Abstract
Study Design
Cross-sectional, paired cohort study.
Objectives
To replicate the finding of impaired immunocyte function following spinal cord injury (SCI). To determine whether cellular immune function in SCI subjects with decentralized sympathetic nervous system (SNS) (T6 and above) varies from SCI subjects with intact SNS (below T6).
Setting
University of Medicine and Dentistry of New Jersey–New Jersey Medical School, Newark, NJ, USA.
Method
In vitro immune assays: (1) natural killer (NK) cell cytotoxicity using a K562 target cell line in a 4-h chromium51 release assay. The mean of three samples for each effector-to-target (E:F) ratio (25:1, 50:1, 100:1) was used in the analyses. (2) Cell enumeration was performed using commercially available antibodies and standard flow cytometry techniques.
Results
Participation of 36 SCI subjects and 36 individually age- and sex-matched healthy controls. SCI subjects were stratified into two groups, that is, neurologic level of injury (NLI) at T6 or above (26 subjects) and NLI below T6 (10 subjects). No statistically significant differences were identified between NLI T6 and above and NLI below T6 groups for the NK cytotoxicity assay. There was a statistically significant reduction in NK cell numbers in all subjects with SCI as compared to their paired controls. There was a statistically significant reduction in NK cell cytotoxicity in SCI subjects, relative to the controls for E:F ratio of 100:1 (F = 6.18, d.f. = 34, P = 0.02).
Conclusion
We replicated the finding of decreased NK cell number and cytotoxicity in SCI subjects. The mechanism behind these findings needs to be further investigated, with the long-term goal of developing therapeutic strategies to improve immune function.
Keywords: natural killer cells, spinal cord injury, cytotoxicity, cell enumeration, sympathetic nervous system
Introduction
In the years following spinal cord injury (SCI), septicemia and pneumonia remain leading causes of death.1 Further infections of the urinary tract and respiratory tract are leading reasons for rehospitalization.2 This finding of increased morbidity and mortality because of infections is most pronounced in subjects with complete tetraplegia, and least pronounced in subjects with incomplete paraplegia.1 While mechanical factors such as impaired ventilation and neurogenic bladder have long been considered the cause of heightened infections in SCI subjects, Campagnolo et al.3,4 have considered a complimentary hypothesis, that is, a level of injury-dependent immune system alteration exists and contributes to infection acquisition. If correct, this hypothesis would predict a more pronounced infection profile in subjects with tetraplegia when compared to subjects with paraplegia, which is consistent with the epidemiology of infections following SCI. Supporting this, we have previously found cellular immune system impairment in a small group of subjects with tetraplegia (5 and 10 subjects in these two investigations). Furthermore, eight persons with low paraplegia were later studied and these alterations in immunocyte function were not identified.5 The objective of the current study was to replicate the finding of impaired immunocyte function in a larger cohort of SCI subjects, and to determine whether the subjects with neurologic level of injury (NLI) at or above the sympathetic outflow levels (T6 and above group) would demonstrate altered immune function when compared to the subjects with NLI below the sympathetic outflow levels (below T6 group), thus clarifying whether dysregulation of the sympathetic nervous system (SNS) impacts immune function after SCI. A secondary objective in this study was to explore whether depressive symptomatology, frequently found in SCI persons, predicts immune changes as it does in subjects without neurological injury.
Materials and methods
Subjects were recruited from the Northern New Jersey Spinal Cord Injury System (University of Medicine and Dentistry of New Jersey–University Hospital and Kessler Institute for Rehabilitation) between June 2001 and April 2004. The institutional review board approved this study and all subjects consented prior to their participation. A paired design was utilized. Two experimental subject groups were studied, that is, a cohort of subjects with NLI at or above T6 and a cohort of subjects with NLI below T6. A control group consisting of one individually age ( ± 5 years) and sex-matched healthy control for each SCI subject was also studied. On two occasions, a single control subject served as the control for two separate SCI subjects. Blood was drawn under non-fasting conditions in the morning hours for all subjects. Matched pairs of blood samples were put through laboratory assays at the same time and on the same day with the laboratory personnel blinded to the case–control identification.
Subjects were included if they met the following criteria: (1) 18 years of age or older; (2) living in the community (non-hospitalized) and (3) neurologically complete SCI (as defined by the American Spinal Injury Association, Atlanta, GA, USA) occurring 3 or more months prior to the study. Exclusion criteria were fever (oral temperature > 100 °F) in 2 days preceding study procedures; current pressure ulcers; surgeries during 2 months prior to study procedures; history of HIV seropositivity; or use of the following substances known to affect immune function such as α- and β-blockers, NSAIDS, alcohol, opiates, chemotherapies and corticosteroids. All subjects underwent a general screening examon the day of study and a full American Spinal Injury Association (ASIA) exam. For in vitro immune assays, blood was collected in a heparinized (preservative-free) syringe. Laboratory analyses were performed within 2h of phlebotomy.
Natural killer cell assay
Natural killer (NK) cell cytotoxicity was assayed using the target cell line K562 in the 4-h chromium51 release assay as previously described.4,5 The mean of three samples for each effector-to-target (E:T) ratio (25:1, 50:1, 100:1) was used in the analyses.
Cell enumeration
Cell enumeration was performed using commercially available antibodies and standard flow cytometry techniques.
Data analyses
Data were first examined descriptively for skewness and kurtosis. The variables that exhibited substantial skewness and/or kurtosis were transformed, using either a log or a square root transformation, to achieve approximate normality for the statistical analyses. Comparison of cell counts between the SCI subjects and individually matched controls was analyzed using multilevel mixed modeling software to estimate a repeated measures analysis of variance for unbalanced data (because of two control subjects each serving as the matched control to two SCI subjects). For ease of interpretation, the untransformed least square group means and standard errors were plotted, but significance levels were based on transformed data. The percentages of different lymphocyte subtypes were analyzed in the same manner.
NK cytotoxicity response differences between the SCI groups as a whole compared to the individually matched controls were analyzed in a similar manner, except that there were two repeated factors, group and E:T ratio. The finding of a significant overall group effect was followed by the examination of whether/how the group difference varied by E:T ratio. One SCI subject and matched control were excluded from the analysis of NK cytotoxicity due to obviously invalid data that could not be resolved.
Differences between the T6 and above group and the below T6 group were examined by first residualizing the scores of the subjects with their matched controls, thereby controlling for day-to-day laboratory assay variance. The residuals were then compared using a t-test.
Relationships between immune assay data and potential mediators of immune function were explored with Pearson’s correlations. The potential mediators examined in this study were depressive symptoms, as measured by the Beck Depression Inventory-II (BDI-II) total score, social support by the social provisions scale (SPS), stress by the perceived stress scale (PSS), pain by the pain intensity scale (PIS) and physical disability by the functional independence measure (FIM). For the PIS, subjects were asked to describe the intensity or physical sensation of pain ranging from 0 for none to 10 for ‘extremely intense’ pain. All analyses were performed with SAS for Windows Version 8.2 (SAS, Cary, NC, USA).
Results
Experimental and control groups
Thirty-six SCI subjects were studied along with the 34 individually age- and sex-matched controls. The SCI group consisted of 26 persons whose neurological level of injury was T6 and above (T6 and above group) and 10 with injury level below T6 group (below T6). The distribution of age, race and sex in these groups as well as the matched control (able-bodied) group is shown in Table 1. It shows that the two SCI groups were similar in socioeconomic areas such as education, employment status prior to their injury and employment status at the time they were studied. There was not a statistically significant difference in time (months) since the spinal cord injury in the T6 and above group (n = 26, mean = 13, s.d. = 26.2) as compared to the below T6 group (n = 10, mean = 41, s.d. = 71.1) (Mann–Whitney test, P = 0.64).
Table 1.
Groups characteristics (age, gender, months from injury, race, education and employment) with standard deviation
| Group | T6 and above, n = 26 | Below T6, n = 10 | Control n = 34 |
|---|---|---|---|
| Age (mean years (s.d.)) | 37.7 (14.2) | 35.6 (13.6) | 36.4 (13.0) |
| Sex (% male; % female) | 88.5; 11.5 | 100; 0 | Matched for sex |
| Months from injury (mean, s.d.) | 13 (26.2) | 41 (71.1) | Not applicable |
| Race (%) | |||
| African American | 57.7 | 40.0 | 17.6 |
| Caucasian | 26.9 | 30 | 52.9 |
| Hispanic | 15.4 | 30 | 8.8 |
| Asian | 0 | 0 | 20.6 |
| Education (%) | |||
| Grade school | 16.0 | 0 | 0 |
| High school | 44.0 | 50.0 | 5.9 |
| Trade school | 4.0 | 30.0 | 29.4 |
| College or higher | 36.0 | 20.0 | 64.7 |
| Employed (%) | |||
| Prior to injury | 69.2 | 60.0 | NA |
| At time of study | 11.5 | 10.0 | 89.9 |
Abbreviation: NA, not applicable.
There were no statistically significant differences between SCI subjects and their matched controls in the numbers of white blood cells, lymphocytes, granulocytes, monocytes, eosinophils and basophils (all P > 0.20; see Figure 1). There was a statistically significant reduction in the percent of NK cells (P = 0.04) and a significant increase in the percent of T cells (P = 0.04) and helper (CD3 + CD4 + ; P = 0.03) cells in the SCI subjects as compared to their paired controls (see Figure 2).
Figure 1.
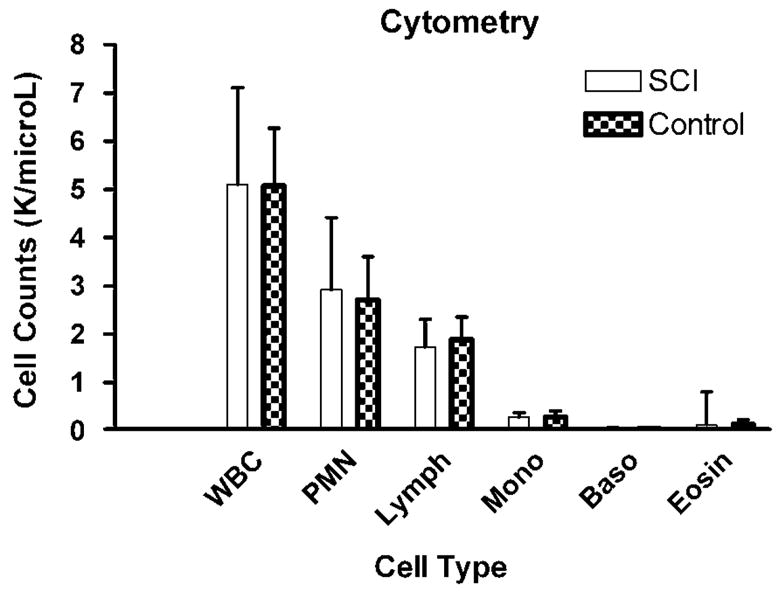
Cell counts (mean K per microliter, s.d.) by standard flow cytometry techniques using commercially available antibodies.
Figure 2.
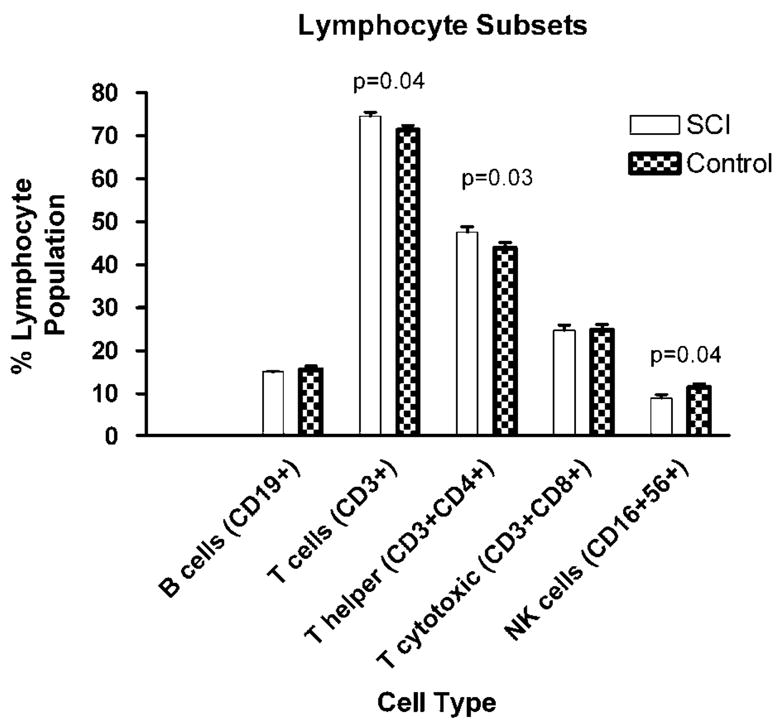
Percent cell types of the total lymphocyte population (mean ± s.e.m.).
Regarding in vitro cytotoxicity, the first set of statistical analyses assessed differences between immune measures in the SCI group as a whole compared with the individually matched controls. We found a statistically significant reduction in NK cell cytotoxicity in SCI subjects, relative to the controls for E:T cell ratio of 100:1 (F = 6.18, d.f. = 34, P = 0.02); for E:T cell ratio of 50:1 the difference was of borderline significance (F = 3.53, d.f. = 34, P = 0.07). No statistically significant difference was found for E:T cell ratio of 25:1 (F = 2.64, d.f. = 34, P = 0.11; see Figure 3) A secondary analysis in which we fit a growth curve model to the complete three cytotoxicity measures revealed the expected main effect for E:T ratio (the higher the E:T ratio, the greater the % NK kill (P = 0.001)) and a nearly significant interaction effect supporting the conclusion that this relationship is about 40% weaker in this group of SCI subjects than in their matched controls (P = 0.07).
Figure 3.
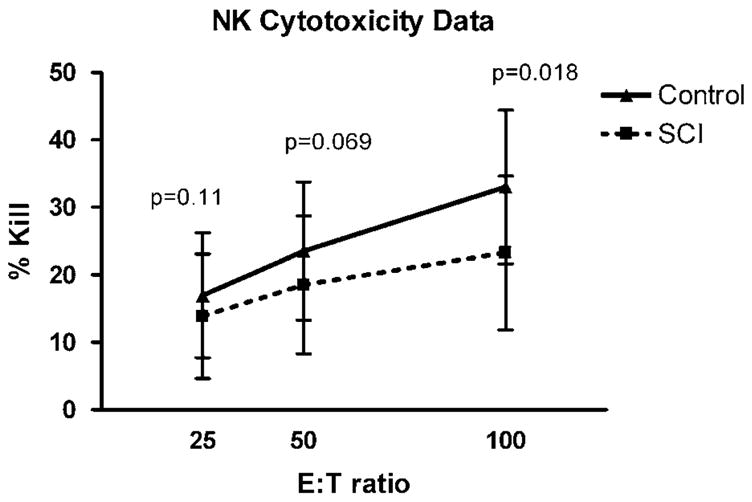
Natural killer (NK) cell activity (mean ± s.e.m.) in spinal cord injury (SCI) subjects (hashed line) and control subjects (solid line). Percent kill of target K562 cells on y axis and effector-to- target (E:F) cell ratio on x axis. This graded effect size was expected, and explained by a less than optimal E:F ratios that range from 100:1 to 25:1.
Subtypes of SCI subjects
The next set of statistical analyses examined the T6 and above group (n = 26) as compared to those in the below T6 group (n = 10). These analyses determined whether a group difference existed between those subjects who physiologically were most at risk for sympathetic derangement (T6 and above) compared to those subjects who were not (below T6). Residuals (from a regression of NK cytotoxicity of SCI subjects on the NK cytotoxicity of their matched controls) were analyzed by t-test. There was no evidence of a difference in NK cytotoxicity (data not shown, all P > 0.30).
In the whole group of SCI subjects, there were no statistically significant relationships, by using Pearson’s correlation, between the immune measures and the measures of depressive symptoms, social support, current pain stress or physical function (as measured by the total score of the BDI-II, SPS PIS and PSS). There was a statistically significant relationship between % NK cells in peripheral blood, NK cytotoxicity at 50:1, and total FIM score (r = 0.36, P = 0.03, n = 36; r = 0.37, P = 0.03, n = 35) (see Table 2). Trends in relationships (r > 0.25) are also highlighted in Table 2.
Table 2.
Pearson’s correlation coefficients
| NK25 | NK50 | NK100 | PER T | PER H | PER NK | |
|---|---|---|---|---|---|---|
| BDI | 0.00964 | −0.16343 | −0.15165 | −0.03029 | −0.17199 | 0.02147 |
| 0.9568 | 0.3557 | 0.3919 | 0.8629 | 0.3232 | 0.9026 | |
| 34 | 34 | 34 | 35 | 35 | 35 | |
| SPS | −0.06860 | −0.15235 | −0.08483 | −0.09288 | 0.05302 | −0.07358 |
| 0.6999 | 0.3897 | 0.6334 | 0.5956 | 0.7623 | 0.6744 | |
| 34 | 34 | 34 | 35 | 35 | 35 | |
| PSS | −0.03173 | −0.09165 | −0.19618 | −0.06258 | −0.15014 | −0.06136 |
| 0.8586 | 0.6062 | 0.2661 | 0.7210 | 0.3893 | 0.7262 | |
| 34 | 34 | 34 | 35 | 35 | 35 | |
| PIS | −0.20427 | −0.18673 | −0.13370 | 0.09458 | −0.16693 | −0.22538 |
| 0.3498 | 0.3936 | 0.5431 | 0.6602 | 0.4356 | 0.2896 | |
| 23 | 23 | 23 | 24 | 24 | 24 | |
| FIM | 0.26530 | 0.36631 | 0.23705 | −0.19661 | −0.03547 | −0.35904 |
| 0.1235 | 0.0304 | 0.1703 | 0.2504 | 0.8373 | 0.0315 | |
| 35 | 35 | 35 | 36 | 36 | 36 |
Abbreviations: BDI, Beck Depression Inventory-II; FIM, functional independence measure; NK, natural killer; PIS, pain intensity scale; PSS, perceived stress scale; SPS, social provisions scale.
P > |r| under H0: Rho = 0.
Highlights are statistically significant relationships and trends with r > 0.25. Pearson’s correlation coefficients for immune measures (NK cytotoxicity (NK25, NK50, NK100) and percent NK cells, T helper, T cytotoxic (PER NK, PER H, PER T) and potential mediators (BDI-II total score, SPS, PSS, pain by the PIS and FIM.
Discussion
The investigators’ previously published work suggested that NLI impacted in vitro immune function in SCI subjects. The subjects with NLI of T6 and above are known to have limited ability to fully regulate their SNS. Links shown between the immune system and the SNS, as well as the negative effects of chronic stress on NK cell numbers and function,6–8 lead the investigators to hypothesize that this SNS derangement may contribute to altered immune function. This provided the rationale for stratifying subjects into the two groups used. The data from this comparison of our two experimental groups (those with limited ability to control SNS responses compared to those without limitation) do not support this notion as SCI subjects with NLI at or above T6 (decentralized autonomic system) did not differ with respect to NK activity and peripheral white blood cell counts from those with NLI below the majority of SNS outflow. In the SCI group as a whole, however, we again showed a reduction of NK cell cytotoxicity relative to the healthy controls. The triggering of NK-cell cytotoxicity against the absence of killing activity is determined by a delicate balance between activating and inhibitory signals delivered by many families of cell-surface receptors. This process is not totally understood and is under ongoing investigation.9 Furthermore, our finding of increased percentage of total T cells and T-helper cells in the SCI group as compared to controls is consistent with the understanding that NK cells do influence cells of the adaptive immune system including T cells.9 For example, in human autoimmune disease, it has been suggested that NK cells trigger autoreactive T cells.9 Our finding of increase in percent T cells and T-helper cells may be a compensatory change related to reduced numbers of NK cells. The mechanism and details of interaction between the innate and adaptive immune have yet to be fully worked out.
Our past finding of decreased percentage of NK cells as well as decreased cytotoxicity of those NK cells is replicated in this larger study of 36 SCI subjects. NK cells are large granular lymphocytes that constitute about 10–15% of the total lymphocyte pool (in our control subjects 11.5%). They are defined phenotypically by expression of cluster differentiation CD56 and lack of expression of CD3.10 Having reproduced our earlier finding of reduced total numbers of NK cells in SCI survivors raises the question of why this is happening. One possible explanation is a problem with production of NK cells. These cells develop within the microenvironment of the bone marrow (BM), in which sensory and autonomic nerve fibers have been identified, suggesting a regulatory role in the BM.11,12 It has been shown that neural input to the BM regulates the granulocytic lineage via direct effects of SNS fibers (adrenergic receptors) and neuropeptides.13,14 Spinal cord injury in humans presents a unique opportunity to study possible early release of progenitors from the bone marrow in subjects with injury to the central nervous system.15 Iversen et al.15,16, after two studies, concluded that terminal differentiation of immune cells did not require supraspinal control; however, more investigation was needed to fully explore this possibility.
A second proposed explanation for reduced numbers of NK cells involves a compartmental shift. For example, large numbers of NK cells are found in the human uterus, in marked contrast to their relatively fewer numbers in peripheral blood.17 Furthermore, there is evidence that NK cells may play a regulatory role in central nervous system diseases,18 as these cells have also been shown to present in inflammatory CNS lesions.19,20 At this point, we do not know what regulatory or repair function NK cells maintain in the CNS, but it would likely be important, within a site of damage and inflammatory response, such as the area of SCI, where the blood–brain barrier has been compromised. Therefore, we speculate that the decreased numbers of NK cells in the peripheral circulation of our SCI cohort when compared with their matched controls may represent a shift from the peripheral blood compartment into another area, possibly the CNS compartment.
One limitation of this study is the comparison of a subgroup of subjects with level of injury from T1 to T6, to the subjects with cervical level of injury and those in the below T7 group. This may have provided more information regarding dependency on level of injury; however, this subgroup analysis was not possible due to the small number of subjects (six) that fell into the T1–T6 category. Another limitation of this study is that we did not perform detailed phenotypic analyses that would further identify the NK subsets in our population. Therefore, we do not know whether there is a change in the subsets of NK cells, which would possibly explain the reduced cytotoxicity, as certain NKs have different cytotoxic ability. We are currently studying this. Additionally, cytokines are the chemical messengers that immunocytes use to communicate with each other. An examination of cytokine production in these subjects is underway to fully assess the possible shift in pro-inflammatory (Th1) compared to anti-inflammatory (Th2) cytokines. We believe it is important to continue to study these innate immune as well as innate/adaptive interactions, as there are potential therapeutic strategies to enhance immune function broadly and specifically to enhance NK cell function. These would then be incorporated into routine care of SCI subjects. Nash7,8 acknowledges that the orchestration of immunity following SCI is multifactorial, with interplay of contributing factors such as drugs, physical inactivity and diet. Knowing more about the innate immune response in this vulnerable population will serve to guide recommendations to optimize these contributory factors in the future.
Acknowledgments
The study is supported by PVA Spinal Cord Research Foundation Grant 2161 and the National Institutes of Health K24 HD043819-01A1. We thank Dr Richa Sood for her assistance in preparing this paper.
References
- 1.DeVivo MJ, Krause JS, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- 2.Cardenas DD, Hoffman JM, Kirshblum S, McKinley W. Etiology and incidence of rehospitalization after traumatic spinal cord injury: a multicenter analysis. Arch Phys Med Rehabil. 2004;85:1757–1763. doi: 10.1016/j.apmr.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 3.Campagnolo DI, Keller SE, DeLisa JA, Glick TJ, Sipski ML, Schleifer SJ. Alteration of Immune system function in tetraplegics: a pilot study. Am J Phys Med Rehabil. 1994;73:387–393. doi: 10.1097/00002060-199411000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Campagnolo DI, Bartlett JA, Keller SE, Sanchez W, Oza R. Impaired phagocytosis of Staphylococcus aureus in complete tetraplegics. Am J Phys Med Rehabil. 1997;76:276–280. doi: 10.1097/00002060-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Campagnolo DI, Bartlett JA, Keller SE. Influence of neurological level on immune function following spinal cord injury: a review. J Spinal Cord Med. 2000;23:121–128. doi: 10.1080/10790268.2000.11753519. [DOI] [PubMed] [Google Scholar]
- 6.Perna FM, Schneiderman N, LaPerriere A. Psychological stress, exercise and immunity. Int J Sports Med. 1997;18(Suppl 1):S78–S83. doi: 10.1055/s-2007-972703. [DOI] [PubMed] [Google Scholar]
- 7.Nash MS. Immune dysfunction and illness susceptibility after spinal cord injury: an overview of probable causes, likely consequences, and potential treatments. J Spinal Cord Med. 2000;23:109–110. doi: 10.1080/10790268.2000.11753517. [DOI] [PubMed] [Google Scholar]
- 8.Nash MS. Known and plausible modulators of depressed immune functions following spinal cord injuries. [Review] [95 refs] J Spinal Cord Med. 2000;23:111–120. doi: 10.1080/10790268.2000.11753518. [DOI] [PubMed] [Google Scholar]
- 9.Shi F, Van Kaer L. Reciprocal regulation between natural killer cells and autoreactive T cells. Nat Rev Immunol. 2006;6:751–760. doi: 10.1038/nri1935. [DOI] [PubMed] [Google Scholar]
- 10.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 11.Iversen PO. Blood flow to the haemopoietic bone marrow. Acta Physiol Scand. 1997;159:269–276. doi: 10.1046/j.1365-201X.1997.00107.x. [DOI] [PubMed] [Google Scholar]
- 12.Rameshwar P. Substance P: a regulatory neuropeptide for hematopoiesis and immune functions. Clin Immunol Immunopathol. 1997;85:129–133. doi: 10.1006/clin.1997.4446. [DOI] [PubMed] [Google Scholar]
- 13.Broome CS, Miyan JA. Neuropeptide control of bone marrow neutrophil production. A key axis for neuroimmunomodulation. Ann NY Acad Sci. 2000;917:424–434. doi: 10.1111/j.1749-6632.2000.tb05407.x. [DOI] [PubMed] [Google Scholar]
- 14.Broome CS, Whetton AD, Miyan JA. Neuropeptide control of bone marrow neutrophil production is mediated by both direct and indirect effects on CFU-GM. Br J Haematol. 2000;108:140–150. doi: 10.1046/j.1365-2141.2000.01808.x. [DOI] [PubMed] [Google Scholar]
- 15.Iversen PO, Hjeltnes N, Holm B, Flatebo T, Strom-Gundersen I, Ronning W, et al. Depressed immunity and impaired proliferation of hematopoietic progenitor cells in patients with complete spinal cord injury. Blood. 2000;96:2081–2083. [PubMed] [Google Scholar]
- 16.Iversen PO, Nicolaysen A, Hjeltnes N, Nja A, Benestad HB. Preserved granulocyte formation and function, as well as bone marrow innervation, in subjects with complete spinal cord injury. Br J Haematol. 2004;126:870–877. doi: 10.1111/j.1365-2141.2004.05085.x. [DOI] [PubMed] [Google Scholar]
- 17.King A, Jokhi PP, Burrows TD, Gardner L, Sharkey AM, Loke YW. Functions of human decidual NK cells. Am J Reprod Immunol. 1996;35:258–260. doi: 10.1111/j.1600-0897.1996.tb00041.x. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K, Aranami T, Endoh M, Miyake S, Yamamura T. The regulatory role of natural killer cells in multiple sclerosis. Brain. 2004;127(Part 9):1917–1927. doi: 10.1093/brain/awh219. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto Y, Kohyama K, Aikawa Y, Shin T, Kawazoe Y, Suzuki Y, et al. Role of natural killer cells and TCR gamma delta T cells in acute autoimmune encephalomyelitis. Eur J Immunol. 1998;28:1681–1688. doi: 10.1002/(SICI)1521-4141(199805)28:05<1681::AID-IMMU1681>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 20.Antel J, Owens T. Multiple sclerosis and immune regulatory cells. Brain. 2004;127(Part 9):1915–1916. doi: 10.1093/brain/awh272. [DOI] [PubMed] [Google Scholar]


