Abstract
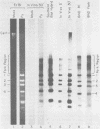
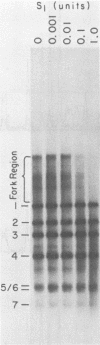
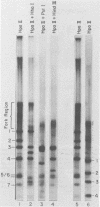
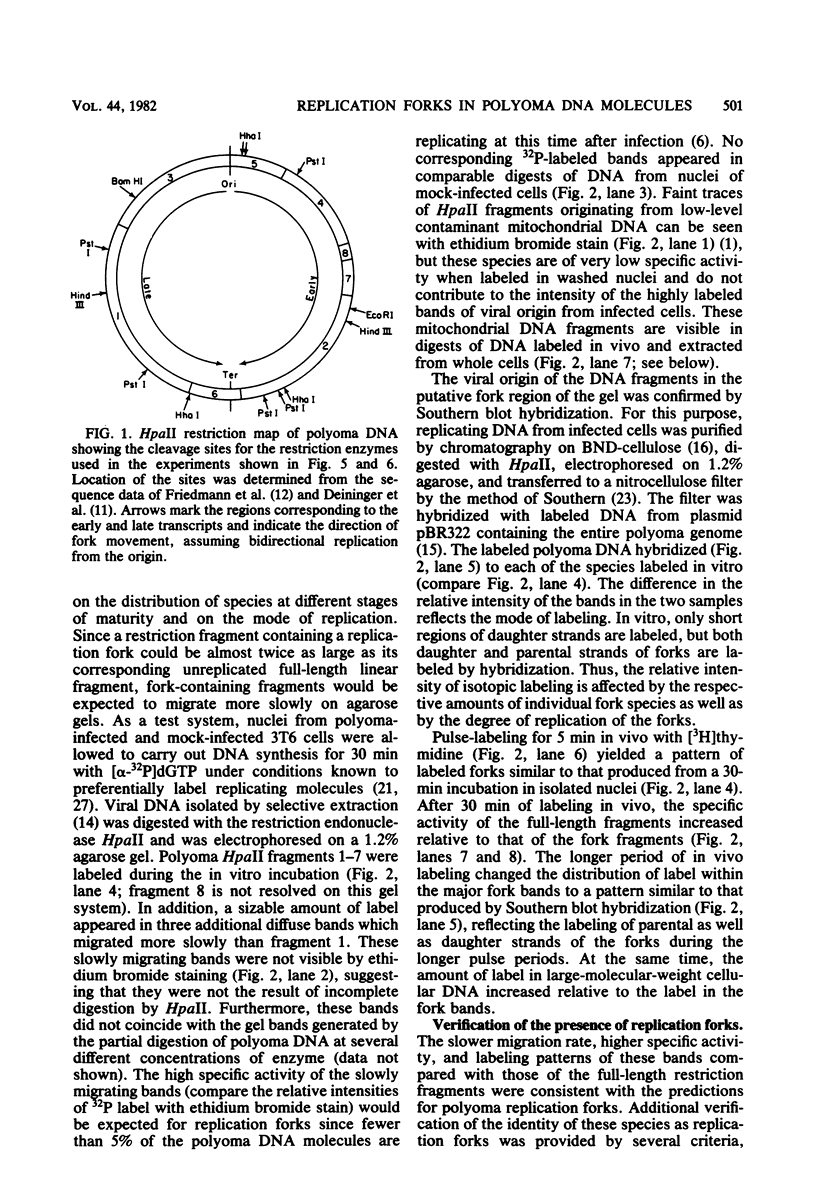
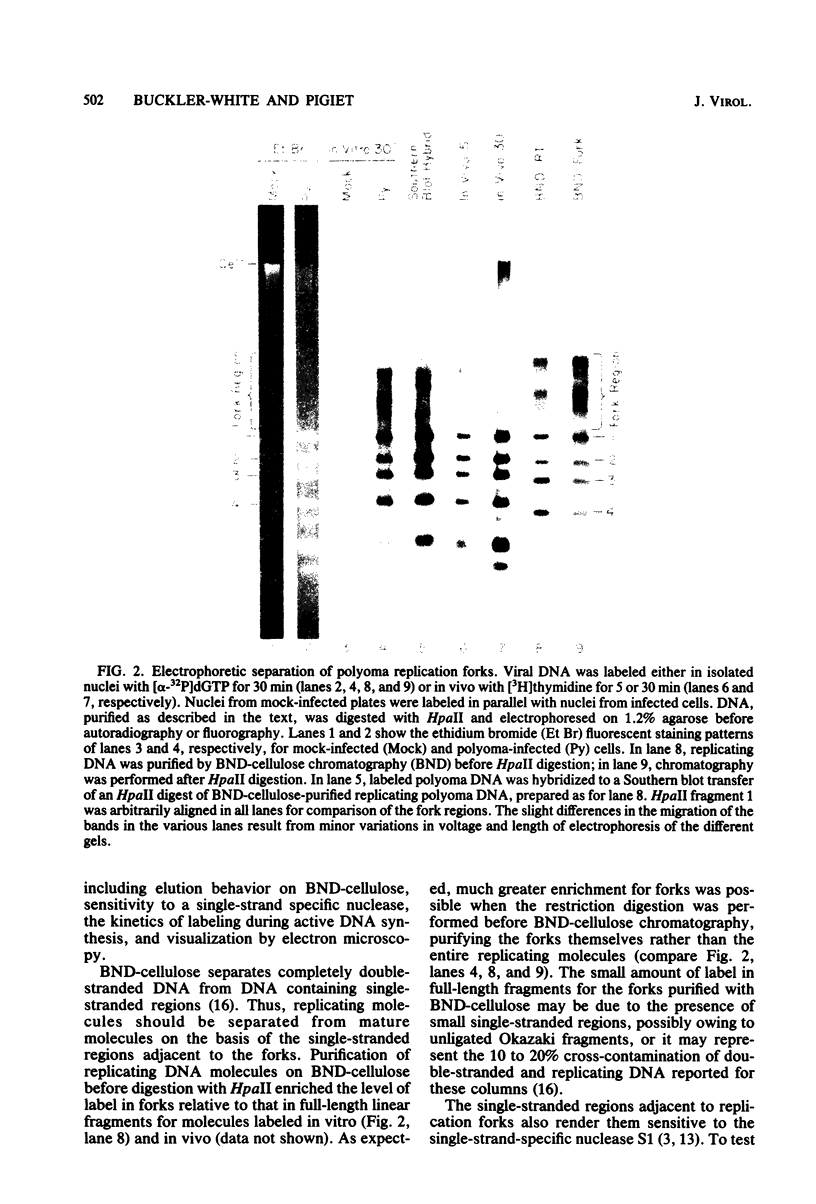
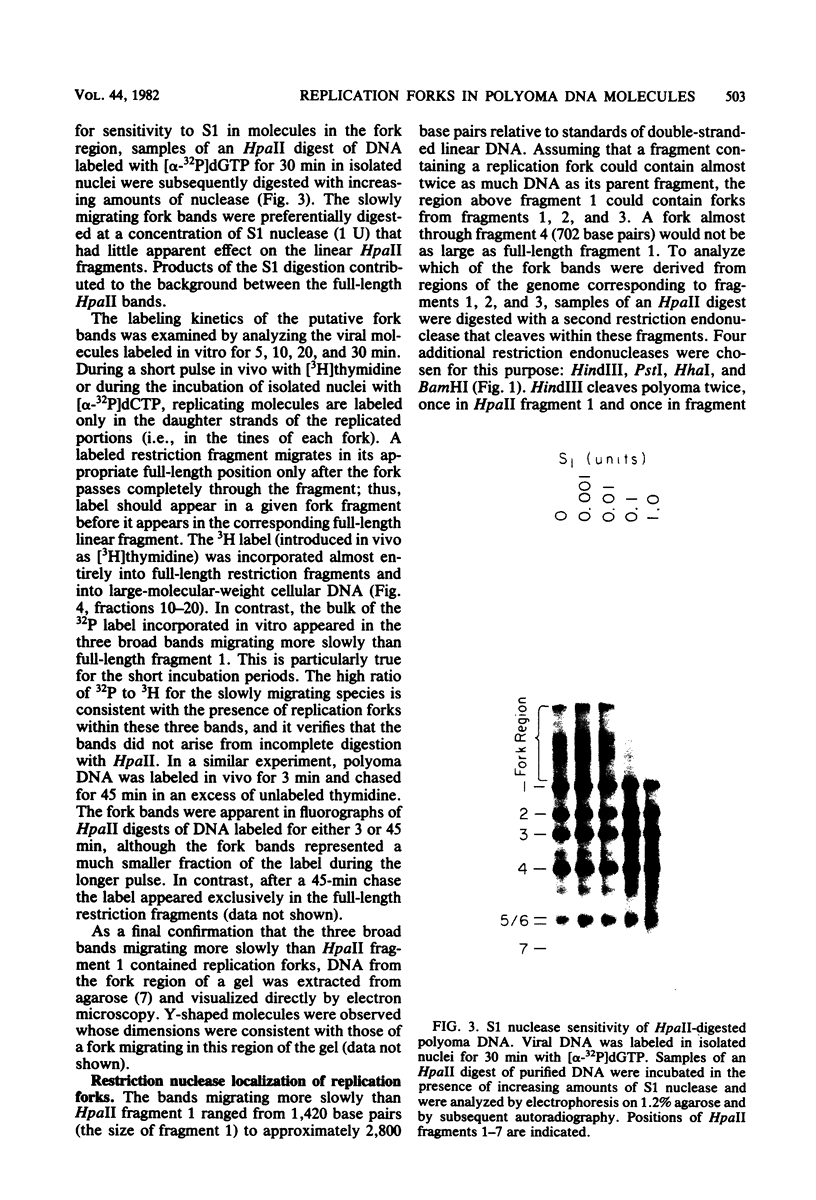
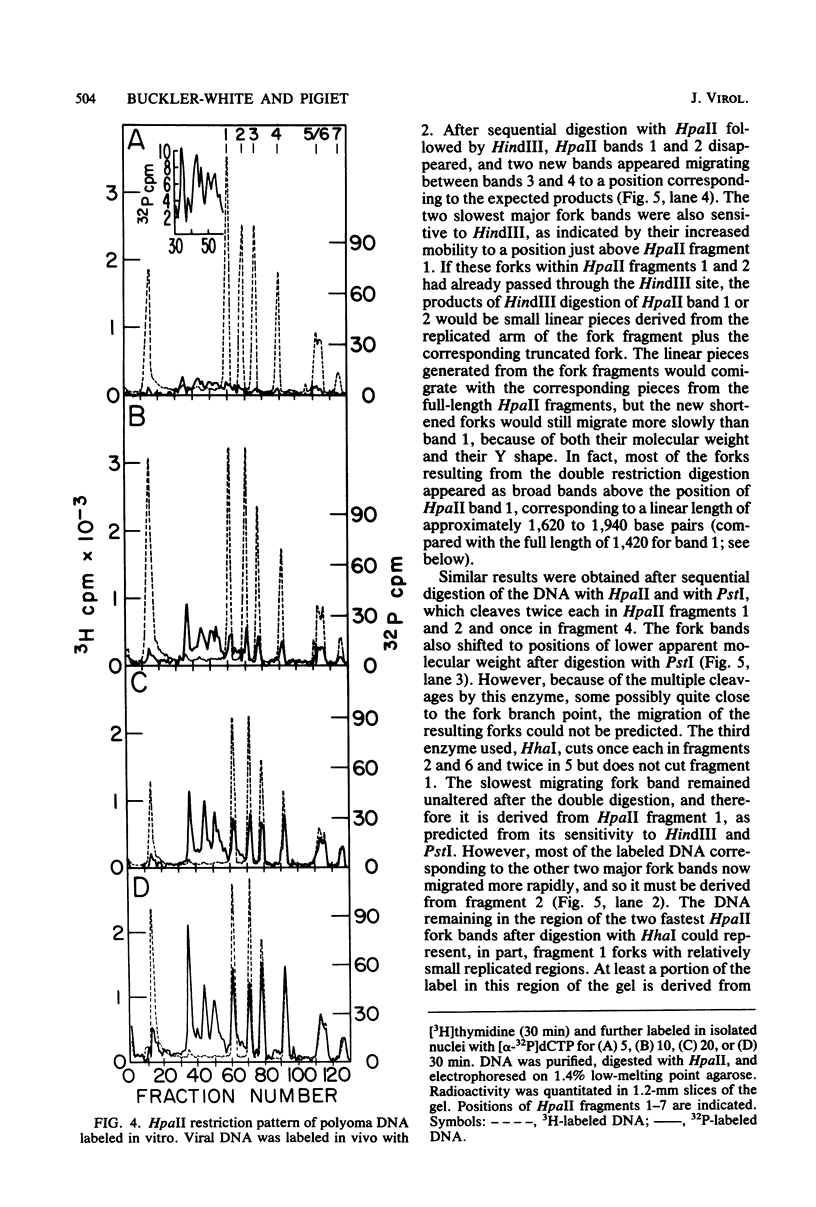
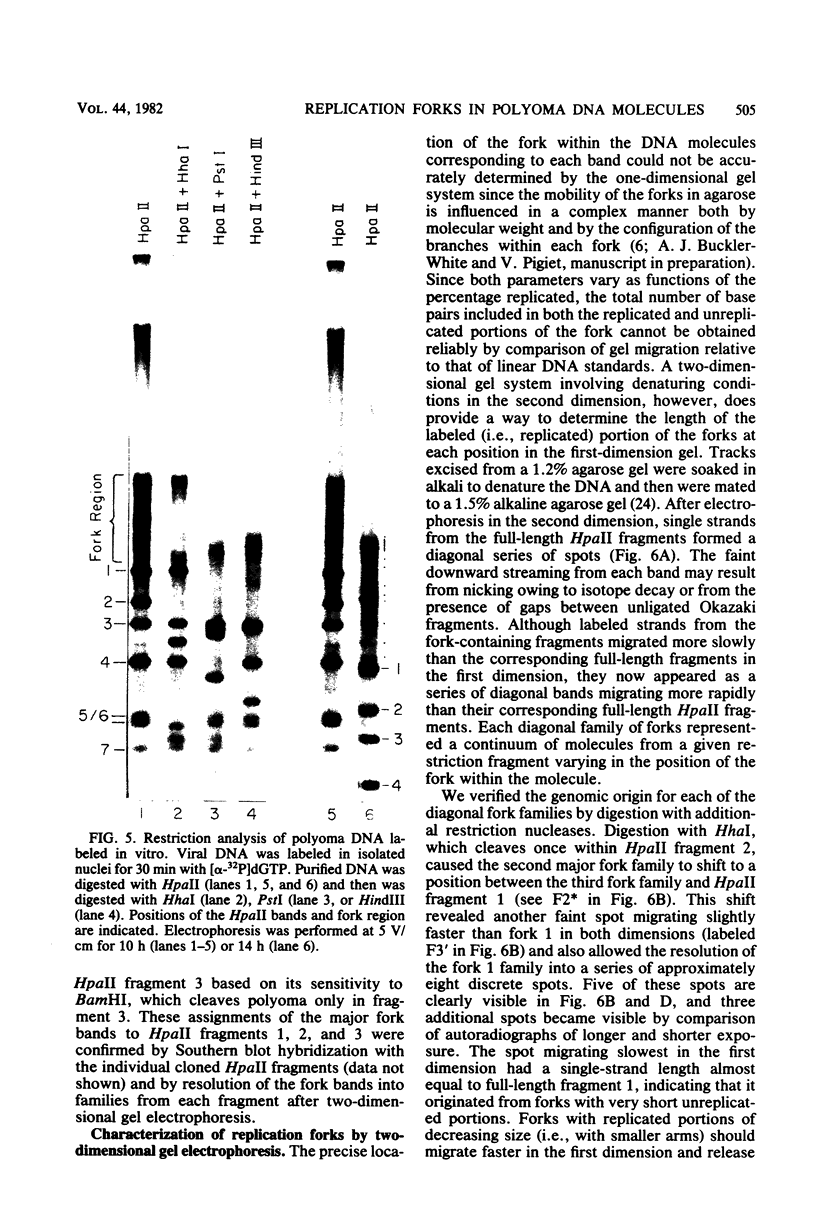
Synthesis of polyoma DNA in nuclei isolated from virus-infected 3T6 mouse fibroblasts leads to the selective labeling of replicative intermediates. Digestion of these replicative intermediates with the restriction endonuclease HpaII resulted in three highly labeled heterogeneous species in addition to the expected full-length fragments. These three species migrated more slowly in agarose than did any of the full-length restriction fragments and were shown to represent families of replication forks by criteria of sensitivity to S1 nuclease, kinetics of labeling both in vitro and in vivo, electron microscopy, and migration behavior during agarose gel electrophoresis. Subsequent digestion with other restriction enzymes showed that the two largest of the three fork bands originated from HpaII fragments 1 and 2. These fragments flank the putative terminus located 180 degrees relative to the origin. The third fork-containing band was less labeled and was derived from fragment 3, which is juxtaposed to the replication origin on the side corresponding to late transcription. A two-dimensional gel system revealed the presence of a fourth fork band, derived from fragment 4, that was obscured by full-length fragments 1 and 2 in the single-dimension electrophoresis. Resolution of the fork families revealed multiple discrete species within the major bands, implying the existence of stops or hesitations during replication of a given region of the genome. This conclusion is consistent with the presence of multiple species upon electrophoresis of the fork bands under denaturing conditions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battey J., Clayton D. A. The transcription map of mouse mitochondrial DNA. Cell. 1978 May;14(1):143–156. doi: 10.1016/0092-8674(78)90309-4. [DOI] [PubMed] [Google Scholar]
- Bjursell G., Magnusson G. Replication of polyoma DNA accumlation of early replicative intermediates during hydroxyurea inhibition. Virology. 1976 Oct 1;74(1):249–251. doi: 10.1016/0042-6822(76)90149-5. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D. A symmetrical model for polyoma virus DNA replication. J Mol Biol. 1971 Dec 28;62(3):513–524. doi: 10.1016/0022-2836(71)90152-5. [DOI] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D., Seiler P. The replication of the ring-shaped DNA of polyoma virus. II. Identification of molecules at various stages of replication. J Mol Biol. 1971 Jul 14;59(1):195–206. doi: 10.1016/0022-2836(71)90421-9. [DOI] [PubMed] [Google Scholar]
- Buckler-White A. J., Humphrey G. W., Pigiet V. Association of polyoma T antigen and DNA with the nuclear matrix from lytically infected 3T6 cells. Cell. 1980 Nov;22(1 Pt 1):37–46. doi: 10.1016/0092-8674(80)90152-x. [DOI] [PubMed] [Google Scholar]
- Buckler-White A. J., Krauss M. R., Pigiet V., Benbow R. M. Asynchronous bidirectional replication of polyoma virus DNA. J Virol. 1982 Sep;43(3):885–895. doi: 10.1128/jvi.43.3.885-895.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. W., Thomas C. A., Jr Recovery of DNA segments from agarose gels. Anal Biochem. 1980 Jan 15;101(2):339–341. doi: 10.1016/0003-2697(80)90197-9. [DOI] [PubMed] [Google Scholar]
- Clertant P., Cuzin F. Initiation of polyoma virus DNA replication in vitro and its dependence on the viral gene A protein. Nucleic Acids Res. 1980 Oct 10;8(19):4377–4392. doi: 10.1093/nar/8.19.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Robbins A. K., Nicklin P. M. Location of the origin and terminus of replication in polyoma virus DNA. J Gen Virol. 1974 Oct;25(1):133–142. doi: 10.1099/0022-1317-25-1-133. [DOI] [PubMed] [Google Scholar]
- Danna K. J., Nathans D. Bidirectional replication of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3097–3100. doi: 10.1073/pnas.69.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deininger P., Esty A., LaPorte P., Friedmann T. Nucleotide sequence and genetic organization of the polyoma late region: features common to the polyoma early region and SV40. Cell. 1979 Nov;18(3):771–779. doi: 10.1016/0092-8674(79)90130-2. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Esty A., LaPorte P., Deininger P. The nucleotide sequence and genome organization of the polyoma early region: extensive nucleotide and amino acid homology with SV40. Cell. 1979 Jul;17(3):715–724. doi: 10.1016/0092-8674(79)90278-2. [DOI] [PubMed] [Google Scholar]
- Herman T. M., DePamphilis M. L., Wassarman P. M. Structure of chromatin at deoxyribonucleic acid replication forks: Okazaki fragments released from replicating SV40 chromosomes by single-strand specific endonucleases are not in nucleosomes. Biochemistry. 1979 Oct 16;18(21):4563–4571. doi: 10.1021/bi00588a017. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Israel M. A., Chan H. W., Rowe W. P., Martin M. A. Molecular cloning of polyoma virus DNA in Escherichia coli: plasmid vector system. Science. 1979 Mar 2;203(4383):883–887. doi: 10.1126/science.217087. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Magnusson G., Nilsson M. G. Replication of polyoma DNA in isolated nuclei: analysis of replication fork movement. J Virol. 1979 Nov;32(2):386–393. doi: 10.1128/jvi.32.2.386-393.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. F. Analysis of polyoma virus DNA replicative intermediates by agarose gel electrophoresis. J Virol. 1977 Sep;23(3):827–832. doi: 10.1128/jvi.23.3.827-832.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A., Levine A. J. DNA replication in SV40-infected cells. 8. The distribution of replicating molecules at different stages of replication in SV40-infected cells. Virology. 1972 Nov;50(2):328–338. doi: 10.1016/0042-6822(72)90384-4. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigiet V., Eliasson R., Reichard P. Replication of polyoma DNA in isolated nuclei. 3. The nucleotide sequence at the RNA-DNA junction of nascent strands. J Mol Biol. 1974 Mar 25;84(1):197–216. doi: 10.1016/0022-2836(74)90222-8. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Kelly T. J., Jr, Thoren M. M., Salzman N. P. Structure of replicating simian virus 40 deoxyribonucleic acid molecules. J Virol. 1971 Oct;8(4):478–490. doi: 10.1128/jvi.8.4.478-490.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell. 1980 Aug;21(1):103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Discontinuous DNA replication: accumulation of Simian virus 40 DNA at specific stages in its replication. J Mol Biol. 1978 Apr 15;120(3):401–422. doi: 10.1016/0022-2836(78)90427-8. [DOI] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Preferred DNA sites are involved in the arrest and initiation of DNA synthesis during replication of SV40 DNA. Cell. 1980 Nov;22(1 Pt 1):97–108. doi: 10.1016/0092-8674(80)90158-0. [DOI] [PubMed] [Google Scholar]
- Winnacker E. L., Magnusson G., Reichard P. Replication of polyoma DNA in isolated nuclei. I. Characterization of the system from mouse fibroblast 3T6 cells. J Mol Biol. 1972 Dec 30;72(3):523–537. doi: 10.1016/0022-2836(72)90172-6. [DOI] [PubMed] [Google Scholar]