Abstract
Medial prefrontal cortex (mPFC) neurons respond to Pavlovian conditioned stimuli, and these responses depend on input from the basolateral amygdala (BLA). In this study, we examined the mPFC efferent circuits mediating conditioned responding by testing whether specific subsets of mPFC projection neurons receive BLA input and respond to conditioned stimuli. In urethane-anesthetized rats, we identified mPFC neurons that projected to the nucleus accumbens (NAcc) or to the contralateral mPFC (cmPFC) using antidromic activation. Stimulation of the BLA and Pavlovian conditioned odors selectively activated a subpopulation of ventral mPFC neurons that projected to NAcc, but elicited virtually no activation in mPFC neurons that projected to cmPFC. BLA stimulation typically evoked inhibitory responses among nonactivated neurons projecting to either site. These results suggest that the ventral mPFC-to-NAcc pathway may support behavioral responses to conditioned cues. Furthermore, because projections from the BLA (which also encode affective information) and the mPFC converge within the NAcc, the BLA may recruit the mPFC to drive specific sets of NAcc neurons, and thereby exert control over prefrontal cortical-striato-thalamocortical information flow.
Keywords: corticostriatal, electrophysiology, emotion, in vivo, infralimbic, fear
Introduction
The medial prefrontal cortex (mPFC) is involved in emotional associative learning, in which actions or stimuli become associated with pleasant or unpleasant consequences. Recently we reported that a subpopulation of mPFC neurons encode emotional stimuli in a Pavlovian fear conditioning paradigm (Laviolette et al. 2005). Interference with mPFC function by blocking dopamine D4 or cannabinoid receptors prevents the encoding of emotional information in these neurons, and reduces the expression of conditioned fear (Laviolette et al. 2005; Laviolette and Grace 2006). However, the precise role of the mPFC in associative learning is complex, and may depend on the type of learning to which the animal is exposed (Garcia et al. 2006; Quirk et al. 2006). Thus, although the mPFC appears critical for fear conditioning, it is not known how the mPFC supports this type of learning.
The ability of the prefrontal cortex to represent emotional stimuli depends on input from the basolateral amygdala (BLA) (Schoenbaum et al. 2003; Laviolette et al. 2005). MPFC neurons fail to encode conditioned stimuli when the BLA is inactivated during conditioning, and only mPFC neurons that receive excitatory input from BLA respond to conditioned stimuli (Laviolette et al. 2005). Because BLA input to mPFC overwhelmingly targets spiny dendrites, many BLA- and conditioned stimulus–responsive neurons are likely to be projection neurons (Bacon et al. 1996; Gabbott et al. 2006); however, it is unclear whether these neurons correspond to subpopulations with distinct projection targets.
The capacity of the mPFC to mediate the expression of conditioned fear depends on its efferent targets, and one target likely to play a role in fear conditioning is the nucleus accumbens (NAcc). As is the case with BLA and mPFC, manipulations of the NAcc can disrupt associative learning (Cardinal et al. 2002). The BLA, mPFC and NAcc share dense, topographic, glutamatergic interconnections (Krettek and Price 1977; Groenewegen et al. 1990; Wright and Groenewegen 1995), and functional disconnection of 2 of these regions often mimics bilateral lesions of either region alone (Coutureau et al. 2000; Parkinson et al. 2000; Setlow et al. 2002; Floresco and Tse 2007). Thus, the connections among these 3 regions appear to play critical roles in the integration of emotional information and the expression of motivated behaviors.
We propose that the BLA, mPFC and NAcc form a functionally interconnected neuronal circuit that encodes emotional information, and that it is the mPFC output neurons that target the NAcc (PFC → NAcc) which receive input from the BLA and respond to conditioned stimuli. To test this hypothesis, we recorded PFC → NAcc neurons during stimulation of the BLA and during Pavlovian fear conditioning and compared their responses to those of a comparable, but largely nonoverlapping subpopulation of mPFC neurons projecting to the contralateral mPFC (cmPFC) (PFC → PFC) (Pinto and Sesack 2000).
Materials and Methods
All experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Pittsburgh Animal Care and Use Committee.
Subjects and Surgery
Experiments were performed on male, Sprague–Dawley rats (290–400 g). For the BLA stimulation experiments, 81 rats were anesthetized with a single intraperitoneal injection of urethane (1.4–1.5 g/kg in deionized water), which provided stable anesthesia throughout the experiments (maximum experiment duration was 8 h). For the 14 rats used in odor conditioning experiments, chloral hydrate (400 mg/kg in water intraperitoneally, maintained via supplemental injections through a femoral vein catheter), was used in order to duplicate the conditions of previous studies (Rosenkranz and Grace 2002; Laviolette et al. 2005). Comparative studies have shown that chloral hydrate and urethane have similar effects on evoked cortical responses (Angel and Gratton 1982).
When the rats no longer displayed a reflexive withdraw in response to a foot pinch, they were placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA). Burr holes were drilled in the skull overlying the electrode implantation targets, and the underlying dura was carefully removed. Up to 4 electrodes were then lowered (1 mm/min or less) into the brain: a recording electrode in the right side mPFC, a combination stimulating electrode/guide cannula (chemotrode) in the ipsilateral BLA, and stimulating electrodes in either the ipsilateral NAcc, cmPFC, or both (Fig. 1A). In a small number of experiments, the mPFC was stimulated and the BLA was the recording site. All coordinates are given relative to the bregma landmark, with the axes abbreviated as follows: AP for anterior-posterior; ML for medio-lateral; DV for dorso-ventral.
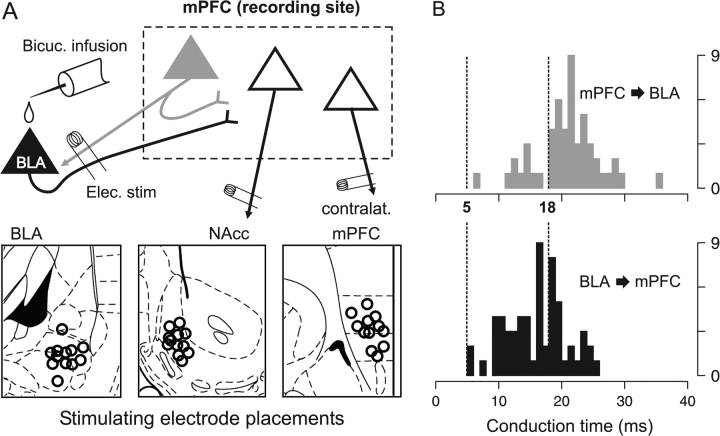
Figure 1.
(A) A diagram illustrating representative electrode placements for BLA stimulation experiments. The primary recording site was the mPFC, where neurons were recorded and identified as projecting to the NAcc, cmPFC or BLA by antidromic stimulation. In addition, in a small number of experiments, the BLA was the recording site, and BLA neurons projecting to mPFC were identified by antidromic stimulation of the mPFC (not illustrated). (B) Histograms of antidromic latencies for BLA → mPFC projection neurons (bottom) and for mPFC → BLA projection neurons (top); y-axis is number of neurons. The latency distributions suggest that BLA stimulation causes orthodromic activation of the BLA → mPFC pathway prior to the antidromic activation of PFC → BLA neurons. Therefore, to isolate orthodromic responses, only responses that occurred within 5–18 ms of BLA stimulation were analyzed.
The recording electrodes were pulled (Narishige, Tokyo, Japan) from 2 mm outside-diameter filamented borosilicate glass tubing (World Precision Instruments, Sarasota, FL). The electrode tips were broken back under microscopic control, and the electrodes were filled with the recording solution (2 M NaCl with Pontamine Sky Blue, impedance 6–12 MΩ measured in situ through the amplifier [Fintronics WDR-420, Orange, CT]). The recording electrodes were lowered slowly through the mPFC (anterior-posterior [AP] +2.5 to +3.5 mm; medio-lateral [ML] 0.3–0.9 mm; dorso-ventral [DV] −3.0 to −5.5 mm) using a hydraulic microdrive (Narishige).
The BLA was implanted with chemotrodes (combined guide tube and stimulating electrodes, Plastics One C313G-MS303/2) with a stimulating electrode protruding 2 mm beyond the guide tube (0.7–1.0 mm exposed at tip), and an accompanying infusion cannula that projected 1.6 mm beyond the guide tube. The chemotrodes were placed such that the negative pole was in the posterior BLA (AP −3.6 mm; ML 4.8–5.0 mm; DV 9.0 mm), the positive pole was in the anterior BLA (AP −2.6 mm, rest same) and the guide tube was located between the 2 poles. The NAcc stimulating electrodes (Rhodes Medical Instruments, Woodland Hills, CA, electrode type NEX-100) were placed in NAcc shell (AP +1.2 to 1.6 mm; ML 0.7–0.9 mm; DV −8.2 mm). The cmPFC stimulating electrodes (Rhodes Medical Instruments, SNEX-100) were placed in the infralimbic (IL), dorsal peduncular (DP) and medial orbital (MO) cortical areas (AP +2.7 to 3.3 mm; ML 0.3–0.7 mm; DV −5.0 to −5.3 mm).
Electrophysiological Procedures and Neuron Identification
Extracellular action potentials from mPFC neurons were amplified and filtered (1000× gain, 100–4000 Hz band pass, Fintronics WDR-420, Orange, CT), passed to an oscilloscope and audio monitor for real time monitoring, and to a computerized data acquisition system (Microstar Laboratories, Bellevue, WA) for storage and off-line analysis with custom software (Neuroscope) and routines for the R software language (R Development Core Team 2005). Electrical stimulation was controlled with a software pulse generator (Neuroscope) and the Master-8 stimulator and stimulus isolation units (A.M.P.I., Jerusalem, Israel). The pulse duration was 0.25 ms for all stimulation sites, and the currents applied varied with the stimulated region, as described below.
The neurons of interest, identified by antidromic stimulation, were those mPFC neurons projecting to the NAcc (PFC → NAcc), those projecting to the mPFC contralateral to the recording site (PFC → PFC), and in some cases those projecting to the BLA (PFC → BLA). Neurons projecting to both NAcc and cmPFC or to both NAcc and BLA were found infrequently (Pinto and Sesack 2000) and were only tested for responses to BLA electrical stimulation. Action potentials were confirmed as antidromic if they met 2 of 3 criteria: a spike latency that varied by 2 ms or less over 20 trials; the ability to follow 2 stimulation pulses delivered 2.5 ms apart (400 Hz, preferred for silent or slow-firing neurons); and the collision of the antidromic response with spontaneous spikes (preferred for spontaneously active neurons) (Fuller and Schlag 1976). As the recording electrode was lowered through the mPFC, a cell searching procedure (Floresco and Grace 2003) was used to find antidromically responding neurons. Stimulation pulses were delivered one at a time to the NAcc, cmPFC and/or BLA at an overall rate no greater than 0.5 Hz, with maximum currents of 500, 400, and 700 μA, respectively. Neurons that were confirmed as projecting to the NAcc or cmPFC were then subjected to electrical and/or chemical stimulation of the BLA, or to conditioned odors (see below). For the odor conditioning experiments, only projection neurons with baseline firing ≥ 1 Hz were recorded in order to adequately measure inhibitory responses. For neurons confirmed as projecting to the BLA, only antidromic response latency was measured.
BLA Electrical Stimulation
Once a suitable neuron was isolated, the baseline activity was recorded, and then the BLA was stimulated for 2–5 min with the following pattern: a continuous 0.3-Hz pulse train, with intermittent 20-Hz trains (1-s train duration) every 20 s. These 2 frequencies fall within the range of firing rates observed in BLA principal neurons in freely moving rats; low frequency firing is typically observed at rest, whereas higher frequency firing occurs in the presence of conditioned stimuli (Schoenbaum et al. 1998, 1999). Thus, neuronal responses to both low and high frequency BLA stimulation may relate to mPFC function in different behavioral states. The BLA stimulation current used in this part of the experiment was typically 400 μA; however, if a neuron exhibited a 100% response ratio at this current intensity, the current was lowered to produce a 50–80% response ratio.
Minimizing the Effects of Antidromic Activation
One potential artifact of BLA stimulation is the antidromic activation of terminals of mPFC neurons that project to the BLA, which may cause recurrent excitation of mPFC neurons. However, we minimized the impact of antidromic/recurrent excitation in the current study by measuring only responses likely to be orthodromic. We measured the antidromic latency (conduction time) of 60 PFC → BLA neurons (mean 20.8 ± 0.7 ms), and of 52 BLA neurons projecting to mPFC (BLA → mPFC, mean 16.2 ± 0.6 ms, significant difference by t test, P < 10−5), recorded during preliminary BLA recording/mPFC stimulation experiments. Thus, BLA neurons projecting to the mPFC exhibited faster conduction times on average than reciprocally projecting neurons; a difference which is consistent with that reported in 2 previous studies (Likhtik et al. 2005; Floresco and Tse 2007). The majority (60%) of BLA → mPFC neurons had conduction latencies between 5 and 18 ms, but only 19% of PFC → BLA neurons had conduction latencies in this range (Fig. 1B). Therefore, within 5–18 ms after a BLA stimulation pulse, the majority of orthodromic input reaches the mPFC, but only a small portion of recurrent antidromic inputs have been activated. Based on this differential distribution of conduction times, we limited our analyses to only the spikes that occurred within 5–18 ms of a BLA stimulation pulse. Although this window does not completely eliminate the contribution of antidromic activity, it minimizes antidromic effects and maximizes the measurement of orthodromic BLA → mPFC activation. Furthermore, the threshold current for most antidromically activated PFC → BLA neurons was greater than the 400 μA current used during the BLA stimulation part of the experiment (510 ± 21 μA mean threshold current; 84% had thresholds greater than 400 μA). Therefore, it is likely that few PFC → BLA neurons were activated antidromically by 400 μA BLA stimulation amplitude.
Population Analysis of BLA-Evoked Responses
The change in probability of spike discharge evoked by BLA electrical stimulation was calculated by subtracting the prepulse spike probability (-200 to 0 ms, calculated from 0.3 Hz stimuli only) from the postpulse spike probability (+5 to +18 ms); the prepulse probability was scaled to be comparable with the shorter poststimulus window. To assess excitatory responses across the population of neurons recorded, cells with greater than 1% increase in spike probability to either 0.3 or 20 Hz stimuli (approximately the smallest detectable increase) were grouped together, and the mean pre- and poststimulus spike probability were calculated. Because the baseline firing rate was typically low, inhibitory responses were not reliably detectable in all neurons (Floresco and Tse 2007). Therefore, to assess inhibitory responses across the population, neurons that did not exhibit a spike probability increase and that also had a prestimulus spike probability greater than or equal to 1% were grouped together, and the mean pre- and poststimulus spike probabilities were calculated. A 1% prestimulus spike probability is equivalent to a spontaneous firing rate of 0.8 Hz.
Single Neuron Analysis of BLA-Evoked Responses
We also assessed whether single mPFC neurons exhibited significant excitation in response to BLA stimulation using statistical criteria similar to previous studies (Maunsell and Gibson 1992; Hanes et al. 1995; Bisley et al. 2004; Gifford et al. 2005). For each neuron, the prestimulus spike count was fitted to a Poisson distribution. This distribution was used to calculate a poststimulus spike count threshold, t, such that the number of poststimulus spikes was expected to be t or fewer for 95% of the time based on prestimulus firing (i.e. we found t such that P(X ≤ t) < 0.95, where X is a random sample from the Poisson fit). Thus, a neuron was considered to exhibit a significant level of excitation if the actual number of poststimulus spikes for either 0.3 or 20 Hz stimuli was greater than its 95% threshold t, corresponding to a significance level of P < 0.05. Among these significantly excited neurons we also identified a subset of “highly excited” neurons, whose poststimulus spike counts exceeded a 99.9% threshold (the spike count exceeded t such that P(X ≤ t) < 0.999), corresponding to a significance level of P < 0.001.
Because most neurons recorded exhibited low spontaneous activity, this method was not appropriate for determining the significance of inhibitory responses. Specifically, in low firing neurons, even a total cessation of firing would not surpass a significant threshold for inhibition. Given the stimulation and analysis parameters used in this study, a spontaneous firing rate of 2.6 Hz or greater would typically be needed to identify a meaningful (i.e. nonzero) inhibition threshold; only 11 of the 136 PFC → NAcc and PFC → PFC neurons recorded exhibited sufficient spontaneous firing to calculate such a threshold. Therefore, although we observed inhibitory responses, we did not attempt to report the significance of inhibition in single neurons.
BLA Chemical Stimulation (Bicuculline Infusion)
To further rule out the contribution of activation of terminals or fibers of passage, in some experiments the BLA was stimulated chemically with a direct infusion of the GABAA antagonist bicuculline. Bicuculline-evoked field potential activity in BLA was recorded simultaneously with projection neuron single unit activity in mPFC. Bicuculline (20–50 ng in 500 nL Dulbecco's phosphate buffered saline) was infused into the BLA over a 2-min period through a cannula (Plastics One) fitted into the chemotrode guide tube, and coupled to polyethylene tubing, a Hamilton syringe, and motorized syringe pump. At most, 2 infusions separated by 90–180 min were performed in the same animal. Using the electrode leads of the BLA chemotrode, extracellular field potentials were recorded and amplified (Cygnus, 10–1000 Hz band pass, 1000× gain). Within 30–120 s after bicuculline infusion, isolated epileptiform discharge events (EDs) appeared, representing the vigorous firing of many nearby neurons (Steriade et al. 1998). The EDs were characterized as spontaneous, all-or-none voltage deflections with an initial negative phase, followed by a large amplitude positive phase. The EDs occurred at a rate of 0.1–0.5 Hz and persisted for 15–25 min following the infusion. Unlike previous studies (Steriade and Contreras 1998; Steriade et al. 1998), the EDs were not followed by seizure activity, and the rats did not display outward signs of seizure. Infusion of 500 nL buffered saline vehicle did not cause EDs (not shown).
ED times were used as reference times to construct peri-event time histograms of mPFC projection neuron activity. The ED times were defined as the peak of the initial negative phase of the voltage deflection. Previous studies have shown that the peak of the initial negative phase of the ED corresponds to the strongest firing in nearby neurons. Therefore, based on the orthodromic BLA → mPFC conduction times (5–26 ms, see above, Fig. 1B), post-ED spikes that occurred within 5–26 ms were interpreted as being consistent with monosynaptic, orthodromic input from the BLA.
Pavlovian Conditioning Experiments
Two groups of male Sprague–Dawley rats (285–365 g) were subjected to Pavlovian odor conditioning. The first group, consisting of 14 rats, was conditioned while awake, with recordings subsequently performed under anesthesia (see below); before the recording procedure, 9 of these 14 rats were subjected to a very brief duration test for the retention of fear conditioning. The second group, consisting of 8 rats, was conditioned while awake and then subjected to more thorough testing for the retention of conditioned fear, similar to that described previously (Laviolette et al. 2005; Laviolette and Grace 2006). None of this latter group was subjected to recording procedures to avoid potential extinction effects of the longer exposure. The protocols and equipment used in these experiments were similar to previous studies from our group (Otto et al. 1997; Rosenkranz and Grace 2002; Laviolette et al. 2005). Rats were allowed to habituate individually in the conditioning room for 20–30 min, and were then put in the conditioning chamber: a fan-ventilated Plexiglas box, with a metal shock grid floor and open top (Coulbourn, Allentown, PA). After 10 min, the first of 2 distinct odors (almond or peppermint [McCormick, Hunt Valley, MD]) was presented for 10 s, and 2 min later, the second odor was presented (no shocks were given during these initial presentations). Subsequently, the 2 odors were presented in alternation at 2-min intervals. One odor (CS+) was consistently paired with a 3-s foot shock (0.4–0.6 mA), and the other odor was presented alone (CS−). The rats received a total of 6 odor/shock pairings. CS+ identity (almond or peppermint) and CS order (CS+ first or CS− first) were counterbalanced across subjects. The rats remained in the chamber for 20 min following the last odor presentation, and were then removed and returned to their home cage for the rest of the day. The conditioning chamber was cleaned and deodorized after each use.
Behavioral Testing
On the day after conditioning, rats in both groups were tested for freezing in the presence of the CS+ odor (9/14 rats from the first group, 8/8 rats from the second group). Testing was performed in the same room and chamber as conditioning; however, the conditioning chamber was altered to reduce contextual cues that might cause a fear response in the absence of any odor: the ventilating air flow was redirected, the shock grid was reoriented, construction paper was applied to the otherwise clear walls, and the open top was partially occluded.
Rats from the first group (9 of 14, which comprised the group from which recordings were made) were tested with a single, brief CS+ presentation to prevent extinction of the conditioned fear response (Shipley 1974): After 20–30 min habituation in the conditioning room, rats were placed in the chamber. After 6 min, the rats were presented with the CS− odor, then CS+ odor (without shock), then CS− odor again (20 s each, 8 min between odors). Rats remained in the chamber for an additional 6 min, and were then returned to their home cage for the rest of the day. The fear response was determined by measuring freezing: a crouching posture with no visible movement other than breathing. Percent freezing (i.e., time freezing divided by total time) was scored and measured over the 60-s period that followed odor presentation (the 2 CS− periods were averaged). The 20-s period during odor presentation was not included because most rats actively sampled the odor (sniffing and whisking) during this time. One rat did not show any freezing throughout the entire testing period, and was excluded from analysis.
Rats from the second group (n = 8, not recorded from) were tested with longer CS+ and CS− presentations, as had been employed in previous studies (Otto et al. 1997; Laviolette et al. 2005; Laviolette and Grace 2006). After 20–30 min of habituation to the conditioning room, the rats were placed in the chamber. After 10 min, the first odor (either CS+ or CS−, counterbalanced) was presented for 5 min; 10 min after the first odor onset, the second odor was presented for 5 min. Freezing was measured over the 5-min CS+ and CS− presentations in bins of 1 min. In addition to freezing, in this group of 8 rats exploratory and grooming activity was also measured using a scoring system adapted from previous studies (Rosenkranz et al. 2003; Laviolette et al. 2005). For each one minute bin, rats were assigned a score based on ambulatory activity: 0 points for no movement; 1 point for movement across one side of the chamber; 2 points for 2 sides of the chamber; 3 points for all 4 sides of the chamber; 4 points for all 4 sides and center of the chamber. To this score, one point was added if the rat engaged in grooming behavior, and one point was added if the rat explored the chamber by rearing, for a total possible score of 6 points.
Electrophysiological Responses to Conditioned Odors in Anesthetized Rats
In the group of rats subjected previously to odor-based Pavlovian fear conditioning (n = 14), PFC → NAcc and PFC → PFC neuron responses to the CS+ odor (previously paired with foot shock) and the CS− odor (unpaired) were recorded. Recordings took place 24 h after conditioning in 5/14 rats; recording took place 48 h after conditioning (24 h after behavioral testing, see above) in 9/14 rats. After the baseline firing of each neuron was measured, the rat was presented with the CS− odor, followed by the CS+ odor, followed again by the CS− odor (10 s each). In 8 rats, the baseline recording period and the time between odors was 4–5 min, and in the remaining 6 rats, it was 8–10 min. Typically, 3 or 4 neurons were recorded in each animal. To reduce habituation of the odor-evoked neural response, the interval between odor presentation sequences was typically 45–60 min.
As with the electrical stimulation experiments, 2 analyses were performed. First, to assess the response across the population of recorded neurons, the firing rate immediately before odor presentation (30 s) was subtracted from the firing rate during odor presentation (10 s) for each neuron. The odors typically evoked both excitatory and inhibitory responses, and the top and bottom quartiles of responses (i.e., greatest excitatory and inhibitory responses) were analyzed separately using ANOVA. Second, single neurons were analyzed to determine whether they exhibited significant excitation by the CS+ or CS− odor: for each neuron the pre-odor firing rate was fitted to a Poisson distribution, and a spike count threshold of 95% (t such that P(X ≤ t) < 0.95) was calculated. Neurons with spike counts during the odor presentation that exceeded this threshold were deemed “significantly excited” (P < 0.05). Neurons that were “highly excited” (P < 0.001) were similarly identified using a 99.9% threshold (t such that P(X ≤ t) < 0.999).
Electrode Marking and Histological Processing
The recording electrode location was marked by iontophoretic ejection of Pontamine Sky Blue dye (-10 μA, 40–60 min). Stimulating electrode locations were marked by passing current between the electrode poles (200μA [reversed polarity], 10 s). The rats were then killed with an overdose of anesthetic and decapitated, and the brains removed and placed for 48 hours in paraformaldehyde with 1% potassium ferricyanide. The brains were transferred to a cryoprotectant solution of 25% sucrose. After 2 days they were sliced coronally and Nissl stained using standard histological procedures.
The locations of electrodes were assessed using the atlases of Paxinos and Watson (1998, 2005) and plotted onto a typical mPFC section. For clarity, the locations of only a representative sample of 33 out of the 66 PFC → NAcc neurons recorded during BLA stimulation are shown (Fig. 3C), including 11/12 excited PFC → NAcc neurons. Because of damage to some tissue sections, 1 of the 12 excited PFC → NAcc neurons that were located in IL cortex could not be precisely plotted onto the representative section.
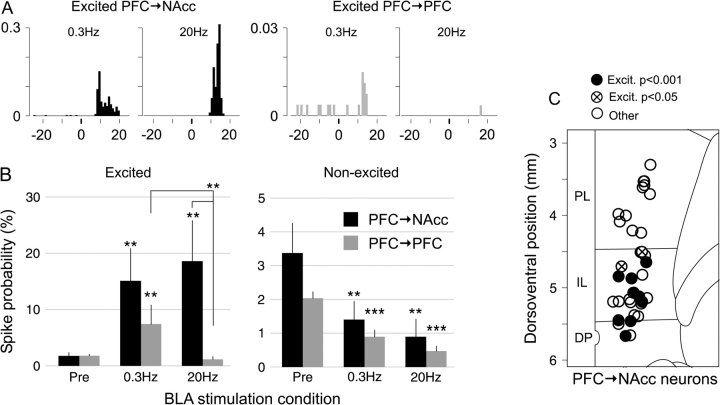
Figure 3.
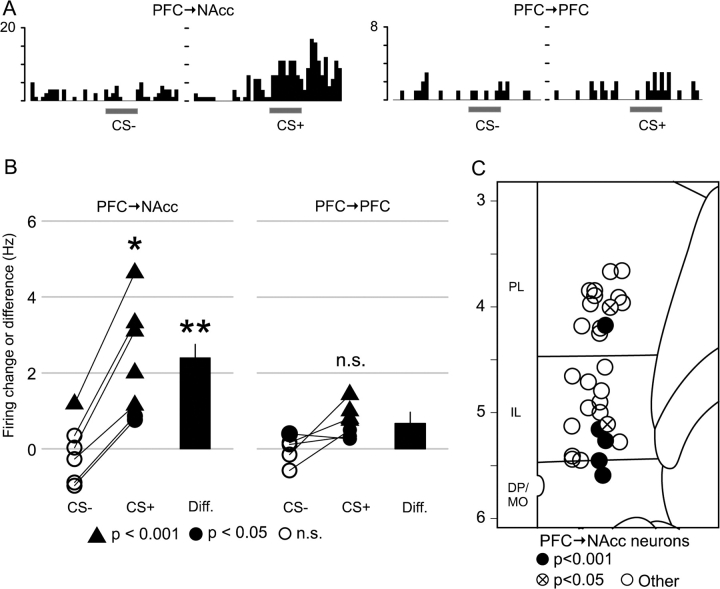
(A) Example peri-stimulus time histograms showing the response of an excited PFC → NAcc (left) and excited PFC → PFC (right) neuron to BLA stimulation at 0.3 and 20 Hz. x-axis is post-BLA stimulus time (ms); y-axis is response ratio per bin. In neurons with significant excitatory responses to both 0.3 and 20 Hz stimuli, the onset of the response to 0.3 Hz was typically 1–2 ms earlier than the response to 20-Hz stimulus trains. (B) 20-Hz BLA stimulation elicited greater excitation in PFC → NAcc than in PFC → PFC neurons (left). For nonexcited neurons with spontaneous activity of both types, BLA stimulation inhibited firing (right). **P < 0.01, ***P < 0.001 (corrected) versus “Pre” condition unless noted otherwise. (C) Location of 33/66 PFC → NAcc neurons, including 11/12 excited neurons; see Methods). All excited PFC → NAcc neurons were located in IL or DP cortex.
Statistics
Differences between groups were assessed using ANOVA with Tukey's Honest Significant Difference post hoc testing (Norman and Streiner 2000), or with paired t tests or Wilcoxon rank sum tests as appropriate. Multiple P values were corrected with Holm's stepwise Bonferroni correction (Norman and Streiner 2000).
Results
Electrical Stimulation of BLA
Projection neurons in the mPFC were identified using antidromic stimulation from their projection sites (Figs 1A and 2A). Two analyses of excitatory BLA-evoked responses were performed. First, using a population analysis the average excitatory response was found to be significantly greater in PFC → NAcc neurons than in PFC → PFC neurons (Fig. 3). This was particularly evident for 20-Hz BLA stimulation, where the response ratio increased by 16.8 ± 7.2% (spikes per stimulus) in PFC → NAcc neurons, and decreased by 0.7 ± 0.7% in PFC → PFC neurons (P < 0.002 Wilcoxon rank sum test). In PFC → NAcc neurons there was no difference in the average excitation evoked by 0.3 and 20 Hz stimuli (Fig. 3B); however the onset latency of 0.3-Hz responses was typically 1–2 ms shorter than for 20-Hz responses (Fig. 3A). Second, analysis of the excitatory response of individual neurons revealed that a larger portion of PFC → NAcc neurons (12/66) had significant increases in spike probability (exceeded a 95% threshold, see Methods) than did PFC → PFC neurons (4/70, P < 0.04, Fisher exact test for proportions) (Table 1). In addition, most excited PFC → NAcc neurons (9/66) fell into the “highly excited” category (exceeded 99.9% threshold), compared with only 1/70 PFC → PFC neuron (P < 0.008, Fisher exact test).
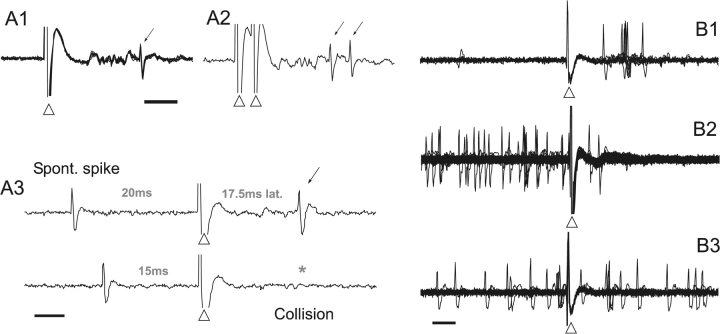
Figure 2.
(A) Antidromic responses (thin arrows) to cmPFC or NAcc stimulation were confirmed using 3 criteria: a constant latency response (A1, 6 overlaid traces) to stimulation (arrowheads); the ability to follow 400-Hz paired pulse stimulation (A2); and the collision of evoked spikes with spontaneous spikes (A3, asterisk). Scale bars = 5 ms. (B) Projection neurons exhibiting spikes in response to BLA stimulation (arrowheads) were classified as “excited” (B1, 10 overlaid traces from a single PFC → NAcc neuron). Neurons classified as “non-excited neurons with spontaneous firing” were typically inhibited (B2, 270 traces from a PFC → NAcc neuron) or showed no response (B3, 90 traces from a PFC → NAcc neuron) to BLA stimulation. The remaining neurons, exhibited few spontaneous or evoked spikes (not shown). Scale bar = 5 ms.
Table 1.
PFC projection neuron locations and classification based on spontaneous activity and responses to BLA electrical stimulation
| Projection target and location | Response to BLA stimulation | Total | ||||
| Excited | Excited | Excited | Nonexcited | Nonexcited | ||
| P < 0.001 | P < 0.05 | (Not signif.) | Baseline ≥1% | Baseline < 1% | ||
| PFC-to-NAcc | ||||||
| PL | 0 | 0 | 2 | 6 | 13 | 21 |
| IL | 8 | 3 | 3 | 7 | 20 | 41 |
| DP/MO | 1 | 0 | 0 | 0 | 3 | 4 |
| Total | 9 | 3 | 5 | 13 | 36 | 66 |
| PFC-to-PFC | ||||||
| PL | 0 | 0 | 4 | 9 | 11 | 24 |
| IL | 1 | 3 | 7 | 11 | 23 | 45 |
| DP/MO | 0 | 0 | 0 | 0 | 1 | 1 |
| Total | 1 | 3 | 11 | 20 | 35 | 70 |
Although PFC → NAcc neurons were recorded throughout the mPFC, all of the significantly excited PFC → NAcc neurons were located in the ventrally situated IL or DP areas (Fig. 3C). The average antidromic latency of PFC → NAcc neurons in the more dorsal prelimbic cortex (PL) was 12.0 ± 0.7 ms and in IL or DP was 8.9 ± 0.5 ms (significant difference P < 0.001, t test). Among IL/DP neurons, there was no latency difference between significantly excited (8.7 ± 0.9 ms) and nonexcited neurons (9.1 ± 0.6 ms, P < 0.7, t-test).
On average, neurons that were not excited and that exhibited spontaneous spike firing showed inhibition to BLA stimulation (Fig. 3B). There was no difference in the magnitude of inhibition when comparing PFC → NAcc and PFC → PFC neurons: −2.0 ± 0.6% versus −1.1 ± 0.2%, P < 0.6 for 0.3 Hz stimuli; −2.5 ± 0.6% versus −1.6 ± 0.3%, P < 0.5 for 20 Hz stimuli (Wilcoxon rank sum tests). However, it was difficult to obtain an accurate estimate of inhibitory responses in neurons with low baseline firing rates (Floresco and Tse 2007). Although for technical reasons (see Methods) we were unable to quantify the significance of inhibitory responses in individual neurons, BLA stimulation (0.3 or 20 Hz) reduced firing in all nonexcited, spontaneously firing cells. Furthermore, stimulation induced a complete cessation of activity within the poststimulus window in 10/14 PFC → NAcc neurons and 15/20 PFC → PFC neurons.
Neurons projecting to both NAcc and cmPFC were rarely encountered (n = 11), and exhibited little spontaneous or evoked activity. Of these 11 neurons: 3 had spontaneous firing (at least 1% prestimulus spike probability) and were inhibited by BLA stimulation; 8 did not have appreciable spontaneous activity; none were significantly excited by the stimulation. In 14 nonexcited projection neurons tested, very high frequency BLA stimulation (80 and 200 Hz) did not evoke excitatory responses (not shown). The responses of nonexcited neurons with little or no spontaneous firing is not shown.
Chemical Stimulation of BLA
To confirm the orthodromic nature of the BLA electrical stimulation effects, the response of mPFC projection neurons to bicuculline infusion in the BLA was examined. Following bicuculline, spontaneous epileptiform discharges (EDs, measured by a field potential electrode) were recorded in the BLA; these EDs have been associated with simultaneous spike discharge in a population of neurons near the recording site (Steriade et al. 1998). The EDs were followed by spike firing in some prefrontal projection neurons, and firing within 5–26 ms of the peak of the EDs (Fig. 4A) was considered to be consistent with orthodromic transmission from BLA to mPFC (see Methods).
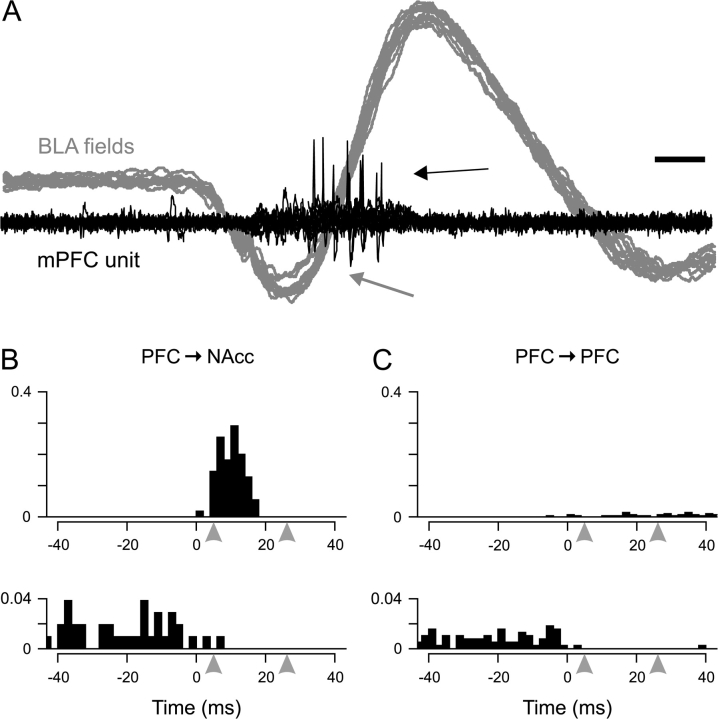
Figure 4.
(A) Simultaneous recording of a PFC → NAcc neuron discharge (black) and extracellular field potentials in the BLA (gray, 10 overlaid traces each). The large deflection in the field traces is an ED (grey arrow) caused by local infusion of bicuculline. The BLA ED occurred prior to spike discharge in the mPFC (black arrow). Scale bar: 10 ms. (B) Peri-event histograms for representative responses in PFC → NAcc neurons: top, an excitatory response from a PFC → NAcc neuron (65 spikes and 55 EDs); bottom, an inhibitory response from a PFC → NAcc neuron (104 EDs). The arrowheads at 5 and 26 ms indicate the expected range of orthodromic BLA → mPFC activation that would be predicted to occur from BLA afferent activation (see Methods). y-axis is response ratio per bin. (C) Peri-event histograms for representative responses in PFC → PFC neurons: top, an excitatory response from a PFC → PFC neuron (11 spikes and 287 EDs); bottom, an inhibitory response from a PFC → PFC neuron (384 EDs). y-axis is response ratio per bin.
Putative orthodromic spike discharge followed BLA EDs in 6 out of 21 PFC → NAcc neurons (Fig. 4B, top), with the following response ratios: 132%, 125%, 93%, 40%, 18%, and 18% (mean 71 ± 8%). Four of these 6 were located in IL or DP cortex (132%, 125%, 93%, and 18%), and the other 2 were located in PL. Consistent with the electrically evoked responses, the 3 neurons with the greatest response ratios were all located in IL or other ventral prefrontal regions. Although most excited PFC → NAcc neurons (4/6) exhibited response ratios greater than 25%, there were no PFC → PFC neurons with an equivalently strong response. Three of 18 PFC → PFC neurons did exhibit post-ED spike discharge (Fig. 4C, top), however these responses were substantially less in magnitude (19%, 4%, and 1%; mean 8 ± 6%). Two of these 3 neurons were located in IL cortex (19 and 1%), and the other was in PL. Excitation in all 9 of these projection neurons was significant beyond a 99.9% threshold as determined by a Poisson-based analysis.
In projection neurons of both types that were not excited and that were spontaneously active, post-ED firing was typically inhibited within the 5–26 ms post-event window (Fig. 4B,C, bottom). In 3 PFC → NAcc neurons and 3 PFC → PFC neurons, spontaneous activity was completely inhibited within the post-ED window.
Pavlovian Odor Conditioning and Recording
A group of 14 rats was conditioned to odors while awake and then anesthetized for subsequent mPFC recordings. Conditioning was done by pairing a distinct odor (CS+) with a foot shock multiple times; a second, nonpaired odor (CS−) was also presented during the conditioning session. After conditioning but before recording, 9 rats were exposed briefly (20 s) to the CS− and CS+ odors (with no shocks). Freezing during the CS+ odor (14.6 ± 4.6%), was significantly greater than freezing during the CS− odor (8.3 ± 2.5%, P < 0.02 paired Wilcoxon rank sum test) in these rats.
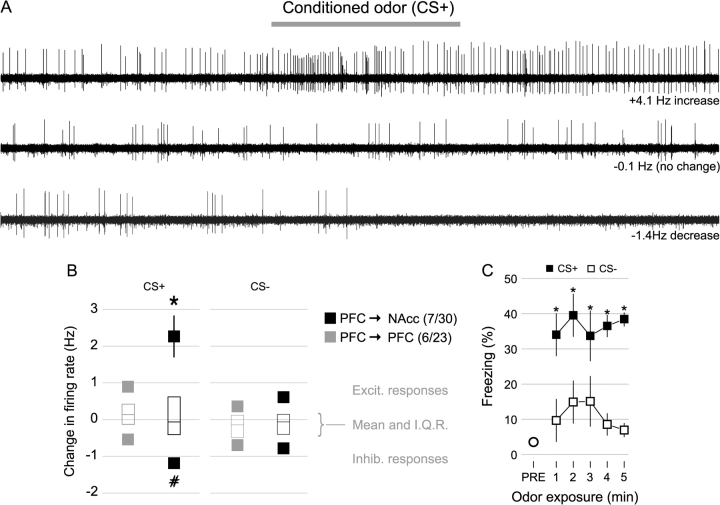
To confirm the effectiveness of the conditioning procedure, a separate group of 8 rats was conditioned in the same way and then tested using longer (5 min) presentations of the CS+ and CS− odors as employed previously (Rosenkranz et al. 2003; Laviolette et al. 2005). Within the first minute of odor presentation freezing in response to the CS+ was greater than in response to the CS−, and remained greater throughout odor presentation (Fig. 5C). The average freezing during the CS+ (36.5 ± 5.1%) was significantly greater than during the CS− (11.7 ± 4.1%, P < 0.008, paired Wilcoxon rank sum test). In addition, exploratory and grooming activity scores measured during odor presentation, whereas not significantly different within the first minute of odor presentation, did show a difference over minutes 2–5 of odor presentation (CS+ score 1.7 ± 0.3; CS− score 0.8 ± 0.6, P < 0.03 paired Wilcoxon rank sum test).
Figure 5.
(A) Traces from 3 neurons show responses to the CS+ odor (gray bar, 10 s): evoked excitation (top), inhibition (bottom), or no discernable response (middle). (B) Modified box-plots showing excitatory and inhibitory odor responses (change in firing rate during odor). The filled boxes show the averages of the top and bottom quartiles (greatest excitatory and greatest inhibitory responses), which were analyzed separately. 30 PFC → NAcc neurons were recorded, with 7 neurons each in the top and bottom quartiles; 23 PFC → PFC neurons were recorded, with 6 neurons in each quartile. PFC → NAcc neurons had greater CS+ inhibitory and excitatory responses than PFC → PFC neurons. Error bars = SEM. *P < 0.05 versus all other excitatory responses. #P < 0.05 versus the inhibitory PFC → PFC/CS+ response only. (C) Freezing in response to CS+ odor (black squares) and CS− odor (white squares) over a 5-min test, one day after odor conditioning in a group of 8 rats not used for electrophysiological recordings. Average freezing over a 5-min period before odor exposure is indicated by the initial circle. *Indicates significant difference (P < 0.04, corrected) from CS− by paired Wilcoxon rank sum test. Error bars represent SEM.
During mPFC recordings performed under anesthesia (n = 14 rats), presentation of CS+ and CS− odors caused both firing rate increases and decreases in PFC → NAcc (n = 30) and PFC → PFC neurons (n = 23) (Fig. 5A), with the mean change in firing rate being approximately zero for all groups (Fig. 5B, middle). Odor-evoked responses in rats that had been tested behaviorally did not differ from responses in untested rats (not shown), however this data set may have insufficient power to determine the effect of behavioral testing.
Population analysis showed that for excitatory odor responses (top quartile), the average response of PFC → NAcc neurons (n = 7) to the CS+ was greater than the response of PFC → PFC neurons (n = 6), and was also greater than PFC → NAcc responses to the CS− (n = 7, Fig. 5B, top; 2-way ANOVA [F3,22 = 7.43, P < 0.002], with significant effects of both neuron type and CS type [F1,22 = 6.42 and 12.80, P < 0.02 and 0.002, respectively]). Although the neuron type by CS type interaction was not significant (F1,22 = 3.08, P < 0.1), post hoc testing revealed significant differences between groups (Fig. 5B, top). A similar population analysis of the inhibitory responses (bottom quartile) showed a greater CS+-evoked inhibition in PFC → NAcc compared with PFC → PFC neurons (n = 7, n = 6, respectively, Fig. 5B, bottom; 2-way ANOVA [F3,22 = 3.66, P < 0.03], with cell type as the only significant factor [F1,22 = 6.33, P < 0.02]). In PFC → NAcc neurons, inhibition evoked by the CS+ was not significantly different from inhibition evoked by the CS− (n = 7, Fig. 5B, bottom). Unlike responses to BLA electrical stimulation, in spontaneously active neurons inhibitory responses that resulted in the total cessation of firing during the CS+ occurred in only one neuron, which was identified as a PFC → PFC neuron.
Analysis of individual neurons revealed that 7 PFC → NAcc neurons exhibited spiking during CS+ odor presentation that was significantly greater than baseline firing during the prestimulus period (P < 0.05 for all 7; P < 0.001 for 5/7, see Methods). The odor response was specific to the CS+ in 5 of the 6 neurons for which CS− data is also available, as only 1 neuron exhibited a significant excitation in response to the CS− (Fig. 6A,B, left). As was the case for BLA stimulation, PFC → NAcc neurons with significant excitatory responses were found primarily in the ventral mPFC areas IL and MO (5/7) (Fig. 6C). A similar analysis identified 7 PFC → PFC neurons with significant CS+ odor responses (P < 0.05 all 7; P < 0.001 for 4/7), with 4 out of the 5 for which CS− data is available having no significant response to the CS− odor (Fig. 6A,B, right). The difference between the CS+ and CS− response was calculated for each neuron as an index of response specificity for the CS+ over the CS−. The mean of this specificity index in PFC → NAcc neurons (2.4 Hz, n = 6) was greater than in PFC → PFC neurons (0.7 Hz, n = 5, P < 0.009, Wilcoxon rank sum test) (Fig. 6B). Five PFC → NAcc neurons exhibited significant excitation (P < 0.05) in response to the CS− odor; however, only 2 of these 5 neurons were highly excited (P < 0.001), and 1 of these 2 exhibited a greater excitation in response to the CS+ (Fig. 6B, left).
Figure 6.
(A) Peristimulus time histograms from a single PFC → NAcc neuron (left) and a single PFC → PFC neuron (right) during presentation of conditioned odors (1-s bins). The gray bar indicates the time when the odor was presented (10 s). y-axis is number of spikes per bin. (B) Odor-evoked changes in firing rate in response to the CS+ and CS− odors for single neurons (triangles and circles); and mean differences between CS+ and CS− responses (bars). In PFC → NAcc neurons with significant excitatory responses to the CS+ (left), CS+ responses were greater than CS− responses (n = 6, P < 0.03 paired Wilcoxon rank sum test); but this was not the case for PFC → PFC neurons with significant CS+ excitation (right, n = 5, P < 0.13). The mean difference between CS+ and CS− responses was greater in PFC → NAcc neurons (n = 6, left bar) than in PFC → PFC neurons (n = 5, right bar; P < 0.009, Wilcoxon rank sum test). The plotting symbols indicate the response to odor presentation: triangles for excitation exceeding a P < 0.001 threshold (highly excited); filled circles for excitation exceeding a P < 0.05 threshold; empty circles for no significant excitation. (C) The locations of all PFC → NAcc neurons recorded during odor testing. Most excited PFC → NAcc neurons (5/7) were located in IL or DP/MO cortex. y-axis is atlas coordinates in millimeters.
Discussion
A subset of mPFC neurons that project to the NAcc, located almost exclusively in the ventral mPFC, was found to be excited by BLA stimulation and by Pavlovian conditioned stimuli. These excitatory responses were greater in magnitude and more consistently evoked than those in a similar group of mPFC projection neurons that did not project to the accumbens. The data strongly suggest that the excitatory responses were due to direct orthodromic input from the BLA, because these responses occurred at short latencies and were evoked both by electrical and cell body-specific chemical stimulation of the BLA. Even though BLA stimulation and conditioned odor responses were not measured in the same neurons, it is most likely that the odor-evoked responses in PFC → NAcc neurons were due to BLA input because our previous work showed that an intact BLA input is required for the induction of odor-evoked conditioned responses in mPFC neurons (Laviolette et al. 2005).
Behavioral Responses to Conditioned Stimuli
In the rats that were exposed to conditioning and then tested behaviorally prior to recording (n = 9 of 14), freezing responses to the CS+ odor were low compared with earlier studies (Otto et al. 1997; Laviolette et al. 2005) and were less than 2-fold greater than freezing during the CS− odor. This was most likely due to the very brief odor presentations (20 s) that were used to limit the possibility of exposure-induced extinction in the electrophysiological tests (Shipley 1974). Indeed, in a separate group of rats (n = 8) that were exposed to an identical conditioning procedure, the more standard odor presentation durations (5 min) elicited freezing responses that were comparable with previous reports (Otto et al. 1997), with the CS+ response more than 3-fold higher than the CS− response (Laviolette et al. 2005). Therefore, the conditioning procedure employed was effective in eliciting robust fear behavior in these rats.
Responses to Amygdala Stimulation
Although some previous studies in awake rats did not report excitatory responses to conditioned stimuli (Garcia et al. 1999; Milad and Quirk 2002), others have found a subset of mPFC neurons that were excited by conditioned cues (Baeg et al. 2001; Gilmartin and McEchron 2005). Studies focusing on the BLA input to mPFC have found a significant minority of mPFC neurons that are excited by BLA stimulation (Perez-Jaranay and Vives 1991; Ishikawa and Nakamura 2003), including identified projection neurons (Floresco and Tse 2007). Thus, the conflicting findings in awake rats may be due to the heterogeneity of BLA input onto mPFC neurons. We focused our current study specifically on BLA-evoked excitatory responses, which we found previously correlate best with Pavlovian conditioned responses (Laviolette et al. 2005; Laviolette and Grace 2006). The fact that BLA stimulation excites some PFC → NAcc neurons but produces inhibition in a substantial number of other mPFC neuron types (Floresco and Tse 2007) suggests that the amygdala input may function as a bias signal, facilitating firing of outputs to selected targets while attenuating firing in all others.
Only 18% (12/66) of PFC → NAcc neurons were significantly excited by BLA stimulation, with most other neurons exhibiting inhibition. Even considering only neurons in IL or other ventral mPFC regions, excitatory responses were in the minority (12/45, 27%). Although this sparse excitatory response could be due to the low stimulation current or the narrow response window used, excitatory responses to chemical stimulation and conditioned odors occurred with similar frequencies (6/21 and 7/30, respectively). Furthermore, even though we only recorded from a subset of mPFC neurons, the proportions of BLA-evoked excitatory responses were comparable with those found in uncategorized (with respect to projection site) mPFC neurons using similar methods (Ishikawa and Nakamura 2003; Floresco and Tse 2007). Finally, anesthesia is unlikely to account for the sparse excitation observed here, as fast-onset excitatory responses to conditioned stimuli were found in only 15% of uncharacterized IL neurons (Gilmartin and McEchron 2005) and in 24% of regular-spiking mPFC neurons (Baeg et al. 2001) in studies using awake rats. Thus, our results suggest that the magnitude of excitatory BLA input to PFC → NAcc neurons is similar to mPFC neurons in general, and that less excitation (or more inhibition) is evident in PFC → PFC neurons.
Similar studies of the hippocampus-to-mPFC pathway have found comparable proportions of excitatory responses in mPFC neurons (Degenetais et al. 2003; Ishikawa and Nakamura 2003). Intracellular recording from prefrontal neurons in vivo have shown that hippocampal stimulation typically evokes a complex postsynaptic response, exhibiting both excitatory and inhibitory conductances that have been attributed to monosynaptic glutamatergic input and di-synaptic GABAergic input, respectively (Degenetais et al. 2003). BLA stimulation may also evoke a similar response in mPFC projection neurons (Dilgen and O'Donnell 2004). Thus, the response of an mPFC neuron to BLA stimulation likely depends on the balance of glutamatergic and GABAergic inputs it receives. Furthermore, the typically low number of excitatory responses could be due to occlusion by fast-onset inhibitory inputs, such as those observed in somatosensory cortex pyramidal neurons in response to thalamic stimulation (Cruikshank et al. 2007).
Prefrontal-Accumbens Interactions and Conditioned Fear
The selective activation of PFC → NAcc neurons by conditioned stimuli suggests that this pathway is involved in the expression of conditioned fear. Despite evidence that lesions of the mPFC or NAcc do not consistently impair conditioned fear responses (Kubos et al. 1987; Riedel et al. 1997; Quirk et al. 2000; Levita et al. 2002; Jongen-Relo et al. 2003; Cassaday et al. 2005), we and others have found that acute inactivation or pharmacological manipulations of these areas can impair conditioned fear (Laviolette et al. 2005; Resstel et al. 2006; Schwienbacher et al. 2006; Sierra-Mercado et al. 2006; Corcoran and Quirk 2007). This apparent discrepancy may be due to the differences in which mPFC function is altered. Thus, lesions followed by a recovery period may allow time for compensatory mechanisms to intervene (Maren et al. 1997; Anglada-Figueroa and Quirk 2005). Indeed, acute interference with neuronal activity or excitability, such as by silencing activity in the target area or by altering normal patterns of firing, often reveals the functional impact of a region that is not made apparent when permanent lesions are used (Quirk et al. 2000; Gale et al. 2001; Anglada-Figueroa and Quirk 2005). Therefore, the PFC → NAcc neurons may support conditioned fear in intact rats, whereas other systems may compensate for this essential survival skill in rats with permanent lesions.
Recent work by Corcoran and Quirk (2007) has shown that PL inactivation reduces conditioned, but not innate, fear. They have suggested that the specific function of the PL in conditioned fear is to integrate sensory, contextual and goal-directed information and to promote fear responses only at appropriate times and locations. The IL also receives multimodal sensory and motivational input (Conde et al. 1995), and thus the IL → NAcc pathway may support conditioned fear in a similar way. Indeed, we have previously proposed that the NAcc performs just such a gating function, constraining behavior based on contextual, affective and goal directed information (Grace 2000). Although we and others have observed that inactivation of both IL and PL reduce several measures of conditioned fear (Laviolette et al. 2005; Laviolette and Grace 2006; Resstel et al. 2006), there is also strong evidence that IL neurons signal safety and promote the extinction (rather than the expression) of fear behaviors (Milad and Quirk 2002; Milad et al. 2004; Burgos-Robles et al. 2007). This discrepancy may be explained by the different fear conditioning paradigms used in these studies; for example, manipulation of IL had no effect on conditioned fear in response to a tone CS (Quirk et al. 2000; Burgos-Robles et al. 2007), but abolished fear responses to a context CS (Resstel et al. 2006) or odor CS (Laviolette et al. 2005). Therefore, considering our previous work demonstrating that IL manipulations reduce fear to an odor CS, our current results suggest that IL → NAcc neurons promote conditioned fear responses in this paradigm.
Other mPFC efferent pathways may also play a role in conditioned fear behaviors. For example, although the PL appears to promote conditioned freezing (Corcoran and Quirk 2007), our results imply that these behaviors are mediated by PL projections to targets other than the NAcc or cmPFC. However, the identity of these other projection targets is not known. Corticostriatal neurons are thought to send collateral axons to diverse secondary targets (Levesque et al. 1996), and PFC → NAcc neurons in particular are known to send collaterals to cmPFC, BLA and the ventral tegmental area (Pinto and Sesack 2000). We found no significant excitation in the 11 neurons projecting to both NAcc and cmPFC; however, due to the small sample size it is unclear whether these neurons are more or less excitable than PFC → NAcc neurons in general. Therefore, it is not known how secondary targets of PFC → NAcc neurons contribute to conditioned fear responses, nor whether PFC → NAcc neurons with different secondary targets exhibit different responses to conditioned stimuli.
Conclusions and Implications
Our data suggest that the amygdala is capable of influencing conditioned responses at 2 sites: via its projections to the mPFC neurons projecting to the NAcc, and by projections to NAcc neurons receiving mPFC input. The BLA projects to the mPFC (Krettek and Price 1977), as well as to the NAcc division that receives the strongest mPFC input, (the shell, Groenewegen et al. 1990), with many single BLA neurons projecting to both areas (Shinonaga et al. 1994). MPFC and BLA afferents target the NAcc shell cell clusters (Wright and Groenewegen 1995), and strong evidence suggests they converge and form excitatory synapses onto the same medium spiny neurons (French and Totterdell 2002, 2003). Thus, activation of the BLA alone may be sufficient to recruit both pathways that converge onto and drive NAcc neurons.
Although our study has focused on the role of the mPFC in conditioned fear, there is also evidence that this amygdala-prefrontal-striatal circuit has other roles. In a study where BLA and mPFC were disconnected, rats failed to seek larger rewards that required greater effort to obtain, suggesting that the influence of reward value on decision making is diminished by this lesion. In mPFC/NAcc disconnection studies, rats exhibit deficits that suggest this pathway is necessary for switching strategies (Block et al. 2007) or modifying behavior based on task feedback (Christakou et al. 2004). Therefore, the amygdala activation of IL → NAcc neurons may represent a mechanism by which reward value informs behavioral choice (Cardinal et al. 2002). Indeed, in humans with focal lesions of the amygdala, choice-related signals in the anterior cinglulate cortex (comparable with rodent mPFC; Ongur and Price 2000) are far weaker than in nonlesioned individuals (Hampton et al. 2007). Furthermore, a similar function has been attributed to the BLA-to-orbitofrontal cortex pathway in reversal learning tasks in rodents (Schoenbaum et al. 1999, 2003). Amygdala-evoked excitation in mPFC can be modulated by dopamine and by hippocampal inputs (Ishikawa and Nakamura 2003; Floresco and Tse 2007), suggesting that the affective drive of a strategy or behavioral set is plastic, and subject to being modified by other salient cues or task constraints.
Funding
National Institutes of Health (MH57440) to A.A.G.; and University of Pittsburgh School of Arts and Sciences, Andrew Mellon Fellowship to V.B.M.
Acknowledgments
We wish to thank Christy Smolak and Nicole Macmurdo for technical assistance, and Brian Lowry for development of the Neuroscope software. Clinton McCracken, Daniel Lodge, and Stan Floresco provided useful discussions and comments on the manuscript. Conflict of Interest: None declared.
References
- Angel A, Gratton DA. The effect of anaesthetic agents on cerebral cortical responses in the rat. Br J Pharmacol. 1982;76:541–549. doi: 10.1111/j.1476-5381.1982.tb09252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglada-Figueroa D, Quirk GJ. Lesions of the basal amygdala block expression of conditioned fear but not extinction. J Neurosci. 2005;25:9680–9685. doi: 10.1523/JNEUROSCI.2600-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon SJ, Headlam AJ, Gabbott PL, Smith AD. Amygdala input to medial prefrontal cortex (mPFC) in the rat: a light and electron microscope study. Brain Res. 1996;720:211–219. doi: 10.1016/0006-8993(96)00155-2. [DOI] [PubMed] [Google Scholar]
- Baeg EH, Kim YB, Jang J, Kim HT, Mook-Jung I, Jung MW. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb Cortex. 2001;11:441–451. doi: 10.1093/cercor/11.5.441. [DOI] [PubMed] [Google Scholar]
- Bisley JW, Krishna BS, Goldberg ME. A rapid and precise on-response in posterior parietal cortex. J Neurosci. 2004;24:1833–1838. doi: 10.1523/JNEUROSCI.5007-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb Cortex. 2007;17:1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Cassaday HJ, Horsley RR, Norman C. Electrolytic lesions to nucleus accumbens core and shell have dissociable effects on conditioning to discrete and contextual cues in aversive and appetitive procedures respectively. Behav Brain Res. 2005;160:222–235. doi: 10.1016/j.bbr.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Christakou A, Robbins TW, Everitt BJ. Prefrontal cortical-ventral striatal interactions involved in affective modulation of attentional performance: implications for corticostriatal circuit function. J Neurosci. 2004;24:773–780. doi: 10.1523/JNEUROSCI.0949-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde F, Maire-Lepoivre E, Audinat E, Crepel F. Afferent connections of the medial frontal cortex of the rat. II. Cortical and subcortical afferents. J Comp Neurol. 1995;352:567–593. doi: 10.1002/cne.903520407. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J Neurosci. 2007;27:840–844. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutureau E, Dix SL, Kilcross AS. Involvement of the medial prefrontal cortex–basolateral amygdala pathway in fear-related behaviour in rats. Eur J Neurosci. 2000;12(Suppl 11):156. [Google Scholar]
- Cruikshank SJ, Lewis TJ, Connors BW. Synaptic basis for intense thalamocortical activation of feedforward inhibitory cells in neocortex. Nat Neurosci. 2007;10:462–468. doi: 10.1038/nn1861. [DOI] [PubMed] [Google Scholar]
- Degenetais E, Thierry AM, Glowinski J, Gioanni Y. Synaptic influence of hippocampus on pyramidal cells of the rat prefrontal cortex: an in vivo intracellular recording study. Cereb Cortex. 2003;13:782–792. doi: 10.1093/cercor/13.7.782. [DOI] [PubMed] [Google Scholar]
- Dilgen JE, O'Donnell PO. Program No. 771.15. 2004. Abstract viewer and itinerary planner. Washington (DC): Society for neuroscience annual meeting; 2004. Basolateral amygdala and ventral hippocampal stimulation evoke complex synaptic responses in prefrontal cortex pyramidal neurons recorded in vivo. [Google Scholar]
- Floresco SB, Grace AA. Gating of hippocampal-evoked activity in prefrontal cortical neurons by inputs from the mediodorsal thalamus and ventral tegmental area. J Neurosci. 2003;23:3930–3943. doi: 10.1523/JNEUROSCI.23-09-03930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Tse MT. Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdala-prefrontal cortical pathway. J Neurosci. 2007;27:2045–2057. doi: 10.1523/JNEUROSCI.5474-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Hippocampal and prefrontal cortical inputs monosynaptically converge with individual projection neurons of the nucleus accumbens. J Comp Neurol. 2002;446:151–165. doi: 10.1002/cne.10191. [DOI] [PubMed] [Google Scholar]
- French SJ, Totterdell S. Individual nucleus accumbens-projection neurons receive both basolateral amygdala and ventral subicular afferents in rats. Neuroscience. 2003;119:19–31. doi: 10.1016/s0306-4522(03)00150-7. [DOI] [PubMed] [Google Scholar]
- Fuller JH, Schlag JD. Determination of antidromic excitation by the collision test: problems of interpretation. Brain Res. 1976;112:283–298. doi: 10.1016/0006-8993(76)90284-5. [DOI] [PubMed] [Google Scholar]
- Gabbott PL, Warner TA, Busby SJ. Amygdala input monosynaptically innervates parvalbumin immunoreactive local circuit neurons in rat medial prefrontal cortex. Neuroscience. 2006;139:1039–1048. doi: 10.1016/j.neuroscience.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Fanselow MS. Cholinergic modulation of Pavlovian fear conditioning: effects of intrahippocampal scopolamine infusion. Hippocampus. 2001;11:371–376. doi: 10.1002/hipo.1051. [DOI] [PubMed] [Google Scholar]
- Garcia R, Chang CH, Maren S. Electrolytic lesions of the medial prefrontal cortex do not interfere with long-term memory of extinction of conditioned fear. Learn Mem. 2006;13:14–17. doi: 10.1101/lm.60406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–296. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- Gifford GW, 3rd, MacLean KA, Hauser MD, Cohen YE. The neurophysiology of functionally meaningful categories: macaque ventrolateral prefrontal cortex plays a critical role in spontaneous categorization of species-specific vocalizations. J Cogn Neurosci. 2005;17:1471–1482. doi: 10.1162/0898929054985464. [DOI] [PubMed] [Google Scholar]
- Gilmartin MR, McEchron MD. Single neurons in the medial prefrontal cortex of the rat exhibit tonic and phasic coding during trace fear conditioning. Behav Neurosci. 2005;119:1496–1510. doi: 10.1037/0735-7044.119.6.1496. [DOI] [PubMed] [Google Scholar]
- Grace AA. Gating of information flow within the limbic system and the pathophysiology of schizophrenia. Brain Res Brain Res Rev. 2000;31:330–341. doi: 10.1016/s0165-0173(99)00049-1. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Wolters JG, Lohman AH. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog Brain Res. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. discussion 116–118. [DOI] [PubMed] [Google Scholar]
- Hampton AN, Adolphs R, Tyszka MJ, O'Doherty JP. Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron. 2007;55:545–555. doi: 10.1016/j.neuron.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Hanes DP, Thompson KG, Schall JD. Relationship of presaccadic activity in frontal eye field and supplementary eye field to saccade initiation in macaque: Poisson spike train analysis. Exp Brain Res Exp Hirnforsch. 1995;103:85–96. doi: 10.1007/BF00241967. [DOI] [PubMed] [Google Scholar]
- Ishikawa A, Nakamura S. Convergence and interaction of hippocampal and amygdalar projections within the prefrontal cortex in the rat. J Neurosci. 2003;23:9987–9995. doi: 10.1523/JNEUROSCI.23-31-09987.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongen-Relo AL, Kaufmann S, Feldon J. A differential involvement of the shell and core subterritories of the nucleus accumbens of rats in memory processes. Behav Neurosci. 2003;117:150–168. doi: 10.1037//0735-7044.117.1.150. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex to the cerebral cortex and thalamus in the rat and cat. J Comp Neurol. 1977;172:687–722. doi: 10.1002/cne.901720408. [DOI] [PubMed] [Google Scholar]
- Kubos KL, Moran TH, Robinson RG. Differential and asymmetrical behavioral effects of electrolytic or 6-hydroxydopamine lesions in the nucleus accumbens. Brain Res. 1987;401:147–151. doi: 10.1016/0006-8993(87)91174-7. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. Cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala inputs. J Neurosci. 2006;26:6458–6468. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque M, Charara A, Gagnon S, Parent A, Deschenes M. Corticostriatal projections from layer V cells in rat are collaterals of long-range corticofugal axons. Brain Res. 1996;709:311–315. doi: 10.1016/0006-8993(95)01333-4. [DOI] [PubMed] [Google Scholar]
- Levita L, Dalley JW, Robbins TW. Disruption of Pavlovian contextual conditioning by excitotoxic lesions of the nucleus accumbens core. Behav Neurosci. 2002;116:539–552. doi: 10.1037//0735-7044.116.4.539. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Pare D. Prefrontal control of the amygdala. J Neurosci. 2005;25:7429–7437. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behav Brain Res. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maunsell JH, Gibson JR. Visual response latencies in striate cortex of the macaque monkey. J Neurophysiol. 1992;68:1332–1344. doi: 10.1152/jn.1992.68.4.1332. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Vidal-Gonzalez I, Quirk GJ. Electrical stimulation of medial prefrontal cortex reduces conditioned fear in a temporally specific manner. Behav Neurosci. 2004;118:389–394. doi: 10.1037/0735-7044.118.2.389. [DOI] [PubMed] [Google Scholar]
- Norman DR, Streiner DL. Biostatistics: the bare essentials. Hamilton, Ontario: B.C. Decker, Inc.; 2000. [Google Scholar]
- Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Otto T, Cousens G, Rajewski K. Odor-guided fear conditioning in rats: 1. Acquisition, retention, and latent inhibition. Behav Neurosci. 1997;111:1257–1264. [PubMed] [Google Scholar]
- Parkinson JA, Willoughby PJ, Robbins TW, Everitt BJ. Disconnection of the anterior cingulate cortex and nucleus accumbens core impairs Pavlovian approach behavior: further evidence for limbic cortical-ventral striatopallidal systems. Behav Neurosci. 2000;114:42–63. [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th ed. San Diego (CA): Academic Press; 1998. [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. San Diego (CA): Academic Press; 2005. [Google Scholar]
- Perez-Jaranay JM, Vives F. Electrophysiological study of the response of medial prefrontal cortex neurons to stimulation of the basolateral nucleus of the amygdala in the rat. Brain Res. 1991;564:97–101. doi: 10.1016/0006-8993(91)91357-7. [DOI] [PubMed] [Google Scholar]
- Pinto A, Sesack SR. Limited collateralization of neurons in the rat prefrontal cortex that project to the nucleus accumbens. Neuroscience. 2000;97:635–642. doi: 10.1016/s0306-4522(00)00042-7. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Joca SR, Guimaraes FG, Correa FM. Involvement of medial prefrontal cortex neurons in behavioral and cardiovascular responses to contextual fear conditioning. Neuroscience. 2006;143:377–385. doi: 10.1016/j.neuroscience.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Riedel G, Harrington NR, Hall G, Macphail EM. Nucleus accumbens lesions impair context, but not cue, conditioning in rats. Neuroreport. 1997;8:2477–2481. doi: 10.1097/00001756-199707280-00013. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during Pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Neural encoding in orbitofrontal cortex and basolateral amygdala during olfactory discrimination learning. J Neurosci. 1999;19:1876–1884. doi: 10.1523/JNEUROSCI.19-05-01876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39:855–867. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Schwienbacher I, Schnitzler HU, Westbrook RF, Richardson R, Fendt M. Carbachol injections into the nucleus accumbens disrupt acquisition and expression of fear-potentiated startle and freezing in rats. Neuroscience. 2006;140:769–778. doi: 10.1016/j.neuroscience.2006.02.052. [DOI] [PubMed] [Google Scholar]
- Setlow B, Holland PC, Gallagher M. Disconnection of the basolateral amygdala complex and nucleus accumbens impairs appetitive Pavlovian second-order conditioned responses. Behav Neurosci. 2002;116:267–275. doi: 10.1037//0735-7044.116.2.267. [DOI] [PubMed] [Google Scholar]
- Shinonaga Y, Takada M, Mizuno N. Topographic organization of collateral projections from the basolateral amygdaloid nucleus to both the prefrontal cortex and nucleus accumbens in the rat. Neuroscience. 1994;58:389–397. doi: 10.1016/0306-4522(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Shipley RH. Extinction of conditioned fear in rats as a function of several parameters of CS exposure. J Comp Physiol Psychol. 1974;87:699–707. doi: 10.1037/h0036997. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Jr, Corcoran KA, Lebron-Milad K, Quirk GJ. Inactivation of the ventromedial prefrontal cortex reduces expression of conditioned fear and impairs subsequent recall of extinction. Eur J Neurosci. 2006;24:1751–1758. doi: 10.1111/j.1460-9568.2006.05014.x. [DOI] [PubMed] [Google Scholar]
- Steriade M, Amzica F, Neckelmann D, Timofeev I. Spike-wave complexes and fast components of cortically generated seizures. II. Extra- and intracellular patterns. J Neurophysiol. 1998;80:1456–1479. doi: 10.1152/jn.1998.80.3.1456. [DOI] [PubMed] [Google Scholar]
- Steriade M, Contreras D. Spike-wave complexes and fast components of cortically generated seizures. I. Role of neocortex and thalamus. J Neurophysiol. 1998;80:1439–1455. doi: 10.1152/jn.1998.80.3.1439. [DOI] [PubMed] [Google Scholar]
- Team RDC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. [Google Scholar]
- Wright CI, Groenewegen HJ. Patterns of convergence and segregation in the medial nucleus accumbens of the rat: relationships of prefrontal cortical, midline thalamic, and basal amygdaloid afferents. J Comp Neurol. 1995;361:383–403. doi: 10.1002/cne.903610304. [DOI] [PubMed] [Google Scholar]