Abstract
Acute lung injury disrupts the alveolar septal barrier, leading to patchy alveolar flooding and hypoxemia. While calcium entry into endothelial cells is critical for loss of barrier integrity, the cation channels participating in disruption of this barrier in acute lung injury have not been identified. We hypothesized that activation of the vanilloid transient receptor potential channel TRPV4 disrupts the alveolar septal barrier. Expression of TRPV4 was confirmed via immunohistochemistry in the alveolar septal wall in human, rat, and mouse lung. In isolated rat lung, the TRPV4 activators 4α-phorbol-12,13-didecanoate and 5,6- or 14,15-epoxyeicosatrienoic acid, as well as thapsigargin, a known activator of calcium entry via store-operated channels, all increased lung endothelial permeability, assessed by measurement of the filtration coefficient, in a dose- and calcium-entry dependent manner. However, ruthenium red blocked the permeability response to the TRPV4 agonists, but not to thapsigargin. Light and electron microscopy of rat and mouse lung revealed that TRPV4 agonists preferentially produced blebs or breaks in the endothelial and epithelial layers of the alveolar septal wall, while thapsigargin disrupted inter-endothelial junctions in extra-alveolar vessels. The permeability response to 4α-phorbol-12,13-didecanoate was absent in TRPV4−/− mice, whereas the response to thapsigargin remained unchanged. Collectively, these findings implicate TRPV4 in disruption of the alveolar septal barrier, and suggest its participation in the pathogenesis of acute lung injury.
Keywords: permeability, TRP channels, TRPV4, acute lung injury
INTRODUCTION
Acute lung injury and its more severe form the adult respiratory distress syndrome are characterized by disruption of the alveolar septal barrier, leading to patchy alveolar flooding and hypoxemia 1. Effective clinical treatments are limited and mortality remains high 2, 3. Such endothelial barrier disruption is often dependent upon entry of Ca2+ from the extracellular space 4, 5. For example, in the intact rat lung, thapsigargin-induced store depletion and Ca2+ entry via store-operated channels increase endothelial permeability 6, 7. Extra-alveolar vessel endothelium is targeted while the septal microvasculature appears to be spared 7, 8. Although endothelial cells derived from extra-alveolar pulmonary arteries and septal microvessels are phenotypically distinct 9, 10 and both potentially could be targeted in acute lung injury, disruption of the septal barrier is more likely to promote alveolar flooding and impair gas exchange than disruption in extra-alveolar vessels. Importantly, Ca2+ entry pathways involved in regulation of barrier integrity in the alveolar septal compartment have not been elucidated.
The consensus of data suggests that TRPC1 and TRPC4, members of the canonical subfamily of transient receptor potential (TRP) channels 11–15, comprise subunits of store-operated Ca2+ channels in lung endothelium 16–18. In the isolated rat lung, the permeability response to thrombin-induced store depletion was attenuated in TRPC4−/− mice 18. Further, loss of the permeability response to thapsigargin-induced store depletion in rat lung after heart failure was associated with down-regulation of TRPC1 and TRPC4, channels which are normally expressed in extra-alveolar endothelium 19. Retention of the permeability response to 14,15-epoxyeicosatrienoic acid (14,15-EET), a P450 epoxygenase-derived arachidonic acid metabolite, in this heart failure model was intriguing since the permeability responses to both thapsigargin-induced store depletion and to 14,15-EET are dependent upon Ca2+ entry. This striking observation drives home the point that EETs must target Ca2+ entry pathways in lung endothelium distinct from store-operated channels.
We propose that Ca2+ entry via TRPV4, a member of the vanilloid subfamily of TRP channels 13, 20–24, contributes critically to regulation of endothelial permeability and barrier integrity in the lung. This hypothesis is based on several observations: 5,6-EET and 8,9-EET have been linked to activation of TRPV4 and subsequent Ca2+ entry in aortic endothelial cells 25, 26, 5,6-EET and 14,15-EET increase endothelial permeability in rat and canine lung 6, 27, and the permeability response to 14,15-EET, at least, is dependent upon entry of extracellular Ca2+ 6. In the current study, we tested the hypothesis that Ca2+ entry via TRPV4 regulates lung endothelial permeability and barrier integrity. Further, we tested whether Ca2+ entry via TRPV4 and that occurring through store-operated channels evoke barrier disruption in spatially distinct compartments of the pulmonary vasculature.
MATERIALS AND METHODS
Animals
Protocols were approved by IACUC, conforming to the NIH Guide for the Care and Use of Laboratory Animals. Adult male CD40 rats (n=139, Charles River) or 8–10-week-old TRPV4 wild type mice (TRPV4+/+) and null littermates (TRPV4−/−)28 of either sex (n=39) were anesthetized with sodium pentobarbital (50 mg/kg body wt, i.p.). Lungs were isolated for ex vivo perfusion as previously described 6.
TRPV4 Expression
Human lung resection specimens (n=3), obtained under a protocol approved by the Institutional Review Board, were fixed by immersion in 10% formalin or 100% ethanol. Rat and mouse lungs (n=2–3 in each group) were perfusion-fixed with 4% paraformaldehyde. Sections (5 μm) were processed for immunohistochemistry using a goat anti-TRPV4 polyclonal antibody (Alomone), stained with diaminobenzidine and counterstained with hematoxylin. Western blots were prepared from lysates (40 μg total protein) of rat pulmonary artery and microvascular endothelium and TRPV4 detected using enhanced chemiluminescence. Total RNA from mouse lung was reverse transcribed, then PCR performed using primers designed to amplify the pore-loop region of TRPV4 (see Supplemental Data).
Microscopic Assessment of Acute Lung Injury
Light microscopy and transmission electron microscopy (EM) were used to evaluate structural changes in glutaraldehyde-fixed lung 19, after treatment with vehicle (910 μL ethanol), 4αPDD (3 or 10 μmol/L in rat or mouse lung, respectively), 14,15-EET (3 μmol/L, rat lung only) or thapsigargin (150 nmol/L) for 60 min (n=3 per group). Using 1 μm thick sections, extra-alveolar vessel cuffing and alveolar flooding were evaluated. Cuff frequency and the cuff volume (Vc) fraction of total wall volume (Vc/Vw) were determined, the latter using a point-counting strategy 29. Thin sections (80 nm) from the same blocks were examined via transmission EM. Junctional discontinuities (gaps), blebs, or breaks in septal capillaries were enumerated and point-counting used to determine alveolar fluid volume (Vaf) fraction in the alveolar space (Vaf/Vas). Means for cuff frequency, volume fractions and blebs or breaks per capillary were determined separately for each lung, then overall descriptive statistics derived for each group.
Isolated Lung and Assessment of Endothelial Permeability
Both rat and mouse lungs were perfused at constant flow with buffer (in mmol/L: 116.0 NaCl, 5.2 KCl, 0.9 MgSO4, 1.0 Na2HPO4, 2.2 NaHCO3, 8.3 D-glucose) containing 4% bovine serum albumin and either physiologic (2.2 mmol/L) or low (0.02 mmol/L) CaCl2 at pH 7.4 (38 °C). Hemodynamics and the filtration coefficient (Kf) were measured as previously described 6, 19, 30, 31, using zone 3 conditions. Kf was calculated as the rate of weight gain obtained 13–15 min after a 7–10 cmH2O increase in pulmonary venous pressure, normalized per g lung dry weight. Kf, the product of specific endothelial permeability and surface area for exchange, is a sensitive measure of lung endothelial permeability when surface area is fully recruited 32.
Protocols
In rat lungs perfused with physiologic [Ca2+], Kf and hemodynamics were measured at baseline and 60 min after treatment with the TRPV4 agonist 4α-phorbol-12,13-didecanoate (4αPDD, 1–10 μmol/L, n=3 per dose) or the TRPV1 agonist 4α-phorbol-12,13-didecanoate-20 homovanillate (4αPDDHV, 10 μmol/L, n=4). Using 0.02 mmol/L [Ca2+] perfusate, Kf and hemodynamics were measured at baseline and 45 min after treatment (n=5 per group) with 4αPDD (3 μmol/L), 5,6-EET or 14,15-EET (3 μmol/L, Biomol) or thapsigargin (150 nmol/L), with or without the TRPV antagonist ruthenium red (1 μmol/L). Subsequently, CaCl2 was added to achieve physiologic [Ca2+] (Ca2+ add-back) and measurements repeated 15 min later. Lungs isolated from TRPV4−/− mice or wild type littermates (n=4–5 per group) were perfused with buffer containing 4% albumin and physiologic [Ca2+]. Kf and hemodynamics were measured baseline and 60 min after treatment with vehicle (50 μL DMSO), 4αPDD (10 μmol/L), or thapsigargin (150 nmol/L). Vehicle controls in rat lungs (n=5 each) included ethanol (910 μL or 2%) or DMSO (50 μL or 0.1%); DMSO was also evaluated in lungs from wild type mice (n=4). All drugs were added to the perfusate; final circulating concentrations are noted.
Statistical Analysis
Quantitative data are presented as mean ± SEM. Group means were compared using a paired t-test (two-tailed) or analysis of variance (ANOVA), as appropriate; the Newman-Keuls multiple comparison test was used to identify specific differences. P values < 0.05 were considered statistically significant.
RESULTS
Baseline Parameters in Isolated Rat and Mouse Lung
Total pulmonary vascular resistance, the distribution of vascular resistance and baseline Kf were similar in rat and mouse lungs (Table 1) and were not impacted by the choice of perfusate [Ca2+]. There were no differences with respect to baseline measurements between TRPV4+/+ and TRPV4−/− mice.
Table 1.
Baseline hemodynamics and permeability in isolated rat and mouse lung
| Rat lung | Mouse lung | ||
|---|---|---|---|
| Perfusate [Ca2+], mmol/L | 2.2 | 0.02 | 2.2 |
| N | 51 | 76 | 23 |
| Body weight, g | 344±6 | 377±5 | 22±1 |
| Q, mL/min/g body wt | 0.041±0.001 | 0.037±0.001 | 0.055±0.002 |
| Q, L/min/100 g PLW | 1.05±0.03 | 0.93±0.02 | 1.23±0.04 |
| Pa, cmH2O | 17.0±0.1 | 14.8±0.2 | 16.5±0.3 |
| Pc, cmH2O | 10.2±0.1 | 9.0±0.1 | 10.4±0.2 |
| Pv, cmH2O | 4.0±0.0 | 4.0±0.0 | 4.2±0.1 |
| Ra, cmH2O/L/min/100g PLW | 6.8±0.2 | 6.4±0.2 | 5.0±0.2 |
| Rv, cmH2O/L/min/100g PLW | 6.1±0.2 | 5.5±0.2 | 5.2±0.3 |
| Rt, cmH2O/L/min/100g PLW | 12.9±0.4 | 11.9±0.3 | 10.2±0.4 |
| Kf, mL/min/cmH2O/g dry wt | 0.0102±0.0003 | 0.0106±0.0004 | 0.0123±0.0003 |
Expression of TRPV4 in Lung: Functional Consequences
TRPV4 was expressed in the septal compartment of lungs from humans, rats and mice (Figure 1A-C, respectively), as well as in bronchiolar epithelium (not shown). TRPV4 was also expressed in smooth muscle in human and rat extra-alveolar vessels. Although TRPV4 expression in cultured rat pulmonary artery endothelium was similar to that in microvascular endothelium (Figure 1D), TRPV4 was not consistently expressed in extra-alveolar vessel endothelium in intact lung (Figure 1A-C). In rat lung, 1, 5, and 10 μmol/L 4αPDD increased Kf by 1.7-, 4.2- and 5.6-fold, respectively (Figure 1E), supporting the notion that TRPV4 may play a role in regulating endothelial permeability. In contrast, the TRPV1 agonist 4αPDDHV had no impact on endothelial permeability despite use of a > EC100 dose 33. To determine whether activation of TRPV4 increased permeability in a Ca2+ entry-dependent fashion, Kf was re-evaluated using the low Ca2+/Ca2+ add-back strategy. The Ca2+ concentration chosen for the low Ca2+ segment of these experiments was based on the lowest concentration which allowed stable Kf for at least 1 hr (see Supplemental Data). Ca2+ add-back provides a normal inward Ca2+ gradient, and if Ca2+ permeant channels have been activated by the treatment, Ca2+ entry results and endothelial permeability increases. The ~3-fold increase in Kf induced by 4αPDD was clearly dependent upon Ca2+ entry (Figure 2A) and was blocked by ruthenium red (Figure 2B), which potently blocks TRPV4 by binding to an extracellular domain on the channel 24, 33. We confirmed that the permeability response to 14,15-EET and thapsigargin in rat lung is dose- (see Supplemental Data) and Ca2+ entry-dependent (Figure 2A), and documented a similar pattern for 5,6-EET (Supplemental Data and Figure 2A). At 0.02 mmol/L [Ca2+], both 5,6-EET and 14,15-EET evoked a small increase in permeability, though Ca2+ add-back was required for the development of the normal permeability response to the EETs 6. In the absence of Ca2+ add-back, the increase in Kf induced by 14,15-EET in 0.02 mmol/L [Ca2+] (0.010±0.001 to 0.024±0.003 mL/min/cmH2O/g dry wt, p=0.0007, n=5) was transient and Kf returned to baseline within 15 min (p=0.007). Ruthenium red (Figure 2B) blocked the Ca2+ entry-dependent component of the permeability response to 5,6- and 14,15-EET, but had no impact on the Ca2+ entry-dependent permeability response to thapsigargin. In the absence of treatment, 0.02 mmol/L [Ca2+] had no impact on Kf with or without Ca2+ add-back (see Supplemental Data). EETs activate large conductance Ca2+-activated potassium channels (BKCa), providing an increased driving gradient for Ca2+ entry 34, 35. However, blockade of BKCa channels did not alter the EET-induced permeability response (see Supplemental Data).
Figure 1.
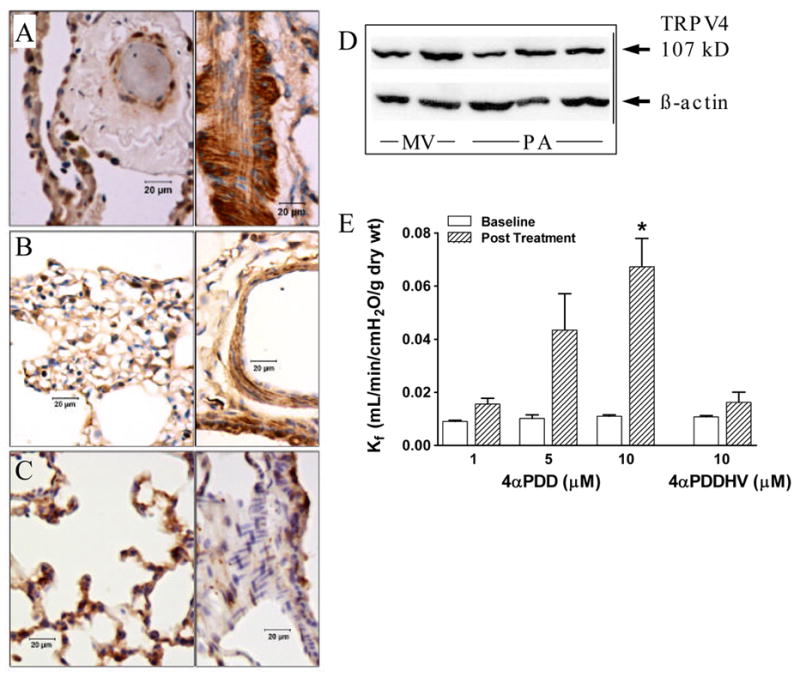
Activation of TRPV4 expressed in lung increases endothelial permeability. In human (A), rat (B), and mouse (C) lung, TRPV4 was expressed in the alveolar septal compartment (left panels) and in bronchial epithelium (not shown). TRPV4 expression in vascular smooth muscle in extra-alveolar vessels (right panels) was observed in human and rat lung, whereas little was seen in mouse lung. Western blotting (D) showed similar TRPV4 expression in rat microvascular (MV) and pulmonary artery (PA) endothelium (TRPV4/β-actin band density was 1.1 in both groups). However, TRPV4 was not consistently expressed in endothelium of extra-alveolar vessels in intact lung (A–C). The TRPV4 agonist 4αPDD increased the filtration coefficient Kf in isolated rat lung (p=0.021) in a dose-dependent fashion (p=0.002); *p<0.05 vs 1 or 5 μmol/L. The TRPV1 agonist 4α-phorbol-12,13-didecanoate-20-homovanillate (4αPDDHV) had no effect (p=0.201, paired t-test).
Figure 2.
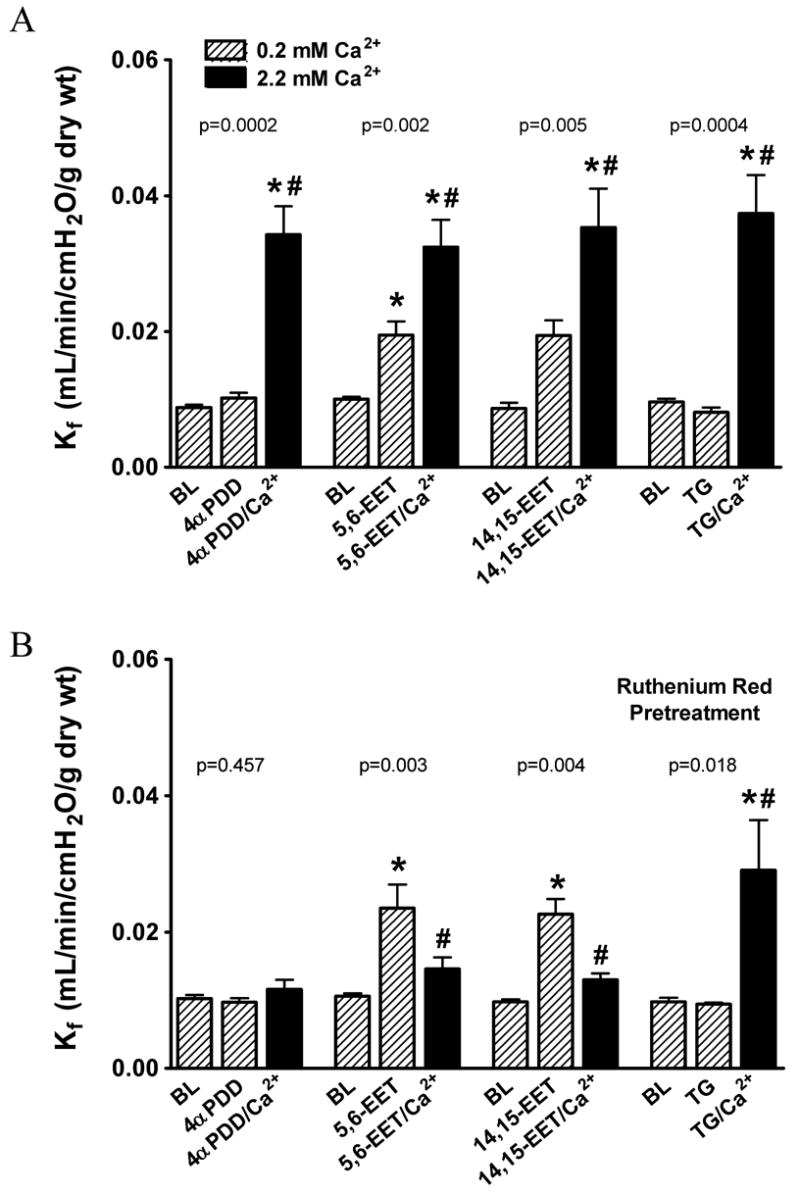
Activation of TRPV4 or store-operated channels increases permeability in a Ca2+ entry-dependent fashion. In low (0.02 mmol/L) extracellular [Ca2+] (striped bars), Kf was measured at baseline (BL) and 45 min after treatment with the TRPV4 agonists 4αPDD or EETs or thapsigargin which activates store-operated channels. A final Kf was measured 15 min after Ca2+ add-back (2.2 mmol/L, closed bars). Vehicle (A) or the TRPV antagonist ruthenium red (1 μmol/L, panel B) was added 15 min prior to treatment. Ca2+ entry was required for the permeability response to all agonists (A), yet only the response to 4αPDD or EETs was blocked by ruthenium red (B). P values for ANOVA are shown above each group; post hoc tests identified specific differences: *p<0.05 vs. BL, #p<0.05 vs agonist in low Ca2+.
Compartmentalization of Barrier Disruption
Results of the morphometric analysis from light microscopy (Figure 3) and transmission EM (Figure 4) are shown in Table 2. 4αPDD and thapsigargin resulted in frequent extra-alveolar cuffs in rat (p=0.006), but not in mouse lung, compared to control. Nonetheless, Vc/Vw was not different between groups in either rat or mouse lung. Activation of TRPV4 resulted in blebs or breaks in septal endothelium in both rat and mouse lung (p=0.009 and p=0.019, respectively, vs control or thapsigargin). Further, the TRPV4 agonists disrupted type I alveolar epithelial cells, manifested as separation of the epithelium from the basal lamina. 4αPDD increased Vaf/Vas, compared to control and thapsigargin, in both rat and mouse lung (p=0.003 and 0.011, respectively). While 14,15-EET caused significant septal injury, the increase in Vaf/Vas was more variable, and as a result the 4-group ANOVA was not significant. In contrast, thapsigargin primarily targeted inter-endothelial junctional complexes in extra-alveolar vessels, leading to formation of gaps; the septal capillary network was spared and Vaf/Vas did not increase. Together, these data support the notion that activation of TRPV4 (via 4αPDD or EETs) and activation of store-operated channels (via thapsigargin) promote barrier disruption in discrete vascular compartments of the lung even though these agents cause similar increases in total lung Kf in a Ca2+ entry-dependent fashion.
Figure 3.
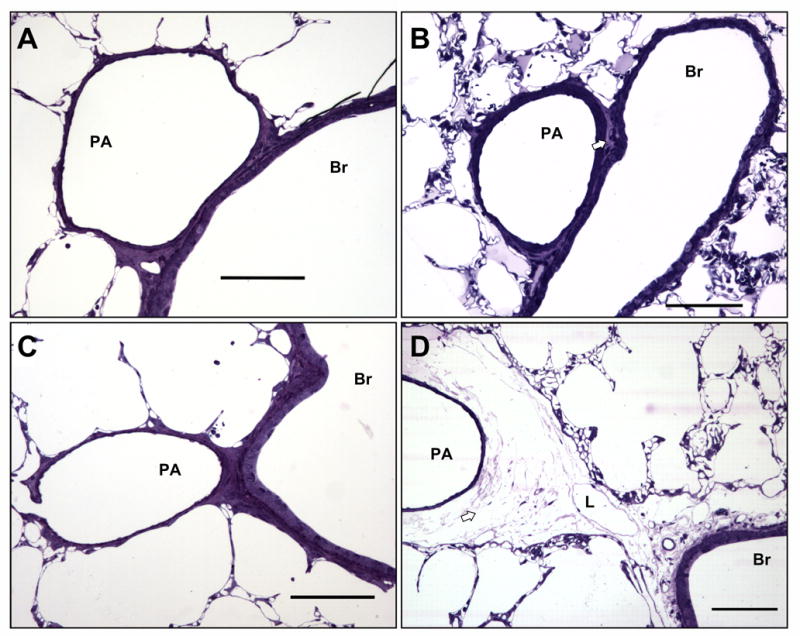
Microscopic assessment of perivascular cuffing in extra-alveolar vessels. Perivascular cuffs were infrequently observed in control rat lungs (A). Perivascular cuffing induced by 4αPDD (B), 14,15-EET (C), or thapsigargin (D) was heterogeneous, and when cuffing appeared (arrow), cuff volume fraction was no different between groups (see Table 2). Extra-alveolar vessels often appeared no different control. Scale bars are 100 μm. PA, pulmonary arteriole; Br, bronchiole; L, lymphatic.
Figure 4.
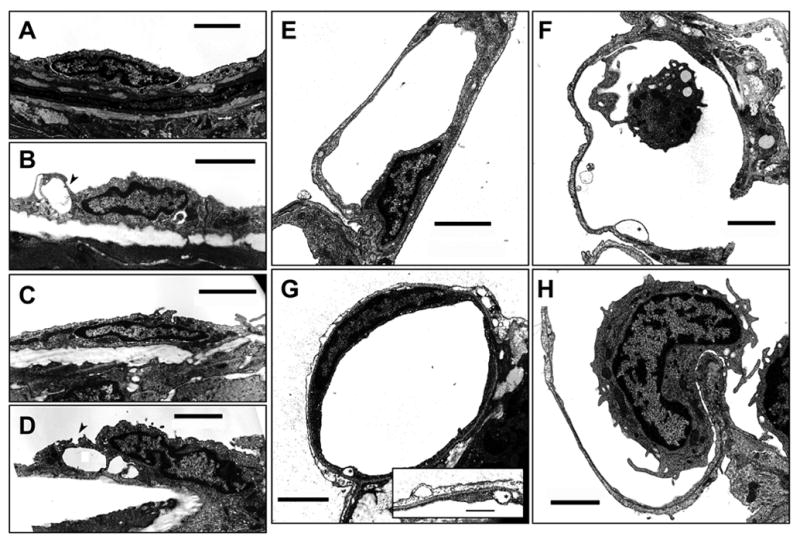
Assessment of endothelial ultrastructure in rat lung. Endothelial cell integrity was assessed using transmission electron microscopy. Random blocks were selected from glutaraldehyde-fixed lung one hr after treatment with vehicle or drug, and measurement of Kf to document endothelial permeability. Representative micrographs from extra-alveolar vessels (A–D) and septal capillaries (E–H) are shown. Endothelial ultrastructure was retained in control lung (A and E), though occasional blebs or breaks in septal capillaries were observed. 4αPDD (B and F) and 14,15-EET (C and G) rarely altered junctional morphology. However, both agonists resulted in endothelial breaks and blebs (asterisk) in the septal capillary endothelium (F and G), as well as blebs in the alveolar epithelium (inset in panel G). Thapsigargin resulted in development of gaps at junctions between endothelial cells in extra-alveolar vessels (arrowhead, D), but had no impact in the septal compartment (H). Scale bars (A–H) are 2 μm; scale bar for inset in G is 500 nm.
Table 2.
Extra-alveolar cuffs and alveolar septal barrier disruption
| Extra-alveolar vessels, n | % of extra-alveolar vessels with cuffs | Cuff volume fraction, Vc/Vw | Septal capillaries, n | Blebs, breaks per capillary | Alveolar fluid volume fraction, Vaf/Vas | |
|---|---|---|---|---|---|---|
| Isolated Rat Lung | ||||||
| Control | 231 | 6.0 ± 3.6 | 0.158 ± 0.054 | 367 | 0.15 ± 0.05 | 0.008 ± 0.008 |
| 4αPDD | 129 | 29.0 ± 2.6 *† | 0.320 ± 0.118 | 223 | 1.34 ± 0.25 *‡ | 0.055 ± 0.007 |
| 14,15-EET | 219 | 8.3 ± 5.2 | 0.237 ± 0.081 | 470 | 1.29 ± 0.36 *‡ | 0.172 ± 0.157 |
| Thapsigargin | 190 | 36.2 ± 7.0 *† | 0.458 ± 0.089 | 397 | 0.33 ± 0.08 | 0.003 ± 0.003 |
| Isolated Mouse Lung (TRPV4+/+) | ||||||
| Control | 434 | 13.8 ± 0.2 | 0.157 ± 0.049 | 528 | 0.16 ± 0.03 | 0.012 ± 0.003 |
| 4αPDD | 454 | 17.1 ± 4.9 | 0.254 ± 0.068 | 408 | 1.49 ± 0.42 *‡ | 0.108 ± 0.027 *‡ |
| Thapsigargin | 348 | 21.4 ± 7.3 | 0.202 ± 0.025 | 403 | 0.28 ± 0.15 | 0.015 ± 0.009 |
Data are mean ± SEM (3 lungs per group). Injury in the extra-alveolar compartment was not quantified, since at this magnification the entire vessel perimeter is not visualized.
p<0.05 vs control;
p<0.05 vs 14,15-EET;
p<0.05 vs thapsigargin.
The 4αPDD and EET-mediated permeability responses were inhibited with ruthenium red, a pleiotropic antagonist that blocks TRPV4. Although activation of TRPV1 had no impact on endothelial permeability in rat lung, it was possible that ruthenium red blocked other TRPV isoforms, contributing to its impact on the permeability response. Thus, we utilized mice genetically engineered for a targeted deletion of exon 12 in the trpv4 gene 28, encoding the transmembrane domains 5 and 6, which includes the predicted pore loop region of the channel (TRPV4−/−). Genotyping (see Supplemental Data) and PCR (Figure 5A) were used to document expression of TRPV4 in wild-type mice. While 3 μmol/L 4αPDD had no impact on permeability in TRPV4+/+ mice, 10 μmol/L 4αPDD increased Kf 2.8-fold (Figure 5B); this dose did not alter Kf in lungs from TRPV4−/− littermates. Thapsigargin increased Kf ~3-fold in both TRPV4+/+ and TRPV4−/− mice. These results confirm that activation of TRPV4 increases endothelial permeability in the mouse lung.
Figure 5.
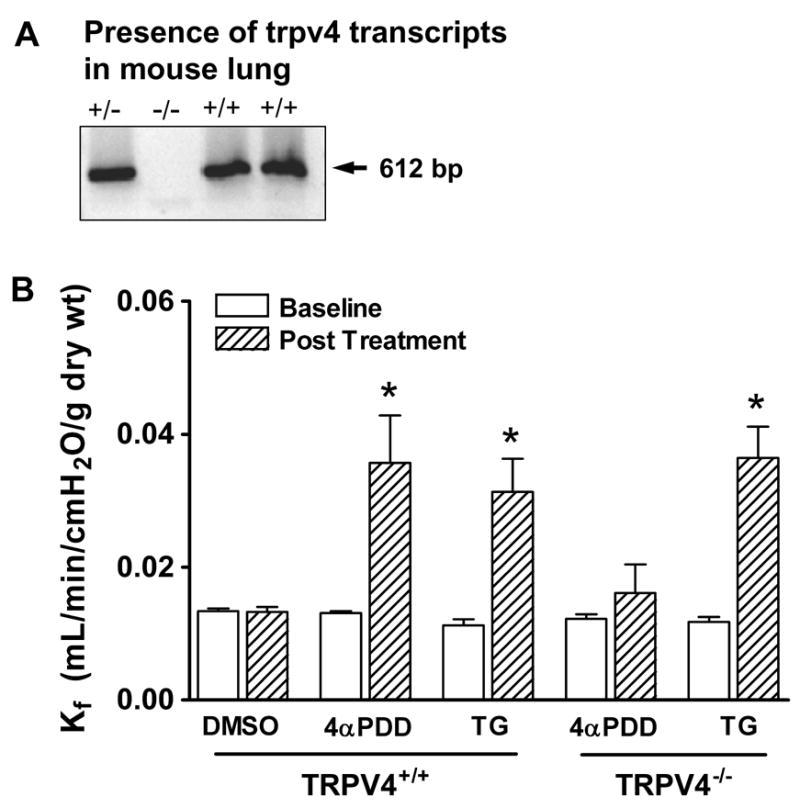
Activation of TRPV4 increases endothelial permeability in mouse lung. A. Using primers designed to amplify across the pore-loop region of TRPV4, the predicted 612 bp product was observed in wild type and heterozygote mice, but not in null animals. B. In isolated mouse lung, we showed that the TRPV4 agonist 4αPDD (10 μmol/L) increased endothelial permeability (Kf) only in TRPV4+/+ mice (p=0.036, paired t-test). In contrast, activation of store-operated channels via thapsigargin (150 nmol/L) increased permeability in both wild type and null mice (p=0.032 and 0.008, respectively, paired t-test). These results confirm the role of TRPV4 in mediating acute lung injury. *p<0.05 vs. Baseline (paired t-test).
DISCUSSION
This study provides the first evidence of a functional role for TRPV4 in lung endothelium, implicating TRPV4 in Ca2+ entry-dependent regulation of endothelial permeability and barrier integrity in the alveolar septal network. Despite similar impact on Kf, activation of TRPV4 and store-operated channels led to injury in distinct vascular compartments. Activation of TRPV4 by 4αPDD and 14,15-EET preferentially targeted the alveolar septal microvessels, whereas thapsigargin-induced store depletion targeted extra-alveolar vessels. The TRPV4 agonists typically caused endothelial blebs and/or breaks, whereas thapsigargin induced formation of gaps at endothelial cell junctions. These data suggest that distinct endothelial compartments, and possibly distinct subcellular compartments of lung endothelial cells, must be targeted subsequent to Ca2+ entry via these TRP channels. The observation that the Ca2+ entry-dependent component of the permeability response to 5,6- and 14,15-EET, as well as the TRPV4 agonist 4αPDD, was ablated by pretreatment of rat lung with ruthenium red, suggests an important role for TRPV4 in regulation of lung endothelial permeability. This notion is corroborated by the loss of the permeability response to the TRPV4 agonist 4αPDD in lungs from TRPV4−/− mice. In light of the critical vulnerability of the alveolar septal barrier in acute lung injury 2, 3, our findings in the rodent models of lung injury and the finding of TRPV4 expression in human alveolar septum lead us to hypothesize that TRPV4 is likely to play a role in the development of acute lung injury and the acute respiratory distress syndrome in humans.
TRP channels and permeability
Ca2+ entry into endothelial cells occurs via activation of selective Ca2+ channels or non-selective cation channels, such as those encoded by the large superfamily of TRP channel proteins. Much of the focus in lung endothelium has been on the TRPC subfamily. Endothelial cells express mRNA and protein for TRP channels, including TRPC1, TRPC3, TRPC4, TRPC6 and TRPC7 14, 36, 37. In rat lung, TRPC1, TRPC3, TRPC4, and TRPC6/7 protein is expressed in endothelium of extra-alveolar vessels. While mRNA for several members of the TRPV subfamily, including TRPV4, are expressed in human pulmonary artery endothelium 36, only a subset of TRP proteins may actually play a physiologic role in regulation of lung endothelial permeability. Two studies have addressed a role for TRPC channels in regulation of permeability in the intact lung. Tiruppathi et al. reported a partial loss of the permeability response to thrombin in TRPC4−/− mice 18 and we recently found that in an aortocaval fistula model of heart failure, endothelial TRPC1 and TRPC4 expression in extra-alveolar vessels is down-regulated and the permeability response to thapsigargin is lost 19. Although Ca2+ entry can occur via receptor-operated TRP channels (TRPC3, TRPC6 and TRPC7) 38, 39, and these channels are indeed expressed in rat lung endothelium, activation of these channels with a diacylglycerol analog had no impact on endothelial permeability in rat lung 19. A role in regulation of lung endothelial permeability has not been explored for TRP proteins outside the canonical TRP family. Aside from thrombin and thapsigargin, we do not know whether the Ca2+ entry-dependent increases in endothelial permeability are due to gating of TRP channels, nor which TRP channels are involved. Our previous work evaluating the impact of EETs, P450 epoxygenase derivatives of arachidonic acid, on endothelial permeability in normal rat lung and that from animals with chronic heart failure 6, 19 supports the notion of heterogeneity in Ca2+-dependent regulation of endothelial permeability in lung. P450 epoxygenases metabolize arachidonic acid to form four regioisomers: 5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET 40. EETs are released from human lung after inflammatory challenge 41, suggesting their potential involvement in acute lung injury. In canine lung, blockade of EET synthesis attenuated the pulmonary edema and hypoxemia resulting from ethclorovynol 42. Further, exogenous 5,6-EET and 14,15-EET increased endothelial permeability in canine and rat lung 6, 27, in Ca2+ entry-dependent manner. The notion that EETs must target Ca2+ permeant channels distinct from TRPC1/TRPC4 is based on the following evidence: 1) the permeability response to both EETs and thapsigargin in rat lung requires Ca2+ entry, 2) the response to 14,15-EET was retained after experimentally-induced heart failure while that to store depletion was lost, and 3) TRPC1 and TRPC4 expression were down-regulated in extra-alveolar vessels from rats with heart failure 6, 19. Our current results support the notion that TRPV4 expressed in lung septal microvascular endothelium plays a critical role in the permeability response to EETs and 4αPDD.
Functional role for TRPV4 channels in the alveolar septal network
TRPV4, a channel originally described as activated by hypotonicity 20, 23, 43, appears to participate in detection of changes in extracellular fluid osmolality 28. While TRPV channels are appreciated for their role in sensory transduction 44–47, non-sensory functions are now recognized 21, 48–50. Work from Nilius and colleagues 25, 26, 51 suggests that TRPV4 likely plays a significant role in endothelial Ca2+ signaling. Both 4αPDD and 5,6-EET activate TRPV4 heterologously expressed in HEK-293 cells as well as endogenous TRPV4 in aortic endothelial cells, promoting Ca2+ entry which was inhibited by ruthenium red 26. Further, EET-induced Ca2+ entry in aortic endothelial cells was diminished in endothelium derived from TRPV4−/− mice 25. Collectively, these data suggested that TRPV4 could play a role in Ca2+ entry-dependent regulation of endothelial permeability. Our results provide a functional role for TRPV4 expressed in the lung alveolar septal network. The TRPV4 agonist 4αPDD increased endothelial permeability in rat lung in a Ca2+ entry-dependent fashion. This response, and the Ca2+ entry-dependent permeability response to 5,6- and 14,15-EET, could be blocked with ruthenium red. Further, the permeability response to 4αPDD observed in TRPV4+/+ mice was lacking in TRPV4−/− littermates.
Transmission EM documented that both 4αPDD and 14,15-EET caused disruption of the alveolar epithelium, in addition to an impact on the septal endothelial barrier, which very likely contributed to the increase in Kf and alveolar flooding induced by these TRPV4 agonists. TRPV4 has previously been demonstrated to be expressed in respiratory epithelial cells 52–54, though its functional role in bronchiolar or alveolar epithelium in the intact lung has not been clarified. Our results suggest that an exploration of the signaling cascade linking Ca2+ entry via TRPV4 in alveolar type I cells to detachment of those cells from the basement membrane would be informative.
In summary, this work provides critical evidence of a functional role for TRPV4 in regulation of barrier integrity in the lung alveolar septal network. While Ca2+ entry via store-operated TRP channels causes gap formation in extra-alveolar vessels, the functional consequences of barrier disruption in this compartment are likely distinct from those resulting from TRPV4-dependent barrier disruption in the septal compartment. Activation of store-operated channels in extra-alveolar endothelium leads to fluid accumulation in perivascular cuffs, although this likely has little impact on alveolar gas exchange. In contrast, disruption of the vulnerable alveolar septal barrier, such as that resulting from Ca2+ influx via TRPV4, leads to alveolar flooding and thus would be predicted to impair gas exchange. Indeed this is a hallmark of acute lung injury. The implication of this work for translational biomedical research is that TRPV4 is likely a novel molecular target for therapeutic intervention in acute lung injury.
Supplementary Material
Acknowledgments
This work has been supported by grants from the NIH grants (HL081851 and MH064702), and a fellowship from the American Heart Association (0315049B). The authors would like to thank Sue Barnes, Freda McDonald, and Doug Drake for their technical support.
References
- 1.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 2.Matthay MA, Zimmerman GA, Esmon C, Bhattacharya J, Coller B, Doerschuk CM, Floros J, Gimbrone MA, Jr, Hoffman E, Hubmayr RD, Leppert M, Matalon S, Munford R, Parsons P, Slutsky AS, Tracey KJ, Ward P, Gail DB, Harabin AL. Future research directions in acute lung injury: summary of a National Heart, Lung, and Blood Institute working group. Am J Respir Crit Care Med. 2003;167:1027–1035. doi: 10.1164/rccm.200208-966WS. [DOI] [PubMed] [Google Scholar]
- 3.Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353:2788–2796. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 4.Moore TM, Chetham PM, Kelly JJ, Stevens T. Signal transduction and regulation of lung endothelial cell permeability. Interaction between calcium and cAMP. Am J Physiol. 1998;275:L203–L222. doi: 10.1152/ajplung.1998.275.2.L203. [DOI] [PubMed] [Google Scholar]
- 5.Tiruppathi C, Minshall RD, Paria BC, Vogel SM, Malik AB. Role of Ca2+ signaling in the regulation of endothelial permeability. Vascul Pharmacol. 2002;39:173–185. doi: 10.1016/s1537-1891(03)00007-7. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez DF, Gjerde EA, Townsley MI. Role of EETs in regulation of endothelial permeability in rat lung. Am J Physiol Lung Cell Mol Physiol. 2004;286:L445–L451. doi: 10.1152/ajplung.00150.2003. [DOI] [PubMed] [Google Scholar]
- 7.Chetham PM, Babal P, Bridges JP, Moore TM, Stevens T. Segmental regulation of pulmonary vascular permeability by store-operated Ca2+ entry. Am J Physiol. 1999;276:L41–L50. doi: 10.1152/ajplung.1999.276.1.L41. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez DF, King JA, Townsley MI. Evaluation of endothelial permeability by a corrosion casting technique. Microsc Microanal. 2004;10 (Suppl 2):200–201. [Google Scholar]
- 9.Gebb S, Stevens T. On lung endothelial cell heterogeneity. Microvasc Res. 2004;68:1–12. doi: 10.1016/j.mvr.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 10.King J, Hamil T, Creighton J, Wu S, Bhat P, McDonald F, Stevens T. Structural and functional characteristics of lung macro- and microvascular endothelial cell phenotypes. Microvasc Res. 2004;67:139–151. doi: 10.1016/j.mvr.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Clapham DE, Runnels LW, Str bing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 12.Freichel M, Philipp S, Cavalie A, Flockerzi V. TRPC4 and TRPC4-deficient mice. Novartis Found Symp. 2004;258:189–199. [PubMed] [Google Scholar]
- 13.Harteneck C, Plant TD, Schultz G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–166. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- 14.Nilius B, Droogmans G, Wondergem R. Transient receptor potential channels in endothelium: solving the calcium entry puzzle? Endothelium. 2003;10:5–15. doi: 10.1080/10623320303356. [DOI] [PubMed] [Google Scholar]
- 15.Vazquez G, Wedel BJ, Aziz O, Trebak M, Putney JW., Jr The mammalian TRPC cation channels. Biochim Biophys Acta. 2004;1742:21–36. doi: 10.1016/j.bbamcr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Brough GH, Wu S, Cioffi D, Moore TM, Li M, Dean N, Stevens T. Contribution of endogenously expressed Trp1 to a Ca2+-selective, store-operated Ca2+ entry pathway. FASEB J. 2001;15:1727–1738. [PubMed] [Google Scholar]
- 17.Cioffi DL, Wu S, Stevens T. On the endothelial cell ISOC. Cell Calcium. 2003;33:323–336. doi: 10.1016/s0143-4160(03)00046-0. [DOI] [PubMed] [Google Scholar]
- 18.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4−/− mice interferes with increase in lung microvascular permeability. Circ Res. 2002;91:70–76. doi: 10.1161/01.res.0000023391.40106.a8. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez DF, King JA, Townsley MI. Resistance to store depletion-induced endothelial injury in rat lung after chronic heart failure. Am J Respir Crit Care Med. 2005;172:1153–1160. doi: 10.1164/rccm.200506-847OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liedtke W. TRPV4 plays an evolutionary conserved role in the transduction of osmotic and mechanical stimuli in live animals. J Physiol. 2005;567:53–58. doi: 10.1113/jphysiol.2005.088963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nilius B, Vriens J, Prenen J, Droogmans G, Voets T. TRPV4 calcium entry channel: a paradigm for gating diversity. Am J Physiol Cell Physiol. 2004;286:C195–C205. doi: 10.1152/ajpcell.00365.2003. [DOI] [PubMed] [Google Scholar]
- 23.Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- 24.Voets T, Prenen J, Vriens J, Watanabe H, Janssens A, Wissenbach U, Bodding M, Droogmans G, Nilius B. Molecular determinants of permeation through the cation channel TRPV4. J Biol Chem. 2002;277:33704–33710. doi: 10.1074/jbc.M204828200. [DOI] [PubMed] [Google Scholar]
- 25.Vriens J, Owsianik G, Fisslthaler B, Suzuki M, Janssens A, Voets T, Morisseau C, Hammock BD, Fleming I, Busse R, Nilius B. Modulation of the Ca2+ permeable cation channel TRPV4 by cytochrome P450 epoxygenases in vascular endothelium. Circ Res. 2005;97:908–915. doi: 10.1161/01.RES.0000187474.47805.30. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- 27.Ivey CL, Stephenson AH, Townsley MI. Involvement of cytochrome P-450 enzyme activity in the control of microvascular permeability in canine lung. Am J Physiol. 1998;275:L756–L763. doi: 10.1152/ajplung.1998.275.4.L756. [DOI] [PubMed] [Google Scholar]
- 28.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci U S A. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weibel ER. Morphometry: sterological theory and practical methods. In: Gil J, editor. Models of lung disease. Microscopy and structural methods. New York: Marcel Dekker, Inc; 1990. pp. 199–252. [Google Scholar]
- 30.Townsley MI, Korthuis RJ, Rippe B, Parker JC, Taylor AE. Validation of double vascular occlusion method for Pc,i in lung and skeletal muscle. J Appl Physiol. 1986;61:127–132. doi: 10.1152/jappl.1986.61.1.127. [DOI] [PubMed] [Google Scholar]
- 31.Townsley MI, Fu Z, Mathieu-Costello O, West JB. Pulmonary microvascular permeability. Responses to high vascular pressure after induction of pacing-induced heart failure in dogs. Circ Res. 1995;77:317–325. doi: 10.1161/01.res.77.2.317. [DOI] [PubMed] [Google Scholar]
- 32.Parker JC, Townsley MI. Evaluation of lung injury in rats and mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L231–L246. doi: 10.1152/ajplung.00049.2003. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- 34.Harder DR, Campbell WB, Roman RJ. Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J Vasc Res. 1995;32:79–92. doi: 10.1159/000159080. [DOI] [PubMed] [Google Scholar]
- 35.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 36.Fantozzi I, Zhang S, Platoshyn O, Remillard CV, Cowling RT, Yuan JX. Hypoxia increases AP-1 binding activity by enhancing capacitative Ca2+ entry in human pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1233–L1245. doi: 10.1152/ajplung.00445.2002. [DOI] [PubMed] [Google Scholar]
- 37.Yao X, Garland CJ. Recent developments in vascular endothelial cell transient receptor potential channels. Circ Res. 2005;97:853–863. doi: 10.1161/01.RES.0000187473.85419.3e. [DOI] [PubMed] [Google Scholar]
- 38.Putney JW, Jr, Trebak M, Vazquez G, Wedel B, Bird GS. Signalling mechanisms for TRPC3 channels. Novartis Found Symp. 2004;258:123–133. [PubMed] [Google Scholar]
- 39.Trebak M, St JB, McKay RR, Birnbaumer L, Putney JW., Jr Signaling mechanism for receptor-activated canonical transient receptor potential 3 (TRPC3) channels. J Biol Chem. 2003;278:16244–16252. doi: 10.1074/jbc.M300544200. [DOI] [PubMed] [Google Scholar]
- 40.Spector AA, Fang X, Snyder GD, Weintraub NL. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 41.Kiss L, Schutte H, Mayer K, Grimm H, Padberg W, Seeger W, Grimminger F. Synthesis of arachidonic acid-derived lipoxygenase and cytochrome P450 products in the intact human lung vasculature. Am J Respir Crit Care Med. 2000;161:1917–1923. doi: 10.1164/ajrccm.161.6.9906058. [DOI] [PubMed] [Google Scholar]
- 42.Stephenson AH, Sprague RS, Weintraub NL, McMurdo L, Lonigro AJ. Inhibition of cytochrome P-450 attenuates hypoxemia of acute lung injury in dogs. Am J Physiol. 1996;270:H1355–H1362. doi: 10.1152/ajpheart.1996.270.4.H1355. [DOI] [PubMed] [Google Scholar]
- 43.Wissenbach U, Bodding M, Freichel M, Flockerzi V. Trp12, a novel Trp related protein from kidney. FEBS Lett. 2000;485:127–134. doi: 10.1016/s0014-5793(00)02212-2. [DOI] [PubMed] [Google Scholar]
- 44.Alessandri-Haber N, Dina OA, Yeh JJ, Parada CA, Reichling DB, Levine JD. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J Neurosci. 2004;24:4444–4452. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci. 2005;25:1304–1310. doi: 10.1523/JNEUROSCI.4745.04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liedtke W, Tobin DM, Bargmann CI, Friedman JM. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2003 November 25;100(Suppl 2):14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- 48.Caterina MJ. Vanilloid receptors take a TRP beyond the sensory afferent. Pain. 2003;105:5–9. doi: 10.1016/s0304-3959(03)00259-8. [DOI] [PubMed] [Google Scholar]
- 49.Nilius B, Watanabe H, Vriens J. The TRPV4 channel: structure-function relationship and promiscuous gating behaviour. Pflugers Arch. 2003;446:298–303. doi: 10.1007/s00424-003-1028-9. [DOI] [PubMed] [Google Scholar]
- 50.O'Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflugers Arch. 2005;451:193–204. doi: 10.1007/s00424-005-1424-4. [DOI] [PubMed] [Google Scholar]
- 51.Vriens J, Watanabe H, Janssens A, Droogmans G, Voets T, Nilius B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc Natl Acad Sci U S A. 2004;101:396–401. doi: 10.1073/pnas.0303329101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delany NS, Hurle M, Facer P, Alnadaf T, Plumpton C, Kinghorn I, See CG, Costigan M, Anand P, Woolf CJ, Crowther D, Sanseau P, Tate SN. Identification and characterization of a novel human vanilloid receptor-like protein, VRL-2. Physiol Genomics. 2001;4:165–174. doi: 10.1152/physiolgenomics.2001.4.3.165. [DOI] [PubMed] [Google Scholar]
- 53.Fernandez-Fernandez JM, Nobles M, Currid A, Vazquez E, Valverde MA. Maxi K+ channel mediates regulatory volume decrease response in a human bronchial epithelial cell line. Am J Physiol Cell Physiol. 2002;283:C1705–C1714. doi: 10.1152/ajpcell.00245.2002. [DOI] [PubMed] [Google Scholar]
- 54.Sidhaye VK, Guler AD, Schweitzer KS, D'Alessio F, Caterina MJ, King LS. Transient receptor potential vanilloid 4 regulates aquaporin-5 abundance under hypotonic conditions. Proc Natl Acad Sci U S A. 2006;103:4747–4752. doi: 10.1073/pnas.0511211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parker JC, Ivey CL, Tucker JA. Gadolinium prevents high airway pressure-induced permeability increases in isolated rat lungs. J Appl Physiol. 1998;84:1113–1118. doi: 10.1152/jappl.1998.84.4.1113. [DOI] [PubMed] [Google Scholar]
- 56.Parker JC, Gillespie MN, Taylor AE, Martin SL. Capillary filtration coefficient, vascular resistance, and compliance in isolated mouse lungs. J Appl Physiol. 1999;87:1421–1427. doi: 10.1152/jappl.1999.87.4.1421. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


