Abstract
Background
Chlorhexidine skin cleansing might substantially reduce neonatal infection and mortality in developing countries. Few data exist on the impact of chlorhexidine cleansing on skin colonization of infants during the first day of life or on the absorption potential of chlorhexidine during newborn skin cleansing.
Methods
Hospital born newborns in Kathmandu, Nepal were randomly allocated to full-body skin cleansing with 0.25%, 0.50%, or 1.00% chlorhexidine solution. Skin swabs were collected from the axilla, inguinal and peri-umbilical areas before cleansing (baseline), and at 2 and 24 hours after treatment. Skin flora was quantified and organisms identified. In a sub-sample, heel prick blood was collected 24 hours after the cleansing and percutaneous absorption of chlorhexidine was assessed.
Results
Among 286 enrolled newborns, no adverse effects on skin were reported and body temperature was minimally reduced (mean reduction 0.33°C). In all groups, positive skin culture rates were significantly reduced at 2 hours but generally not at 24 hours; greater reductions were observed with higher concentrations of chlorhexidine. Effect at 24 hours was highest in the 1.00% group (37% lower positive skin culture rate). For 15 of 75 infants with heel pricks, chlorhexidine was detected at trace concentrations (< 8 ng/ml, n=14; 25.8 ng/ml, n=1).
Conclusions
Chlorhexidine skin cleansing appeared safe and reduced skin flora in newborns in a dose-dependent manner 2 hours after treatment. Greater residual effect at the highest concentration (1%) might provide broader benefit and may simplify combined maternal and neonatal regimens by matching the concentration used for vaginal cleansing during labor.
Keywords: chlorhexidine, infection, bacterial colonization, Nepal, skin flora, absorption
INTRODUCTION
Continued efforts are necessary to reduce the burden of neonatal infections in developing countries where infection accounts for at least one third of neonatal deaths; this proportion is closer to 50% in settings with high mortality risk (1). For newborns, percutaneous invasion of pathogens may play a crucial role in overall infection and mortality risk (2–4). Chlorhexidine, a broad-spectrum topical antiseptic with strong residual activity, is increasingly being explored for its potential to reduce infections during the neonatal period (5–7). Chlorhexidine is inexpensive and has a strong safety record, making it a potentially ideal, easily delivered and effective intervention in low-resource communities (6,8). Two hospital-based studies have provided evidence that full-body cleansing of newborns in conjunction with maternal vaginal cleansing during labor can reduce neonatal mortality (9,10) and a recent pilot study among urban home births in Karachi, Pakistan demonstrated the feasibility of extending this intervention outside the facility (11). In Sarlahi district of southern Nepal, large community-based trials have provided evidence that umbilical cord cleansing (12) and one time full-body cleansing of the newborn skin (13) can reduce mortality risk among high-risk neonates.
In the Nepal community trial, a single skin cleansing with baby wipes pre-soaked in 0.25% chlorhexidine solution as soon as possible after birth (median age 5.5 hours) reduced mortality among low birth weight infants by 28% compared with placebo wipes (13). The presumed mechanism of action is immediate and extended reductions in skin colonization, thereby reducing the risk of acquisition of infection via percutaneous invasion. Such an effect might be stronger among infants more likely to have relatively immature development of the skin barrier. To investigate this mechanism, the impact of these pre-soaked wipes on skin colonization of newborns was subsequently compared in a randomized trial among outborn neonates at Dhaka Shishu Hospital in Dhaka, Bangladesh (14). While both chlorhexidine and water-based wipes reduced overall neonatal skin colonization in the 2 hours following the skin cleansing, the chlorhexidine based wipes provided extended residual activity from 24 hours to 3 days.
While further evidence from community settings is required to confirm the potential benefits of newborn skin cleansing as a stand-alone intervention, the combined maternal vaginal and newborn skin cleansing intervention could be implemented in hospital-based settings based on existing evidence (5). Ease of implementation would be facilitated by a single concentration of chlorhexidine for both maternal vaginal and neonatal cleansing components of the intervention (5). For the former, a variety of concentrations have been used in past and ongoing studies, but concentrations as high as 1.0% appear to be acceptable (15). For neonatal cleansing, a 0.25% concentration was studied in the Nepal community-based intervention and one of the hospital-based studies (9), but higher concentrations for the newborn, matching the levels provided for vaginal cleansing (10,15), may provide additional protection from infection risk via improved residual activity, while posing no additional risk.
The purpose of this study was to determine an optimal strength of chlorhexidine for the safe reduction of skin flora. We conducted a randomized trial of chlorhexidine cleansing of the skin (1) to describe the range of bacteria colonizing healthy hospital-born newborns in Nepal, (2) to estimate the impact of varying concentrations (0.25%, 0.50%, and 1.00%) of chlorhexidine on skin colonization, and (3) to examine whether absorption of chlorhexidine through the skin varies by concentration of chlorhexidine.
MATERIALS AND METHODS
Population
The study was conducted in the delivery ward of the Tribhuvan University Teaching Hospital (TUTH), an integral part of the Institute of Medicine, Tribhuvan University (IOM-TU), in Kathmandu, Nepal. The 426-bed hospital serves as both a national tertiary-care hospital and teaching center for all medical training programs. The primary catchment area encompasses both the urban and rural Kathmandu valley population of approximately 2 million, and there are approximately 3700 deliveries each year in this hospital.
Recruitment and Enrollment
During the enrollment period, infants born at IOM-TU were eligible to participate in the study if the following exclusion criteria were absent: > 72 hours since birth, weight at birth < 1500 grams, birth by Caesarean-section, referral for a major surgical procedure (e.g., intestinal resection for necrotizing enterocolitis), clinically-evident skin infection (confirmed by surface culture), generalized skin disease likely to produce a defect in epidermal barrier function (e.g., ichthyosis), and any structural defect of the skin involving > 5% body surface area (e.g., congenital blistering disorder). Infants admitted to the Neonatal Intensive Care Unit (NICU) were also excluded from the study. Newborns were enrolled during daytime hours on Sundays through Thursdays to accommodate the regular working schedules of the study nurses.
Randomization
Eligible newborns whose mothers provided verbal consent were individually randomized to receive full-body cleansing with one of three concentrations of chlorhexidine solution. The three groups were 0.25%, 0.50%, and 1.0% chlorhexidine (equivalent to 0.4%, 0.9%, and 1.8% total digluconate salt, respectively). Computer-generated randomization lists were created for two gestational age strata (< 37 weeks, > 37 weeks). The treatment allocations were coded “C”, “H”, and “X” and were distributed in opaque envelopes. At the time of enrollment of each newborn, the study nurse selected the next envelope from the appropriate gestational age specific list, opened the envelope and recorded the printed allocation code on the newborn’s enrollment record. Study participants, project workers and investigators were all masked to the treatment allocations.
During the initial recruitment and enrollment contact, study workers transferred data from the medical records of infants to the newborn enrollment record. These data included birth date and time, birth weight, gestational age, sex, and maternal characteristics relating to pregnancy, labor, and delivery (e.g., maternal age, pregnancy history, antenatal care visits, maternal immunizations, complications, etc.).
Intervention
To deliver the intervention, six 10-cm × 10-cm pieces of cotton cloth were autoclaved and packaged into a polyethylene sleeve that was double-sealed using a hand-operated poly/cellophane heat sealer. The sealed wipe packets were then placed in a second heat-sealed polyethylene sleeve and stored in the hospital study site in Kathmandu. The full-body cleansing intervention was conducted using these pre-packaged, sterile dry cotton cloths which were soaked in the designated concentration of chlorhexidine solution at the time of delivery of the intervention. A stock solution of 20% (w/v) chlorhexidine digluconate was diluted with purified water to concentrations of 0.25%, 0.50%, and 1.00%; the prepared solutions were stored in opaque plastic bottles, individually marked with “C”, “H”, “X”, respectively. According to the assigned random treatment allocation, the study nurse selected the corresponding bottle, poured the solution into a basin, opened a sealed packet of wipes and soaked the cotton cloths in the solution. The nurse then cleansed the infant following a simple standardized procedure based on a pilot study of cleansing of 32 newborns in Sarlahi (16) and implemented in 23,000 newborns in Sarlahi between 2002 and 2006 (13). Axillary temperature was measured prior to, 15 minutes after, and 2 hours after the cleansing intervention. The proportion of the newborn covered in vernix before and after the intervention was also estimated using three categories: <25% (“none”), 25%–75% (“a little”) and >75% (“ a lot”) and study nurses noted the presence of any skin complications at the time of the intervention and again at 2 and 24 hours post-treatment.
Skin Swabs
Skin swabs were collected from the right axilla, right inguinal region and supra-umbilical region immediately before skin cleansing, and at 2 and 24 hours after treatment (or until hospital discharge). Before each swab collection activity, overall skin condition was assessed using a simple five-category scale described previously (17). At each site, the study nurse delineated a 4-cm2 area of skin using a template, and rubbed a sterile swab, pre-soaked in phosphate-buffered saline, 5 times horizontally and 5 times vertically within the sampling area. The specimens were then returned to 1 ml of phosphate-buffered saline, transported immediately to the laboratory and vortexed. Sheep blood and MacConkey agar plates were inoculated with 10 μl of the neat specimen and three consecutive 10-fold dilutions, and incubated at 37°C. Colony count and bacterial identification was done after 48 hours using standard procedures (18). Mothers and infants often leave the hospital within hours after delivery; infants were discharged from the study when released from the hospital, or immediately after the 24-hour skin swab was collected.
Serum Sample Collection and Processing
During the final two months of the study, approximately 1–2 ml of blood was collected by heel prick from all infants who remained in the hospital until 24 hours after the skin cleansing. The right heel was covered with a sock for the duration of the study to minimize contamination of the blood sample from chlorhexidine on the skin. Samples were frozen at −20°C, transferred for processing at the University of Alabama at Birmingham Comprehensive Cancer Center Mass Spectrometry Shared Facility, USA, and prepared for coupled liquid chromatography - mass spectrometry (LC-MS/MS) analyses after protein precipitation. LC-MS/MS analyses of the samples were performed using a system consisting of a model SIL-HT refrigerated Shimadzu autosampler and HPLC instrument (Shimadzu Scientific Instruments, Inc. Columbia, MD), and an API 4000 mass spectrometer (Applied Biosystems/MDS Sciex, Concord, Ontario, Canada). Chromatography was performed using a 100 × 2.1 mm i.d. Perkin Elmer (Brownlee) Aquapore RP-300 column pre-equilibrated with 0.1% formic acid. The mobile phase consisted of 0.1% formic acid as solvent A and isopropanol containing 0.1% formic acid as solvent B. The gradient was begun at 25% solvent B, followed by a linear increase to 100% solvent B in 5 min and then maintained at 100% for 5 min. The gradient then was returned to the initial condition of 25% B. The total run time was 15 min. The injection volume was 5 μL. The eluent with flow rate of 0.4 ml/min was directed into the mass spectrometer, equipped with an electrospray ionization source operated in the positive ion mode. A blank injection (25% isopropanol containing 0.1% HCOOH) was made before every next injection in order to avoid chlorhexidine carry over. Nitrogen was used as the curtain and collision gas. Multiple reaction monitoring (MRM) with mass transition m/z 253/170 was used to perform mass spectrometric quantification of chlorhexidine. The LC-MS-MS system was controlled by BioAnalyst 1.4 software. A calibration curve (10–100 nM) was generated for chlorhexidine.
Sample Size and Analysis
The primary outcome of the trial was the change in density of skin flora 2 hours after the wipe treatment, compared to baseline. To detect a difference in the mean change in pre- (before treatment) and post- (2 hours after treatment) amounts of bacteria of 1.5 log10 units between any two treatment groups, assuming standard deviations of 1.0 log10 units, the required sample size was 88 infants per group. All analyses were conducted with STATA v9.2 (College Station, TX). We analyzed overall and site-specific average positive culture rates by treatment group. For combined-site analyses, confidence intervals were adjusted for multiple measures per infant. Among infants who were positive, we further examined distribution of colony counts (per cm2) by site and treatment group. We additionally examined colonization at 24 hours among the subset of infants who remained in the hospital at that time. To study any group differences as well as changes over follow-up time within the group, t-tests and binomial regression estimation models were conducted as appropriate. Differences between the distribution of the levels of detectable chlorhexidine in serum between the groups was compared directly using non-parametric rank-sum tests (Kruskal-Wallis for overall comparisons, and two-sample Wilcoxon rank-sum test).
Approval
This study was approved by the Institutional Review Board at the Johns Hopkins Bloomberg School of Public Health (Baltimore, MD) and the Institutional Review Board of the Institute of Medicine, Tribhuvan University (Katmandu, Nepal). The trial was registered at ClinicalTrials.gov (NCT00271440).
RESULTS
Subjects
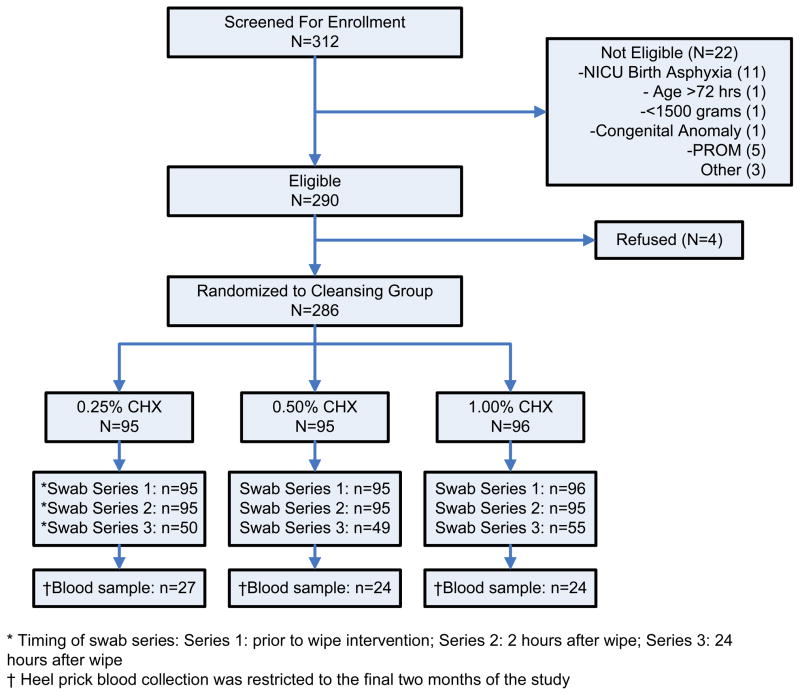
Between January and May 2006, of 312 hospital-born infants screened for eligibility, 290 were eligible for enrollment; half of the exclusions were due to admission to the NICU as a result of severe birth asphyxia (Figure, on-line only). Among 290 eligible infants, four mothers refused to participate and the remaining 286 infants were randomized to one of the three chlorhexidine solutions and included in the trial. Overall, 99.7% of enrolled infants were followed to 2 hours after the intervention. Because of the high early discharge rate, 46.2% of infants were discharged within 24 hours of the intervention and only contributed the first (baseline) and second (2-hour) swabs. The rate of discharge before the third swab did not differ between the treatment groups. Heel prick blood samples were collected from 78 infants; three of these samples were discarded because of insufficient quantity. A comparison of baseline characteristics of the infants is shown in Table 1. There were no significant differences between the three study groups for any baseline characteristics, including the mean (overall mean = 5.3 hours) and median (4.6 hours) age at the time of skin cleansing.
Figure.
Flowchart depicting the eligibility and participation status of newborns in the study
Table 1.
Baseline comparison of selected infant and maternal characteristics
| 0.25% CHX N (%) | 0.50% CHX N (%) | 1.00% CHX N (%) | p-value | |
|---|---|---|---|---|
| Female | 51 (54%) | 50 (53%) | 49 (51%) | 0.93 |
| < 2500 g | 7 (7%) | 6 (6%) | 4 (4%) | 0.63 |
| Mean weight (SD) | 3.1 (0.4) | 3.2 (0.4) | 3.2 ( 0.5) | 0.08 |
| Mean gestational age (SD) | 38.8 (1.5) | 39.1 (1.3) | 39.1 (1.4) | 0.25 |
| Mean maternal age | 24.4 (3.6) | 24.4 (3.4) | 25.3 (4.5) | 0.22 |
| Prima gravida | 43 (45%) | 45 (47%) | 43 (45%) | 0.93 |
| > 4 ANC visits | 77 (83%) | 73 (80%) | 82 (88%) | 0.33 |
|
| ||||
| Mean age at skin cleansing (hrs) (SD) | 5.0 (2.7) | 5.4 (3.1) | 5.6 (3.1) | 0.40 |
Baseline Skin Colonization
The overall proportion of positive swabs at baseline was 60% (513/855 swabs), and colonization (presence of any organisms) was only slightly lower among the peri-umbilical swabs (54%) than the axillary (63%) or inguinal swabs (63%). The overall proportion (combining all three sites) of positive swabs did not vary substantially at baseline between the three chlorhexidine cleansing groups. In the lowest concentration group (0.25%), 57% of swabs were colonized, and rate of colonization was not significantly different in the 0.50% (61%; Relative risk (RR)=1.08 [0.90 – 1.29]) or 1.00% (62%; RR=1.09 [0.91 – 1.30]) groups.
Changes in Skin Colonization
In all three study arms, the positive skin culture rate (presence of any organisms) was significantly lower at each of the three sites sampled 2 hours after the intervention (Table 2). The reduction in proportion colonized overall was greatest among the 1.00% group (63% reduction), and was followed by the 0.50% group (50% reduction) and the 0.25% group (48% reduction). This dose-response trend was generally consistent for each of the separate sites (Table 2). At 24 hours after the cleansing intervention, the positive skin culture rate had returned to approximately baseline levels for all three sites in the 0.25% group. In the 0.50% group, only swabs from the axilla tended to still be lower than observed prior to the intervention, while in the 1.00% group, the positive rate remained lower than baseline at all three sites, and significantly lower among axillary and peri-umbilical swabs (Table 2).
Table 2.
Proportion of positive skin cultures before and after skin cleansing, by site and study group
| 0.25% CHX | 0.50% CHX | 1.00% CHX | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Pos (%) | p-value | N | Pos (%) | p-value | N | Pos (%) | p-value | |
| Axilla | |||||||||
| Baseline | 95 | 55 (58%) | -- | 95 | 68 (72%) | -- | 96 | 57 (60%) | -- |
| 2 h | 95 | 33 (35%) | 0.002 | 95 | 36 (38%) | <0.001 | 95 | 22 (23%) | <0.001 |
| 24 h | 50 | 24 (48%) | 0.26 | 49 | 17 (35%) | <0.001 | 55 | 18 (33%) | 0.002 |
| Umbilical | |||||||||
| Baseline | 95 | 48 (51%) | -- | 95 | 47 (49%) | -- | 95 | 59 (62%) | -- |
| 2 h | 95 | 21 (22%) | <0.001 | 95 | 24 (25%) | 0.001 | 95 | 22 (23%) | <0.001 |
| 24 h | 50 | 31 (62%) | 0.189 | 49 | 25 (51%) | 0.860 | 55 | 18 (33%) | 0.001 |
| Inguinal | |||||||||
| Baseline | 95 | 59 (62%) | -- | 94 | 59 (63%) | -- | 95 | 61 (64%) | -- |
| 2 h | 95 | 31 (33%) | <0.001 | 95 | 27 (28%) | <0.001 | 95 | 22 (23%) | <0.001 |
| 24 h | 50 | 35 (70%) | 0.345 | 49 | 29 (59%) | 0.676 | 55 | 28 (51%) | 0.111 |
Table 3 shows the overall (i.e. any site) positive skin culture rates for each group, by timing of swab collection, combined across the three sampling sites. Compared with the 0.25% group, the proportion positive remained significantly lower at 24 hours in the 1.00% (RR=0.66 [0.49 – 0.87]) but not the 0.50% group (RR=0.83 [0.64 – 1.09]). Comparing time points within study groups, infants were 37% (19% – 52%) and 21% (2% – 37%) less likely to be colonized at 24 hours compared to baseline in the 1.00% and 0.50% groups, respectively, while likelihood of colonization at 24 hours was equal to baseline rates for the 0.25% group.
Table 3.
Comparison of overall positive skin culture rates between intervention groups, combined across axillary, periumbilical and inguinal sites by timing of skin cultures
| Group | N | Positive | Proportion | RR (95% CI)* |
|---|---|---|---|---|
| Baseline | ||||
|
| ||||
| 0.25% CHX | 285 | 162 | 57% | -- |
| 0.50% CHX | 284 | 174 | 61% | 1.08 (0.90 – 1.29) |
| 1.00% CHX | 286 | 177 | 62% | 1.09 (0.91 – 1.30) |
|
| ||||
| 2 hours after wipe | ||||
|
| ||||
| 0.25% CHX | 285 | 85 | 30% | -- |
| 0.50% CHX | 285 | 87 | 31% | 1.02 (0.77 – 1.36) |
| 1.00% CHX | 285 | 66 | 23% | 0.77 (0.56 – 1.07) |
|
| ||||
| 24 hours after cleansing intervention | ||||
|
| ||||
| 0.25% CHX | 150 | 90 | 60% | -- |
| 0.50% CHX | 147 | 71 | 48% | 0.83 (0.64 – 1.09) |
| 1.00% CHX | 165 | 64 | 39% | 0.66 (0.49 – 0.87) |
Confidence intervals adjusted for multiple skin culture swabs per infant
Density of Colonization among Positive Cultures
Colony counts were examined among the positive skin swabs for each group, swab site, and timing of collection (Table 4). A general trend toward large reductions in density at 2 hours followed by rebound at 24 hours was seen for all sites. Combined across the three sampling sites, at baseline, the mean densities (log10 counts per cm2) in the 0.25%, 0.50% and 1.00% groups were 3.69, 3.71, and 3.85, respectively. Two hours after the intervention, these overall densities had been reduced by 0.66 log10 units/cm2, 0.67 log10 units/cm2, and 1.01 log10 units/cm2, respectively for the 3 concentrations of chlorhexidine. The largest reduction was in the 1.00% group, but the increased reduction compared to the 0.25% concentration group (0.35 log10 units/cm2) was not statistically significant (95% CI: −0.12, 0.82). Mean density of skin colonization among positive cultures remained lower than baseline at 24 hours after the cleansing (mean reduction at 24 hours: 0.39 [0.17, 0.61] log10 units/cm2), and there were no differences between the three groups at 24 hours.
Table 4.
Mean skin colony counts (log10 counts per cm2) by site, timing of swab collection, and intervention group
| 0.25% CHX | 0.50% CHX | 1.00% CHX | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | N | Mean | SD | |
| Axilla | |||||||||
| Baseline | 55 | 3.98 | 1.24 | 68 | 3.95 | 1.14 | 57 | 4.40 | 1.28 |
| 2h | 33 | 3.26 | 1.07 | 36 | 3.13 | 1.04 | 22 | 3.08 | 1.02 |
| 24h | 24 | 3.26 | 1.25 | 17 | 3.20 | 1.24 | 18 | 3.02 | 1.02 |
| Umbilical | |||||||||
| Baseline | 48 | 3.24 | 1.07 | 47 | 3.26 | 1.12 | 59 | 3.37 | 1.15 |
| 2h | 20 | 2.86 | 0.96 | 24 | 2.88 | 0.87 | 22 | 2.56 | 0.83 |
| 24h | 31 | 2.97 | 0.95 | 24 | 3.36 | 1.22 | 18 | 2.91 | 0.91 |
| Inguinal | |||||||||
| Baseline | 58 | 3.79 | 1.31 | 58 | 3.08 | 1.23 | 61 | 3.81 | 1.35 |
| 2h | 31 | 2.89 | 1.04 | 27 | 3.08 | 1.03 | 22 | 2.88 | 1.04 |
| 24h | 35 | 3.87 | 1.20 | 29 | 3.46 | 1.05 | 28 | 3.79 | 1.22 |
| Overall | |||||||||
| Baseline | 161 | 3.69 | 1.25 | 173 | 3.71 | 1.20 | 177 | 3.85 | 1.32 |
| 2h | 84 | 3.03 | 1.04 | 87 | 3.05 | 0.99 | 66 | 2.84 | 0.98 |
| 24h | 90 | 3.40 | 1.19 | 70 | 3.37 | 1.15 | 64 | 3.33 | 1.14 |
Range of Organisms
The distribution of specific organisms identified across the three skin sites did not differ at baseline. Among 513 baseline positive skin swabs, the most common organisms identified (multiple organisms possible per positive culture) were coagulase-negative staphylococci (42.1%), Staphylococcus aureus (29.4%), and Escherichia coli (24.0%). A second set of organisms followed: Citrobacter spp. (7.6%), Enterococcus spp. (6.4%) and Acinetobacter spp. (6.0%). There were no differences in any of the organism-specific rates of colonization between the groups at baseline or at 2 or 24 hours after the intervention.
At baseline, gram-positive organisms were more commonly identified (267/513, 52%) than gram-negative organisms (117/513, 23%); there were also 122 (24%) swabs positive for both gram-positive and gram-negative organisms. This ratio did not change substantially across the three time periods (before cleansing, 2 hours, or 24 hours). There was only slight evidence that the impact of cleansing concentration on colonization varied by gram positive/negative status. For example, relative reductions in colonization at 24 hours compared to baseline for 1.00% chlorhexidine was 52% and 35% for gram-positive and gram-negative organisms, respectively. Similarly, in the 0.50% chlorhexidine group, relative reductions at 24 hours compared to baseline were 48% and 9% for gram-positive and gram-negative organisms, respectively.
Safety of Skin Cleansing Procedure
The skin cleansing procedure was well tolerated by all infants. Removal of vernix appeared minimal, as the proportion of newborns with 25%–75% cover of vernix was similar both before (98%) and after the intervention (97%). Mean axillary temperature was reduced from 36.0°C to 35.7°C (change = 0.33°C [0.28°C, 0.39°C]) one minute after the cleansing intervention, but quickly returned to baseline levels (mean=36.0°C) at 15 minutes. There were no differences in body temperature between the three groups before or after the intervention. The cleansing treatment was not associated with any skin irritation. Dry skin with scales was reported in one infant from each of the treatment groups prior to the intervention, and again at 2 hours after the intervention.
Absorption of chlorhexidine
There were 27, 24, and 24 blood samples available from the 0.25%, 0.50% and 1.00% treatment arms, respectively. Detectable chlorhexidine was found in 15 (20%) of the 75 samples; the mean (SD) amount among positives was 4.1 (6.3) ng/ml (range: 0.4 to 25.8 ng/ml) (Table 5). The highest detected level (25.8 ng/ml) was observed in the 0.50% group. The proportion of positive samples increased with increasing concentration of chlorhexidine (Kruskal-Wallis rank-sum test: χ2=9.55, p<0.01). The distribution of the amount of detectable chlorhexidine differed statistically between the 1.00% and the 0.25% group (p=0.003) and was marginally significant for the 0.25% vs. 0.50% group comparison (p=0.09). There was no difference in the proportion of positive samples among low birth weight (<2500 grams) babies (1 positive among 4, 25.0%) compared to normal weight babies (14 positive among 71, 19.7%).
Table 5.
Levels of chlorhexidine in 75 heel-prick blood samples collected
| ng/ml | 0.25% CHX | 0.50% CHX | 1.00% CHX | Total |
|---|---|---|---|---|
| None Detected | 25 | 20 | 15 | 60 |
| 1.00–1.99 | 0 | 1 | 0 | 1 |
| 2.00–2.99 | 0 | 1 | 0 | 1 |
| 3.00–3.99 | 0 | 1 | 0 | 1 |
| 4.00–4.99 | 0 | 0 | 1 | 1 |
| 5.00–5.99 | 0 | 1 | 0 | 1 |
| 6.00–6.99 | 0 | 0 | 1 | 1 |
| 7.00–7.99 | 1 | 0 | 0 | 1 |
| 25.83 | 0 | 0 | 1 | 1 |
|
| ||||
| Total Sample | 26 | 25 | 24 | 75 |
| Positive (%) | 1 (3.9%) | 5 (20.0%) | 9 (37.5%) | 15 (20%) |
| Mean (SD) | 0.55 (0.28) | 1.19 (5.15) | 1.25 (2.07) | 0.82 (3.20) |
DISCUSSION
Both hospital [9,10] and community-based [12] trials of neonatal skin cleansing with chlorhexidine have demonstrated reduced risk of mortality during the neonatal period. The benefit may be a result of reduced skin colonization during the early hours and days of life when infants are at highest risk for percutaneous acquisition of invasive pathogens because of immaturity and compromise in skin barrier function. Studies have used a range of concentrations for maternal vaginal and newborn skin cleansing, yet the impact of variable concentrations of chlorhexidine on the presumed mechanism of beneficial action is not well understood. The data collected in this trial in Nepal add to the scant database regarding the impact of differing concentrations of chlorhexidine on immediate (2 hours) and extended (24 hours) skin colonization in healthy newborns.
Our data demonstrate that all concentrations of chlorhexidine utilized here reduced colonization at all sampled sites (umbilical, inguinal, axilla) and that there was a dose-response trend in impact, with increased immediate reductions in the positive skin culture rate and sustained residual activity with higher concentrations. Among positive cultures, the density of colonization may also be decreased at higher concentrations; the decrease in density of colonization was 52% greater in the 1.00% group compared with the 0.25% group, although this difference was not significant. The overall positive culture rate also remained lower, compared to baseline, at 24 hours after cleansing only in the highest concentration group, indicating that higher concentrations of chlorhexidine might have improved potential for reducing infection through prolonged residual activity. Positive skin culture rates and density of colonization both rebounded at 24 hours, but the 24-hour measures must be interpreted with caution because 46% of the study subjects were discharged early; our study may have been strengthened with greater follow-up and/or follow-up beyond 24 hours of age. Any systematic bias introduced by these early discharges, however, is likely to be limited. Infants who were and were not discharged before the third swab were more likely to be male (54.5%) than female (45.5%), but did not differ in any other baseline characteristics, and had similar positive culture rates at the 2nd (2-hour) swab.
In a recent study in neighboring Dhaka, Bangladesh, 0.25% chlorhexidine cleansing was more effective in reducing Acinetobacter spp levels on the skin (14). In our study, a comparison group with no chlorhexidine was not included as the Data Safety and Monitoring Board in our previous field-based cleansing trials (12,13) had directed us to provide all further enrolled infants in Nepal with the beneficial chlorhexidine intervention, which was a 0.25% concentration. The lack of evidence of any variable impact of chlorhexidine cleansing on specific pathogens or on gram-negative relative to gram-positive organisms may be partly due to an absence of a non-chlorhexidine group.
These data provide further evidence on the safety of this simple intervention. While some concerns have been expressed regarding reconciliation of promotion of newborn skin cleansing and WHO recommendations for delayed bathing, the decrease in temperature that resulted from this non-immersion intervention which leaves little to no detectable residual moisture on the skin (16) was minimal and transient, and similar to that measured in our previous community-based study (16) in Nepal and the hospital-based cleansing study in Dhaka, Bangladesh (14). We had no instances of skin irritation as a result of the cleansing intervention, as reported in the prior studies (14,16) and no differences between the three chlorhexidine concentrations in skin condition at 2 hours and 24 hours after the intervention, as also found in Bangladesh (14).
This study confirms results of previous work demonstrating that some chlorhexidine can be absorbed through the skin of newborns during a single full-body cleansing immediately after birth (15, 19–21) and suggests that the likelihood of absorption increases within higher concentration of chlorhexidine, as approximately one-third of infants had detectable amounts of chlorhexidine in the 1.00% group. Excluding the single outlying value (which might suggest contamination during collection), all other values were lower than the range observed in all previous studies reporting absorption following either skin cleansing (15, 19) or umbilical cord cleansing with chlorhexidine (22, 23). We did not have sufficient low birth weight or preterm infants in our study to examine if absorption potential was greater in these subsets; more data on absorption among such infants is warranted. There is no evidence, however, that trace levels of chlorhexidine in serum samples have any meaningful clinical significance, and thousands of newborns (including low birth weight and preterm) have received one-time cleansing without any negative effects (6,9,10,13,14).
In settings where maternal vaginal cleansing during labor is combined with neonatal skin cleansing, the use of the same concentration of chlorhexidine for both applications may facilitate implementation. These data suggest that increasing the concentration for neonatal skin cleansing to match levels suggested for maternal cleansing (15) (i.e., 1% chlorhexidine ) may provide additional efficacy and residual activity against skin colonization than the 0.25% chlorhexidine solution used for newborn skin cleansing in other studies.
Acknowledgments
We thank Mr. Ray Moore, Mr. Ali Arabshahi and Drs. Jeevan Prasain and Stephen Barnes (University of Alabama at Birmingham) for their contribution in the analysis of the chlorhexidine and manuscript preparation. Operation of the UAB Comprehensive Cancer Center Mass Spectrometry Shared Facility has been supported in part by a NCI Core Research Support Grant to the UAB Comprehensive Cancer (P30 CA13148).
Support: This study was supported by grants from the National Institutes of Health, Bethesda, Maryland (HD 44004, HD 38753), the Bill and Melinda Gates Foundation, Seattle, Washington (810-2054), and Cooperative Agreements between JHU and the Office of Health and Nutrition, US Agency for International Development, Washington DC (HRN-A-00-97-00015-00, GHS-A-00-03-000019-00). The above mentioned funding agencies had no role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, or in preparation, review, or approval of this manuscript.
References
- 1.Lawn JE, Cousens S, Zupan J Lancet Neonatal Survival Steering Team. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 2.Darmstadt GL, Saha SK, Ahmed AS, et al. Effect of topical treatment with skin barrier-enhancing emollients on nosocomial infections in preterm infants in Bangladesh: a randomised controlled trial. Lancet. 2005;365:1039–1045. doi: 10.1016/S0140-6736(05)71140-5. [DOI] [PubMed] [Google Scholar]
- 3.Darmstadt G, Saha S, Ahmed A, et al. The skin as a potential portal of entry for invasive infections in neonates. Perinatol. 2003;5:205–212. [Google Scholar]
- 4.Darmstadt GL, Mao-Qiang M, Chi E, et al. Impact of topical oils on the skin barrier: possible implications for neonatal health in developing countries. Acta Paediatr. 2002;91:546–554. doi: 10.1080/080352502753711678. [DOI] [PubMed] [Google Scholar]
- 5.McClure EM, Goldenberg RL, Brandes N, et al. The use of chlorhexidine to reduce maternal and neonatal mortality and morbidity in low-resource settings. Int J Gynaecol Obstet. 2007;97:89–94. doi: 10.1016/j.ijgo.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullany LC, Darmstadt GL, Tielsch JM. Safety and impact of chlorhexidine antisepsis interventions for improving neonatal health in developing countries. Pediatr Infect Dis J. 2006;25:665–675. doi: 10.1097/01.inf.0000223489.02791.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldenberg RL, McClure EM, Saleem S, Rouse D, Vermund S. Use of vaginally administered chlorhexidine during labor to improve pregnancy outcomes. Obstet Gynecol. 2006;107:1139–1146. doi: 10.1097/01.AOG.0000215000.65665.dd. [DOI] [PubMed] [Google Scholar]
- 8.Denton GW. Chlorhexidine. In: Block SS, editor. Disinfection, sterilization, and preservation. 5. Philadelphia: Lippencott Williams & Wilkens; 2001. pp. 321–326. [Google Scholar]
- 9.Taha TE, Biggar RJ, Broadhead RL, et al. Effect of cleansing the birth canal with antiseptic solution on maternal and newborn morbidity and mortality in Malawi: clinical trial. BMJ. 1997;315:216–219. doi: 10.1136/bmj.315.7102.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakr AF, Karkour T. Effect of predelivery vaginal antisepsis on maternal and neonatal morbidity and mortality in Egypt. J Womens Health. 2005;14:496–501. doi: 10.1089/jwh.2005.14.496. [DOI] [PubMed] [Google Scholar]
- 11.Saleem S, Reza T, McClure EM, et al. Chlorhexidine vaginal and neonatal wipes in home births in Pakistan: a randomized controlled trial. Obstet Gynecol. 2007;110:977–985. doi: 10.1097/01.AOG.0000285653.17869.26. [DOI] [PubMed] [Google Scholar]
- 12.Mullany LC, Darmstadt GL, Khatry SK, et al. Topical applications of chlorhexidine to the umbilical for prevention of omphalitis and neonatal mortality in southern Nepal: a community-based, cluster-randomized trial. Lancet. 2006;367:910–918. doi: 10.1016/S0140-6736(06)68381-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tielsch JM, Darmstadt GL, Mullany LC, Khatry SK, Katz J, LeClerq S, et al. Newborn skin cleansing with a dilute chlorhexidine solution reduces neonatal mortality in southern Nepal: a community-based cluster-randomized trial. Pediatrics. 2007;119:e330–40. doi: 10.1542/peds.2006-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darmstadt GL, Hossain MM, Choi Y, et al. Safety and effect of chlorhexidine skin cleansing on skin flora of neonates in Bangladesh. Pediatr Infect Dis J. 2007;26:492–495. doi: 10.1097/01.inf.0000261927.90189.88. [DOI] [PubMed] [Google Scholar]
- 15.Wilson CM, Gray G, Read JS, et al. Tolerance and safety of different concentrations of chlorhexidine for peripartum vaginal and infant washes: HIVNET 025. J Acquir Immune Defic Syndr. 2004;35:138–143. doi: 10.1097/00126334-200402010-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mullany LC, Darmstadt GL, Khatry SK, LeClerq SC, Tielsch JM. Safety of neonatal skin cleansing in rural Nepal. Indian Pediatrics. 2006;43:117–124. [PMC free article] [PubMed] [Google Scholar]
- 17.Darmstadt GL, Badrawi N, Law PA, et al. Topically applied sunflower seed oil prevents invasive bacterial infections in preterm infants in Egypt: a randomized, controlled clinical trial. Pediatr Infect Dis J. 2004;23:719–725. doi: 10.1097/01.inf.0000133047.50836.6f. [DOI] [PubMed] [Google Scholar]
- 18.Cheesebrough M. Medical Laboratory Manual for Tropical Countries. Vol. 2. London: Tropical Health Technology and Butterworth-Heinemann; 1998. pp. 182–183. [Google Scholar]
- 19.Cowen J, Ellis SH, McAinsh J. Absorption of chlorhexidine from the intact skin of newborn infants. Arch Dis Child. 1979;54:379–383. doi: 10.1136/adc.54.5.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Neill J, Hosmer M, Challop R, Driscoll J, Speck W, Sprunt K. Percutaenous absorption potential of chlorhexidine in neonates. Curr Ther Res. 1982;31:485–489. [Google Scholar]
- 21.O’Brien CA, Blumer JL, Speck WT, Carr H. Effect of bathing with a 4 per cent chlorhexidine gluconate solution on neonatal bacterial colonization. J Hosp Infection. 1984;5(Supp 1):141. [Google Scholar]
- 22.Johnsson J, Seeberg S, Kjellmer I. Blood concentrations of chlorhexidine in neonates undergoing routine cord care with 4% chlorhexidine gluconate solution. Acta Paediatr Scand. 1987;76(4):675–676. doi: 10.1111/j.1651-2227.1987.tb10544.x. [DOI] [PubMed] [Google Scholar]
- 23.Aggett PJ, Cooper LV, Ellis SH, McAinsh J. Percutaneous absorption of chlorhexidine in neonatal cord care. Arch Dis Child. 1981;56:878–880. doi: 10.1136/adc.56.11.878. [DOI] [PMC free article] [PubMed] [Google Scholar]