Abstract
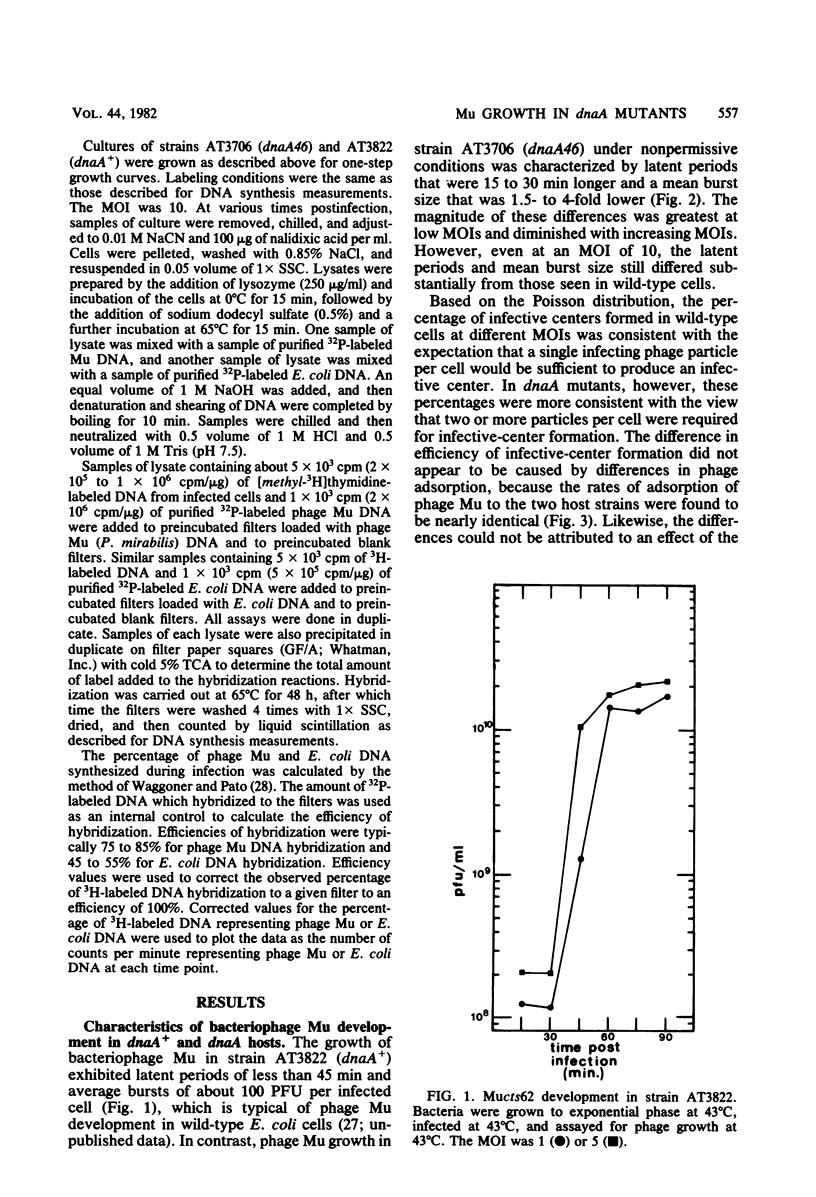
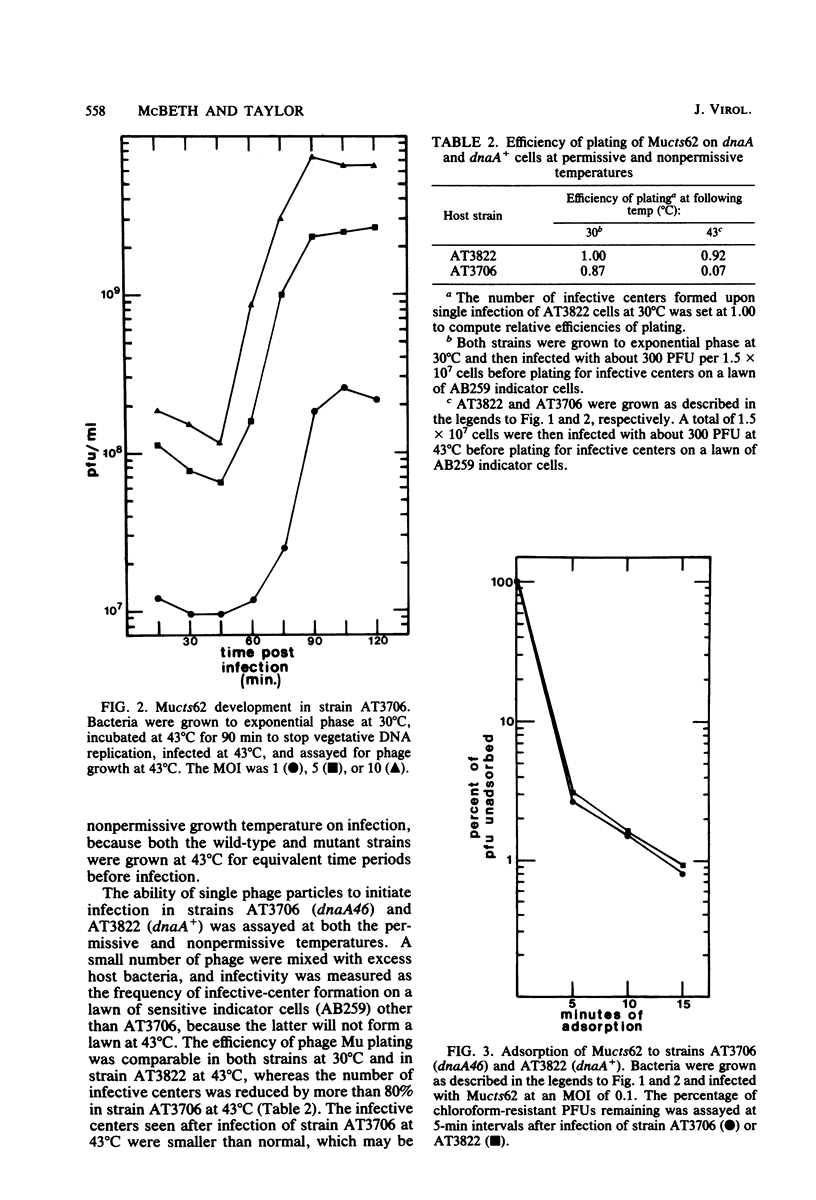
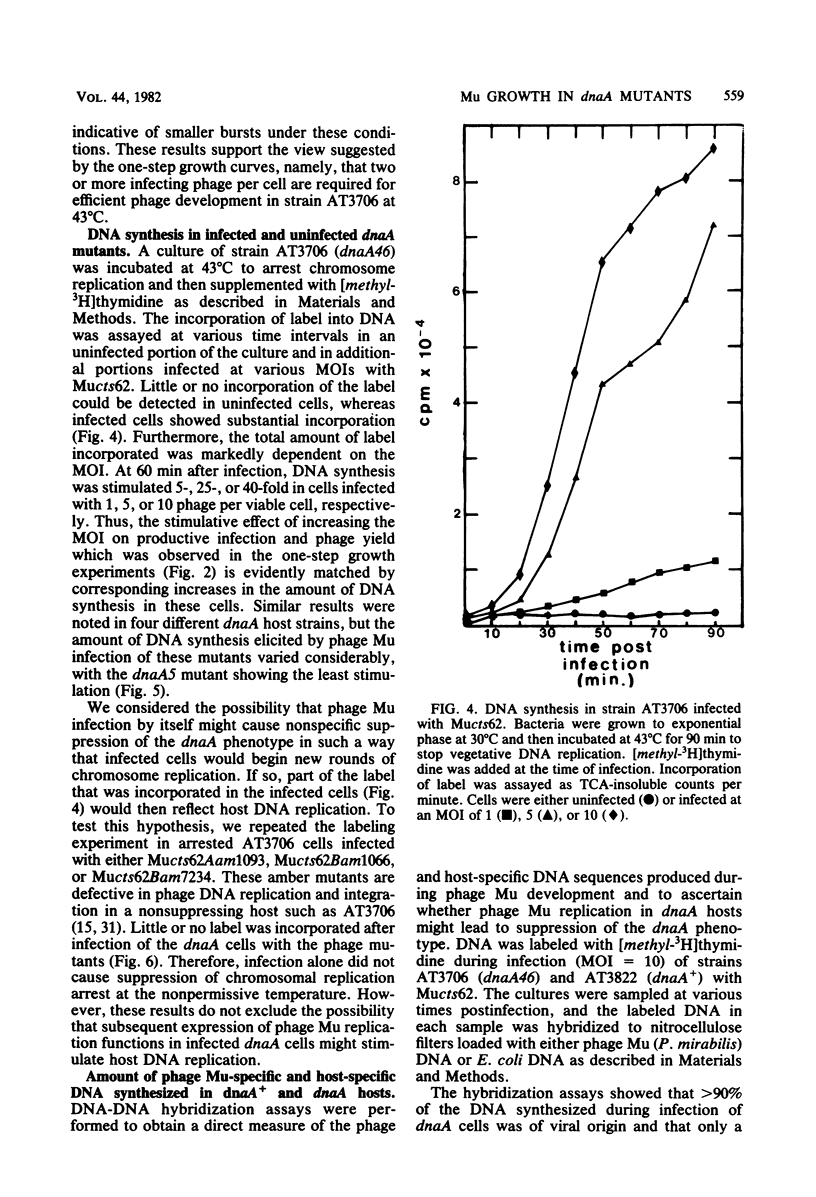
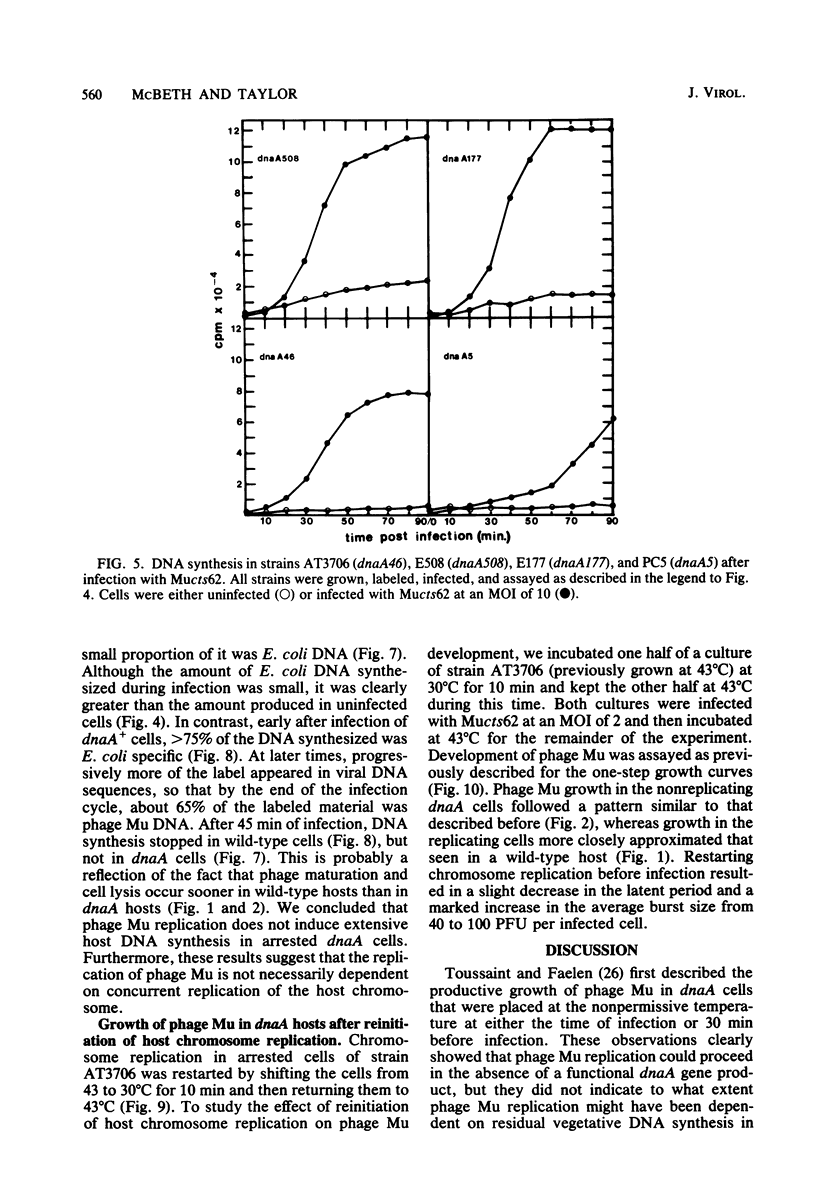
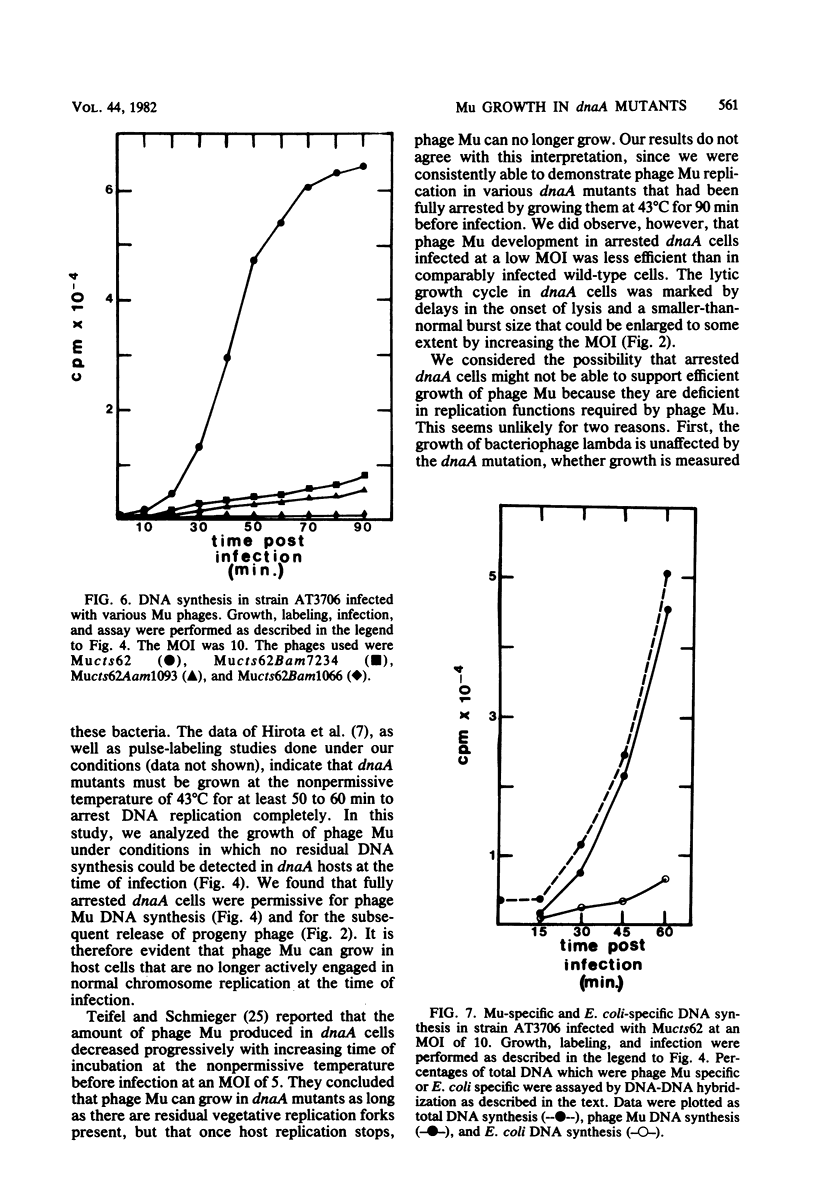
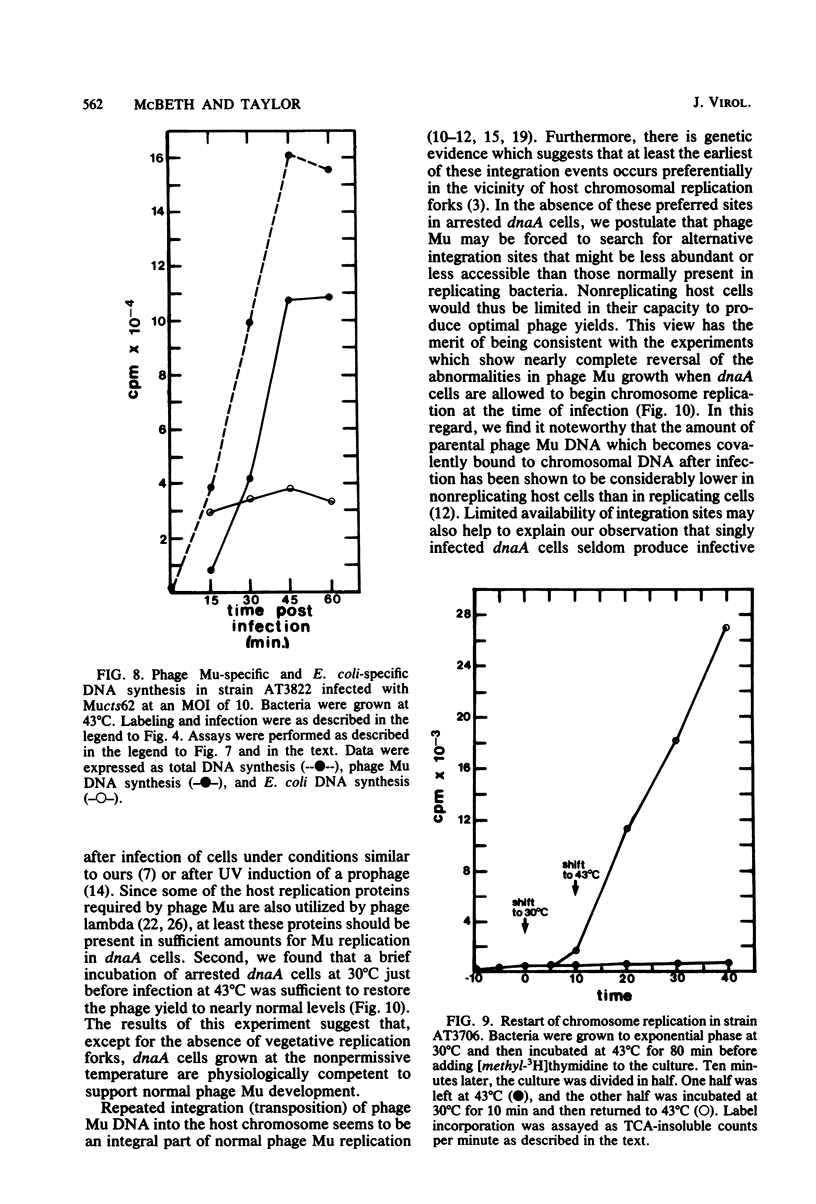
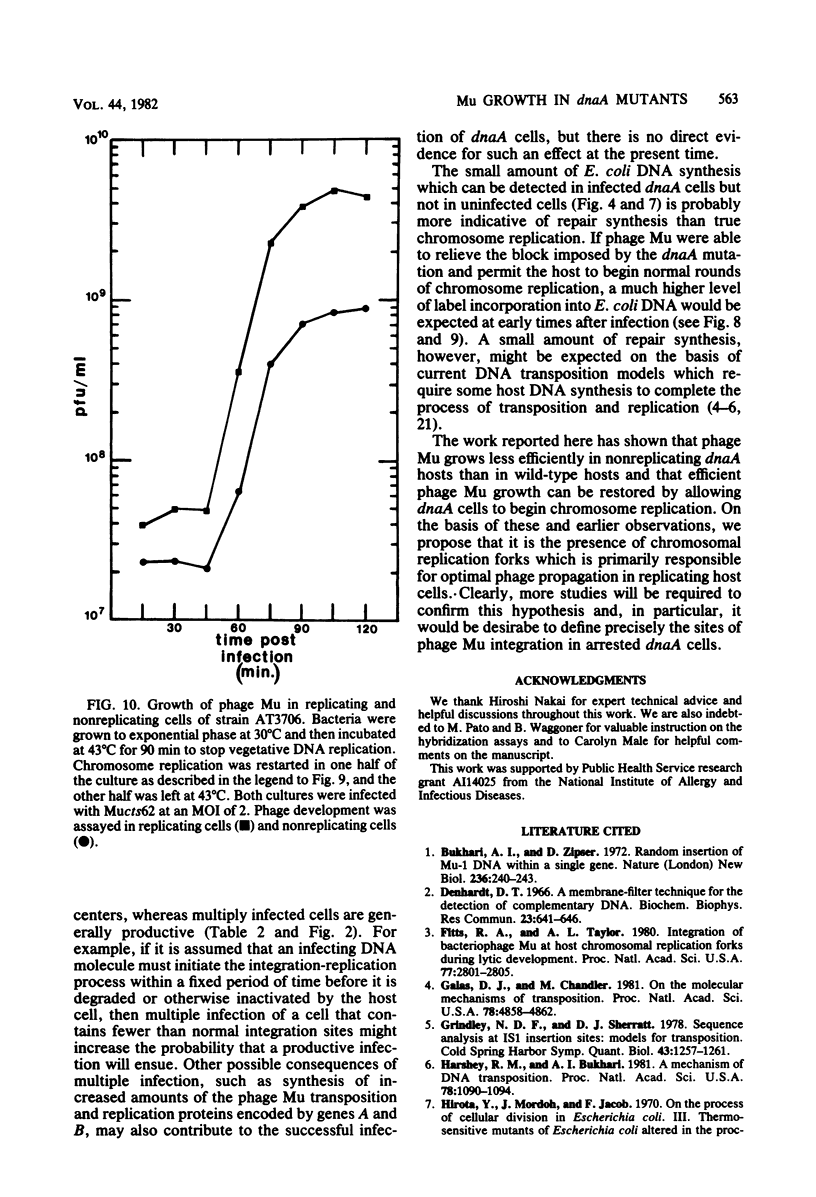
In one-step growth experiments we found that bacteriophage Mu grew less efficiently in nonreplicating dnaA mutants than in dnaA+ strains of Escherichia coli. Phage development in dnaA hosts was characterized by latent periods that were 15 to 30 min longer and an average burst size that was reduced by 1.5- to 4-fold. The differences in phage Mu development in dnaA and dnaA+ strains were most pronounced in cells infected at a low multiplicity and became less pronounced in cells infected at a high multiplicity. Many of these differences could be eliminated by allowing the arrested dnaA cells to restart chromosome replication just before infection. In continuous labeling experiments we found that infected dnaA strains incorporated 5 to 40 times more [methyl-3H]thymidine than did uninfected cells, depending on the multiplicity of infection. DNA-DNA hybridization assays showed that greater than 90% of this label was contained in phage Mu DNA sequences and that only small amounts of the label appeared in E. coli sequences. In contrast, substantial amounts of label were incorporated into both host and viral DNA sequences in infected dnaA+ cells. Although our results indicated that phage Mu development is not absolutely dependent on concurrent host chromosomal DNA replication, they did strongly suggest that host replication is necessary for optimal growth of this phage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Fitts R. A., Taylor A. L. Integration of bacteriophage Mu at host chromosomal replication forks during lytic development. Proc Natl Acad Sci U S A. 1980 May;77(5):2801–2805. doi: 10.1073/pnas.77.5.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Chandler M. On the molecular mechanisms of transposition. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4858–4862. doi: 10.1073/pnas.78.8.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindley N. D., Sherratt D. J. Sequence analysis at IS1 insertion sites: models for transposition. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1257–1261. doi: 10.1101/sqb.1979.043.01.142. [DOI] [PubMed] [Google Scholar]
- Harshey R. M., Bukhari A. I. A mechanism of DNA transposition. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1090–1094. doi: 10.1073/pnas.78.2.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohiyama M. DNA synthesis in temperature sensitive mutants of Escherichia coli. Cold Spring Harb Symp Quant Biol. 1968;33:317–324. doi: 10.1101/sqb.1968.033.01.036. [DOI] [PubMed] [Google Scholar]
- Kuempel P. L. Deoxyribonucleic acid-deoxyribonucleic acid hybridization assay for replication origin deoxyribonucleic acid of Escherichia coli. J Bacteriol. 1972 Jun;110(3):917–925. doi: 10.1128/jb.110.3.917-925.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungquist E., Bukhari A. I. Behavior of bacteriophage Mu DNA upon infecton of Escherichia coli cells. J Mol Biol. 1979 Sep 25;133(3):339–357. doi: 10.1016/0022-2836(79)90397-8. [DOI] [PubMed] [Google Scholar]
- Ljungquist E., Bukhari A. I. State of prophage Mu DNA upon induction. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3143–3147. doi: 10.1073/pnas.74.8.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungquist E., Khatoon H., DuBow M., Ambrosio L., De Bruijn F., Bukhari A. I. Integration of bacteriophage mu DNA. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1151–1158. doi: 10.1101/sqb.1979.043.01.130. [DOI] [PubMed] [Google Scholar]
- Monk M., Gross J. Induction of prophage lambda in a mutant of E. coli K12 defective in initiation of DNA replication at high temperature. Mol Gen Genet. 1971;110(4):299–306. doi: 10.1007/BF00438272. [DOI] [PubMed] [Google Scholar]
- Pato M. L., Waggoner B. T. Cellular location of Mu DNA replicas. J Virol. 1981 Apr;38(1):249–255. doi: 10.1128/jvi.38.1.249-255.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaki T., Bukhari A. I. Events following prophage Mu induction. J Bacteriol. 1975 May;122(2):437–442. doi: 10.1128/jb.122.2.437-442.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro J. A. Molecular model for the transposition and replication of bacteriophage Mu and other transposable elements. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1933–1937. doi: 10.1073/pnas.76.4.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalka A. M. DNA replication--bacteriophage lambda. Curr Top Microbiol Immunol. 1977;78:201–237. [PubMed] [Google Scholar]
- Stubbs J. D., Hall B. D. Level of tryptophan messenger RNA in Escherichia coli. J Mol Biol. 1968 Oct 28;37(2):289–302. doi: 10.1016/0022-2836(68)90268-4. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L. BACTERIOPHAGE-INDUCED MUTATION IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1963 Dec;50:1043–1051. doi: 10.1073/pnas.50.6.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teifel J., Schmieger H. The influence of host DNA replication on the formation of infectious and transducing Mu-particles. Mol Gen Genet. 1981;184(2):308–311. doi: 10.1007/BF00272922. [DOI] [PubMed] [Google Scholar]
- Waggoner B. T., González N. S., Taylor A. L. Isolation of heterogeneous circular DNA from induced lysogens of bacteriophage Mu-1. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1255–1259. doi: 10.1073/pnas.71.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner B. T., Pato M. L. Early events in the replication of Mu prophage DNA. J Virol. 1978 Sep;27(3):587–594. doi: 10.1128/jvi.27.3.587-594.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggoner B., Pato M., Toussaint A., Faelen M. Replication of mini-Mu prophage DNA. Virology. 1981 Aug;113(1):379–387. doi: 10.1016/0042-6822(81)90163-x. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- Wijffelman C., Lotterman B. Kinetics of Mu DNA synthesis. Mol Gen Genet. 1977 Mar 7;151(2):169–174. doi: 10.1007/BF00338691. [DOI] [PubMed] [Google Scholar]



