subgroups among our LAF probands. We found that the ratio of men to women was high in the sporadic LAF probands (4:1), and in the possible familial LAF probands (5:1). In the confirmed familial LAF probands, however, the male predominance was less (ratio = 3:2). In the affected relatives of the confirmed familial LAF probands, there was no gender imbalance. Since the relatives-only group did not include any probands, it was less subject to referral or ascertainment bias.
There are two potential explanations for the preceding observation. First, there may be referral or ascertainment bias that operates to inflate the number of men among the sporadic LAF probands, which does not operate among the affected relatives. However, in our study, women were more likely to experience palpitations and be awakened at night by symptoms, compared with men. This finding is corroborated by a previous study that found women were more likely to have frequent AF episodes.8 Thus, referral or ascertainment bias, if present, should in fact inflate the number of women rather than men. In addition, if referral or ascertainment bias was operating, the male to female ratio among the confirmed familial probands should be similar to sporadic LAF probands–4:1 rather than 3:2. This suggests that while there is an intrinsic male predominance in LAF, it is attenuated as the likelihood of Mendelian inheritance increases. Our data thus indicate that as the diagnostic stringency for familial AF increases, the less prominent the role of any inherent gender bias. In monogenic forms of LAF, the effect of a single gene appears to overshadow any gender-based predilection for the disease.
Predominance of Sporadic LAF Among Men
In X-linked recessive disease, men are affected by the disease, whereas women, including the proband’s mother, are asymptomatic carriers. Increased frequency of sporadic LAF in men versus women can potentially be explained by unrecognized X-linked recessive disease. A male proband may have a negative family history and apparent sporadic disease when in fact his mother and sisters are carriers. Thus, some male probands may be misclassified as having sporadic disease, inflating the proportion of “sporadic” LAF in men.
Limitations
This study was limited principally by our reliance on family history to pursue investigation of potential familial cases. Lack of sensitivity of family history (e.g., due to asymptomatic undiagnosed disease among relatives) may result in some familial cases being erroneously classified as sporadic rather than familial probands. To reduce misclassifications, we applied our definitions consistently. In addition, our study sample was almost entirely of European ancestry. Further investigation in LAF cohorts of African or Asian ancestry is needed to determine whether our findings apply to these population groups.
Conclusions
Intrinsic male predilection for LAF is attenuated as the likelihood of dominant Mendelian inheritance increases. Increased frequency of “sporadic” LAF among men could be partially due to unrecognized X-linked recessive inheritance. Finally, sporadic and familial LAF are clinically indistinguishable.
Acknowledgments
This work was supported by grant RO1 HL075495 from the National Institutes of Health.
References
- 1.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DC, Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P American Heart Association Statistics C; Stroke Statistics S. Heart disease and stroke statistics–2006 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Levy D, Vaziri SM, D’Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 3.Fox CS, Parise H, D’Agostino RB, Sr, Lloyd-Jones DM, Vasan RS, Wang TJ, Levy D, Wolf PA, Benjamin EJ. Parental atrial fibrillation as a risk factor for atrial fibrillation in offspring. JAMA. 2004;291:2851–2855. doi: 10.1001/jama.291.23.2851. [DOI] [PubMed] [Google Scholar]
- 4.Chugh SS, Blackshear JL, Shen WK, Hammill SC, Gersh BJ. Epidemiology and natural history of atrial fibrillation: Clinical implications. J Am Coll Cardiol. 2001;37:371–378. doi: 10.1016/s0735-1097(00)01107-4. [DOI] [PubMed] [Google Scholar]
- 5.Brand FN, Abbott RD, Kannel WB, Wolf PA. Characteristics and prognosis of lone atrial fibrillation. 30-year follow-up in the Framingham Study. JAMA. 1985;254:3449–3453. [PubMed] [Google Scholar]
- 6.Kopecky SL, Gersh BJ, McGoon MD, Whisnant JP, Holmes DR, Jr, Ilstrup DM, Frye RL. The natural history of lone atrial fibrillation. A population-based study over three decades. N Engl J Med. 1987;317:669–674. doi: 10.1056/NEJM198709103171104. [DOI] [PubMed] [Google Scholar]
- 7.Scardi S, Mazzone C, Pandullo C, Goldstein D, Poletti A, Humar F. Lone atrial fibrillation:Prognostic differences between paroxysmal and chronic forms after 10 years of follow-up. Am Heart J. 1999;137:686–691. doi: 10.1016/s0002-8703(99)70224-3. [DOI] [PubMed] [Google Scholar]
- 8.Patton KK, Zacks ES, Chang JY, Shea MA, Ruskin JN, Macrae CA, Ellinor PT. Clinical subtypes of lone atrial fibrillation. Pacing Clin Electrophysiol. 2005;28:630–638. doi: 10.1111/j.1540-8159.2005.00161.x. [DOI] [PubMed] [Google Scholar]
- 9.Brugada R, Tapscott T, Czernuszewicz GZ, Marian AJ, Iglesias A, Mont L, Brugada J, Girona J, Domingo A, Bachinski LL, Roberts R. Identification of a genetic locus for familial atrial fibrillation. N Engl J Med. 1997;336:905–911. doi: 10.1056/NEJM199703273361302. [DOI] [PubMed] [Google Scholar]
- 10.Darbar D, Herron KJ, Ballew JD, Jahangir A, Gersh BJ, Shen WK, Hammill SC, Packer DL, Olson TM. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol. 2003;41:2185–2192. doi: 10.1016/s0735-1097(03)00465-0. [DOI] [PubMed] [Google Scholar]
- 11.Ellinor PT, Shin JT, Moore RK, Yoerger DM, MacRae CA. Locus for atrial fibrillation maps to chromosome 6q14-16. Circulation. 2003;107:2880–2883. doi: 10.1161/01.CIR.0000077910.80718.49. [DOI] [PubMed] [Google Scholar]
- 12.Chen YH, Xu SJ, Bendahhou S, Wang XL, Wang Y, Xu WY, Jin HW, Sun H, Su XY, Zhuang QN, Yang YQ, Li YB, Liu Y, Xu HJ, Li XF, Ma N, Mou CP, Chen Z, Barhanin J, Huang W. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–254. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Xia M, Jin Q, Bendahhou S, Shi J, Chen Y, Liang B, Lin J, Liu Y, Liu B, Zhou Q, Zhang D, Wang R, Ma N, Su X, Niu K, Pei Y, Xu W, Chen Z, Wan H, Cui J, Barhanin J. Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am J Hum Genet. 2004;75:899–905. doi: 10.1086/425342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia M, Jin Q, Bendahhou S, He Y, Larroque MM, Chen Y, Zhou Q, Yang Y, Liu Y, Liu B, Zhu Q, Zhou Y, Lin J, Liang B, Li L, Dong X, Pan Z, Wang R, Wan H, Qiu W, Xu W, Eurlings P, Barhanin J. A Kir2.1 gain-of-function mutation underlies familial atrial fibrillation. Biochem Biophys Res Commun. 2005;332:1012–1019. doi: 10.1016/j.bbrc.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 15.Olson TM, Alekseev AE, Liu XK, Park S, Zingman LV, Bienengraeber M, Sattiraju S, Ballew JD, Jahangir A, Terzic A. Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation. Hum Mol Genet. 2006;15:2185–2191. doi: 10.1093/hmg/ddl143. [DOI] [PubMed] [Google Scholar]
- 16.Jahangir A, Lee V, Friedman PA, Trusty JM, Hodge DO, Kopecky SL, Packer DL, Hammill SC, Shen WK, Gersh BJ. Long-term progression and outcomes with aging in patients with lone atrial fibrillation: A 30-year follow-up study. Circulation. 2007;115:3050–3056. doi: 10.1161/CIRCULATIONAHA.106.644484. [DOI] [PubMed] [Google Scholar]
- 17.Fuster V, Ryden LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Le Heuzey JY, Kay GN, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann S, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Halperin JL, Hunt SA, Nishimura R, Ornato JP, Page RL, Riegel B, Priori SG, Blanc JJ, Budaj A, Camm AJ, Dean V, Deckers JW, Despres C, Dickstein K, Lekakis J, McGregor K, Metra M, Morais J, Osterspey A, Tamargo JL, Zamorano JL. ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Revise the 2001 Guidelines for the Management of Patients With Atrial Fibrillation): Developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]


