Abstract
We report herein that expression of α2β1 integrin increased human erythroleukemia K562 transfectant (KX2C2) cell movement after extravasation into liver parenchyma. In contrast, a previous study demonstrated that α2β1 expression conferred a stationary phenotype to human rhabdomyosarcoma RD transfectant (RDX2C2) cells after extravasation into the liver. We therefore assessed the adhesive and migratory function of α2β1 on KX2C2 and RDX2C2 cells using a α2β1-specific stimulatory monoclonal antibody (mAb), JBS2, and a blocking mAb, BHA2.1. In comparison with RDX2C2 cells, KX2C2 were only weakly adherent to collagen and laminin. JBS2 stimulated α2β1-mediated interaction of KX2C2 cells with both collagen and laminin resulting in increases in cell movement on both matrix proteins. In the presence of Mn2+, JBS2-stimulated adhesion on collagen beyond an optimal level for cell movement. In comparison, an increase in RDX2C2 cell movement on collagen required a reduction in its adhesive strength provided by the blocking mAb BHA2.1. Consistent with these in vitro findings, in vivo videomicroscopy revealed that α2β1-mediated postextravasation cell movement of KX2C2 cells in the liver tissue could also be stimulated by JBS2. Thus, results demonstrate that α2β1 expression can modulate postextravasation cell movement by conferring either a stationary or motile phenotype to different cell types. These findings may be related to the differing metastatic activities of different tumor cell types.
INTRODUCTION
It is well established that β1 integrins represent the major receptors for providing the functional linkage between extracellular matrix (ECM) proteins and cytoskeletal components (for review, Hynes, 1992). The expression of α2β1 integrin as receptors for collagen and laminin has been associated with the morphogenesis of mammary epithelial cells (Berdichevsky et al., 1992; Keely et al., 1995a,b) and differentiation of the human erythroleukemia cell line K562 (Burger et al., 1992). In comparison, α2β1 expression was down-regulated on keratinocytes undergoing terminal differentiation (Adams and Watt, 1990). In addition, α2β1 has also been associated with the metastatic activities of tumor cells. Interestingly, there is both a direct correlation (Dedhar and Saulnier, 1990; Chan et al., 1991; Klein et al., 1991; Mortarini et al., 1991; Danen et al., 1993; Chen et al., 1994; Santala et al., 1994) and an inverse correlation (Pignatelli et al., 1990, 1991; Zutter, et al., 1990, 1995) of α2β1 expression with tumor metastasis. The exact mechanisms whereby α2β1 enhances or, in some cases, reduces the metastatic activities of tumor cells are still unclear. One possibility is that α2β1 may confer distinct cell functions among the tumor cells. The present study focuses on α2β1 interaction with ECM proteins and its in vivo effect on cell movement.
Studies in recent years have demonstrated three distinct modes of ligand binding for α2β1: no ligand-binding activity, binding collagen but not laminin, and binding both collagen and laminin. Integrin α2β1 on human endothelial cells and melanoma cell line LOX bind both collagen and laminin; whereas, on platelets and the fibroblast cell line MRC-5, α2β1 binds only collagen but not laminin (Elices and Hemler, 1989; Languino et al., 1989; Kirchhofer et al., 1990; Lotz et al., 1990). The observed cell type dependence has also been demonstrated with transfectant cells. Expression of α2β1 enhanced adhesion of human rhabdomyosarcoma RD transfectant cells (RDX2C2) to collagen and laminin (Chan et al., 1991, 1992). Interestingly, α2β1 on human erythroleukemia K562 transfectant cells (KX2C2) provided no detectable adhesion to collagen or laminin in static cell adhesion assays (Elices and Hemler, 1989). Certain β1 subunit-specific monoclonal antibodies (mAbs) stimulate ligand-binding activities. mAbs 8A2 and Ts2/16 have been shown to stimulate high-avidity ligand binding of β1 integrins on eosinophils, T and B lymphocytes, myelomonocytic cell line U937, and melanoma A375 (Arroyo et al., 1992; Kovach et al., 1992; van de Wiel-van Kemenade et al., 1992; Arroyo et al., 1993; Kuijpers et al., 1993; Sanchez-Mateos et al., 1993; Faull et al., 1994). In the presence of Ts2/16, the α2β1 on K562 cells was stimulated to bind both collagen and laminin (Chan and Hemler, 1993). Thus, α2β1 can undergo functional transition with respect to their binding properties for collagen and laminin. However, at present, a direct induction of α2β1 to binding collagen, but not laminin, has not been demonstrated.
It is well established that β1 integrins have major roles in cell movement involving the recruitment of cytoskeletal components such as talin and α-actinin, as well as activation of the focal adhesion kinase (for review, Lauffenburger and Horwitz, 1996). The interaction between integrin β1 subunit and cytoskeletal proteins may be regulated by the cytoplasmic domains of α subunits (Chan et al., 1992; Bauer et al., 1993; Kawaguchi et al., 1994; Briesewitz et al., 1995; Kassner et al., 1995). Therefore, β1 integrins are critical in conferring a stationary or motile phenotype to cells on ECM substrates. The ability of α2β1 to confer cell movement appears to be cell type dependent. Integrin α2β1 mediated migration of human melanoma SK-Mel-2, fibrosarcoma HT1080, and keratinocyte HaCaT cells on collagen but not laminin (Yamada et al., 1990; Scharffetter-Kochanek et al., 1992; Knutson et al., 1996). In comparison, α2β1 mediated migration of bladder carcinoma 5637 and melanoma EP, AN, and RU cells on both the matrix proteins (Yamada et al., 1990; Etoh et al., 1993). A biphasic dependence of cell motility on fibronectin in vitro has been reported (Wu et al., 1994; Palecek et al., 1997). Furthermore, the inhibiting and enhancing effects of the soluble integrin-binding inhibitor, echistatin, can be predicted from its effect on adhesion (Wu et al., 1994).
At present, little is known about α2β1 function in cell movement in vivo. In a recent study using in vivo videomicroscopy (IVVM), the expression of α2β1 resulted in an arrest of RD cells (RDX2C2) after extravasation and prevented RD cell migration to the liver subcapsular region (Hangan et al., 1996). This result may be related to a α2β1-mediated increase in the adhesion of RD cells to matrix proteins in the basement membrane; whether α2β1 can confer a migratory function in vivo has not been previously determined. In contrast to RDX2C2 (Hangan et al., 1996), we report herein that K562 transfectant cells expressing α2β1 (KX2C2) were more effective in migration to the subcapsular region of the liver, in comparison with a control transfectant expressing a nonfunctional I-domain deletion variant of α2β1 (KX2C2[I−]). Thus, the functional relationship between adhesion and random cell movement upon stimulation of the receptor function of α2β1 were examined, and the effect of this relationship on the modulation of cell movement in the liver by α2β1 was then characterized using in vivo videomicroscopy.
MATERIALS AND METHODS
Antibodies and Matrix Proteins
mAbs used in this study for human α2β1 were BHA2.1 [blocking (Hangan et al., 1996)], HAS4 (Tenchini et al., 1993), and JBS2 [stimulatory, (Stupack et al., 1994)] and for human β1-subunit was Ts2/16 (Arroyo et al., 1992). mAb P3 (IgG1, κ; Kearney et al., 1979) or normal mouse immunoglobulin (NmIg) was used as control where indicated. Human fibronectin, collagen type I, and mouse Engelbreth-Holm-Swarm (EHS) laminin were obtained from Life Technologies (Gaithersburg, MD).
Flow Cytometry and Immunoprecipitation
Flow cytometry for the determination of β1 integrin expression was carried out by indirect immunostaining with the F(ab)′2 fragment of fluorescein-conjugated antibody (Cedarlane, Oakville, ON). All antibodies were used at predetermined saturating concentrations. Results were analyzed and compared with isotype-matched control mAbs by using a Becton Dickinson FACScan as described (Hangan et al., 1996). Immunoprecipitation of β1 integrins was carried out according to established procedures (Chan and Hemler, 1993). Briefly, cells were labeled with 125I using lactoperoxidase and lysed in 0.5% Nonidet P-40 in the presence of protease inhibitors (aprotinin at 1.0 unit/ml, leupeptin at 0.1 M, and phenylmethylsulfonyl fluoride at 2 mM). Precleared cell lysate was then used for immunoprecipitation by using the specific β1 integrin mAb. The immune complex was isolated using anti-mouse agarose (Sigma, St. Louis, MO). Bound materials were eluted by SDS-PAGE sample buffer under nonreducing condition. Eluted materials from an equivalent of 1 × 106 cells were then analyzed by SDS-PAGE (6% gel). Results were visualized by autoradiography.
Cell Culture and Transfection
Human fibrosarcoma HT1080 and erythroleukemia K562 cell lines were obtained from American Type Culture Collection (Rockville, MD). Transfectant K562 and rhabdomyosarcoma RD cells expressing α2β1 (KX2C2 and RDX2C2) and mock-transfected K562 cells (KpF) were described in the previous studies (Chan et al., 1991; Chan and Hemler, 1993). In this study, K562 transfectant cells expressing the nonfunctional α2β1 variant lacking the α2 I-domain (KX2C2[I−]) were prepared as control of KX2C2. The construction of the I-domain deletion variant of α2 cDNA (X2C2[I−]) has been described in detail previously (Hangan et al., 1996). Briefly, the cDNA construction involved the creation of SpeI restriction enzyme sites at both the 5′ and 3′ ends of α2 I-domain by site-directed mutagenesis. The mutant α2 cDNA was digested by SpeI for the removal of I-domain. The I-domain deletion variant of α2 (X2C2[I−]) was then prepared by religation of the remaining flanking cDNA fragments and cloning into the expression vector pFneo (Ohashi et al., 1985). For preparation of KX2C2(I−), cDNA of X2C2(I−) was transfected into K562 cells using the Lipofectin reagent (Life Technologies) as described previously (Chan and Hemler, 1993). The expression of the I-domain deletion variant of α2β1 on KX2C2(I−) was then characterized by flow cytometry and immunoprecipitation using α2β1 specific mAbs, BHA2.1 and HAS4, as described previously in the preparation of RDX2C2(I−) cells (Hangan et al., 1996). Thus, as described for RDX2C2(I−), HAS4 but not BHA2.1 immunostained and immunoprecipitated the I-domain deletion variant of α2β1 on KX2C2(I−) cells; in comparison, both mAbs were able to stain and immunoprecipitate the complete α2β1 on K562 transfectant cells (KX2C2; our unpublished observation).
Adhesion Assays and Migration Assays
Cell adhesion assays using fluorescence-labeled cells were carried out as described (Chan et al., 1992; Kawaguchi and Hemler, 1993). Briefly, cells were labeled with (2′,7′)-bis(carboxyethyl)-5,6-carboxyfluorescein (BCECF; Sigma) and 5 × 104 labeled cells per 100 μl were allowed to adhere to matrix-coated wells for 45 min at 37°C. Bound fluorescence was measured by a Fluorescence Concentrator Analyser (IDEXX Lab., Westbrook, ME), after the removal of nonadherent cells by gentle washing. For a determination of the adhesive strength, nonadherent cells were removed by centrifugation of the inverted adhesion plate at defined centrifugation g force as described (Wu et al., 1994). The level of bound fluorescence was obtained after subtraction of the fluorescence using bovine serum albumin-coated wells as background. Cell adhesion was expressed as number of bound cells per unit area, based on the fluorescence of 5 × 104 labeled cells, after a subtraction of background fluorescence. Random cell migration assays were carried out according to established procedures using the 48-well chemotaxis chamber (Neuro Probe, Cabin John, MD; Hauzenberger et al., 1994; Kassner et al., 1995). Briefly, polyvinylpyrrolidone-free polycarbonate filters of 10-μm pore size were coated on both sides with matrix proteins at 10 μg/ml in 0.1 M NaHCO3 overnight. The filters were then washed with PBS, air-dried, and assembled with the bottom wells filled with 30 μl of RPMI 1640 culture medium containing 1% bovine serum albumin. Cells were added to top chambers at 2 × 104 cells in 50 μl per well and allowed to migrate into the filter for 6 h at 37°C. Cells that remained at the upper side of the filter were removed by mechanical scraping. Cells migrated into the filter were then stained with Harris’ hematoxylin. The number of migrated cells was obtained as average of cell counts from five random fields from each of the triplicate wells by light microscopy (400×, high-power field) using a gridded objective. All experiments have been repeated for a minimum of three times.
In Vivo Videomicroscopy
The characterization of in vivo cell movement was done using IVVM. This technique involves real-time observation of cells in the vicinity of the microcirculation of undissected organs in living mice (Morris et al., 1994; Hangan et al., 1996). It permits the quantification of 1) intravascular cells whose presence within blood vessels is made apparent by alterations in the blood flow pattern and 2) extravasation of cells and their migration from the liver sinusoids to the avascular subcapsular region. The movement of cells can be observed over a period of hours rather than as a “snapshot” of time. In addition, “optical slicing” allows a three-dimensional picture of the position of cells in relation to surrounding tissue structures. For this technique cells were washed with Opti-MEM medium (Life Technologies), and 7.5 ml of a 1:50 dilution of microspheres (Fluoresbrite carboxylated beads, 0.059–0.067 μm, YG; Polysciences, Warrington, PA) in Opti-MEM was added. The cells were then incubated for 1 h at 37°C with occasional rocking and the cells spontaneously internalized the microspheres. This labeling procedure does not affect the relative plating efficiency or in vivo behavior of the cells (Morris et al., 1994). Six- to 8-wk-old female nu/nu mice (Harlan Sprague Dawley, Indianapolis, IN) were anesthesized with Ketamine (6.7 mg/100 g of body weight) and Xylazine (0.67 mg/100 g of body weight) and the microsphere-labeled cells (3 × 105 cells per mouse) were injected into a mesenteric vein. Mice were given an analgesic (Temgesic, 0.01 mg/100 g of body weight) during their recovery. For a comparison of cell movement between K562 cells expressing the complete α2β1 or α2β1 variant lacking the I-domain, mice were analyzed by IVVM at 24 h after cell injection. At designated times, the injected mice were anesthetized with 6 mg of sodium pentobarbitol/100 g of body weight, and body temperature was kept at 37°C with a heat lamp and adjustable power supply. To examine the liver microcirculation, an incision was made along the midline of the abdomen and transversely beneath the rib cage to expose the intestine and liver. The liver was positioned so that the border of a lobe was placed on a no. 1 coverglass mounted on the viewing platform of an inverted microscope (Zeiss Axiovert 135, Empix Imaging, Mississauga, Ontario) equipped with epifluorescence illumination (excitation wavelength, 450–490 nm) and a fiberoptic light guide positioned at an oblique angle for high-contrast transillumination of the microcirculation. The microcirculation and location of fluorescence-labeled cells were recorded on SVHS videotapes with a videocamera (Hamamatsu C2400, Empix Imaging). For some experiments, mice were injected (intravenously, i.v.) with 100 μg of JBS2, BHA2.1, and/or control mAb P3 12 h after cell injection, the predetermined time when all detectable KX2C2 cells completed extravasation in the liver. Mice were then analyzed by IVVM 3 h after antibody injection. A minimum of 50 cells per mouse and three mice per experimental group was used. Results were compared using Students’ t test with a level of p < 0.05 regarded as statistically significant.
RESULTS
Integrin α2β1 Enhanced K562 Transfectant Cell Movement In Vivo
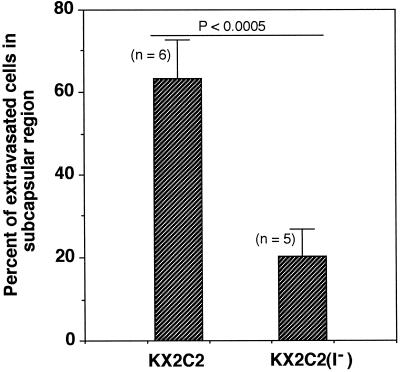
In the previous study (Hangan et al., 1996), we demonstrated by IVVM that expression of α2β1 resulted in an arrest of human rhabdomyosarcoma RD transfectant cells (RDX2C2) immediately after extravasation and prevented their migration to the subcapsular region of the liver. In contrast to its ability to enhance RD cell adhesion to collagen and laminin in vitro, α2β1 lacked detectable ligand-binding function in a static adhesion assay when expressed on K562 transfectant cells (KX2C2; Chan and Hemler, 1993; Elices and Hemler, 1989). It is therefore likely that KX2C2 would have different in vivo effects on cell movement than RDX2C2 cells. To further explore this idea, we used IVVM to determine the effect of α2β1 expression on K562 cell movement in vivo. Specifically, we determined the percentage of extravasated KX2C2 cells that reached the liver subcapsular region at 24 h after cell injection. The K562 transfectant expressing the nonfunctional α2β1 variant lacking the α2 I-domain (KX2C2[I−]) was used as control. As shown in Figure 1, about 60% of KX2C2 reached the liver subcapsular region at 24 h after cell injection. In comparison, only about 20% of KX2C2(I−) cells were able to migrate to the subcapsular region. Therefore, in contrast to RDX2C2, which became arrested after extravasation and lacked the ability to move to the liver subcapsular region for up to 8 d (Hangan et al., 1996), results from the present study clearly suggest a functional involvement of α2β1 in KX2C2 cell movement in vivo.
Figure 1.
α2β1-mediated migration of K562 transfectant cells to the liver surface. K562 transfectant cells expressing α2β1 (KX2C2) or α2β1 variant lacking the α2 I-domain (KX2C2[I−]) were compared for their ability to reach the subcapsular region of the liver. In vivo videomicroscopy was carried out at 24 h after injection of 3 × 105 cells into nu/nu mice via a mesenteric vein. Results are presented as percentage of extravasated cells at the liver subcapsular region.
JBS2 Stimulated α2β1-mediated Adhesion to Collagen but Not Laminin of K562 Cells
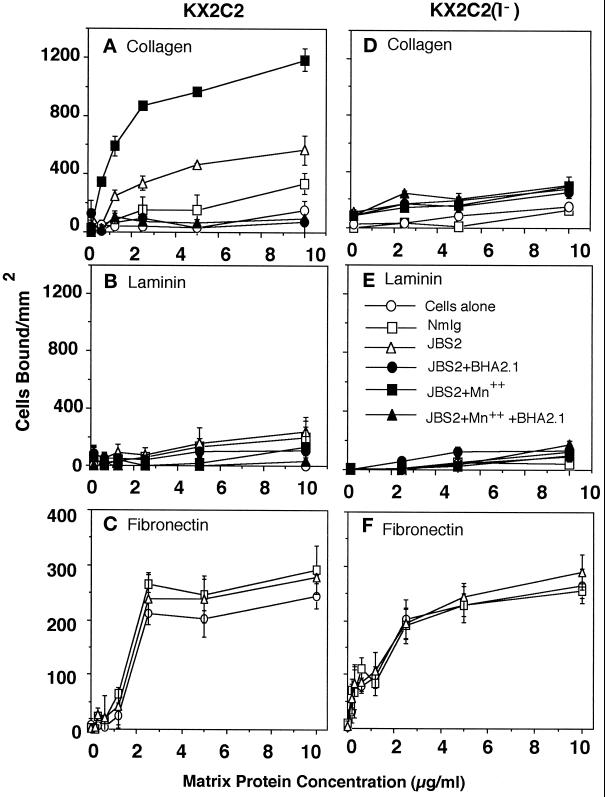
To determine the functional role of α2β1 in providing cell movement, we used stimulatory and inhibitory mAbs in combination with the KX2C2 and KX2C2(I−) cell lines. The interaction of α2β1 integrin with collagen and laminin was further characterized by stimulation of its receptor function. mAb Ts2/16 specific for β1 subunit has been previously shown to stimulate β1 integrin function including the endogenously expressed α5β1 (Chan and Hemler, 1993). In the present study, α2β1-specific mAb JBS2 was used because JBS2 is known to stimulate α2β1 binding of collagen; its effect on adhesion to laminin has not been determined (Stupack et al., 1994; Chou et al., 1996). As shown in Figure 2, A and B, JBS2 (20 μg/ml) stimulated adhesion of KX2C2 to only collagen (p < 0.05) but not laminin. When in combination with 10 mM Mn2+, adhesion to collagen was further stimulated; no adhesion to laminin was detectable under these conditions. Although there was an apparent slight increase in KX2C2 adhesion when in the presence of NmIg, results from other experiments revealed no significant difference in comparison with adhesion using cells alone; these findings were also seen in the previous studies (Chan and Hemler, 1993). Results therefore demonstrated progressive activation of α2β1 adhesive function using JBS2 and JBS2 in combination with Mn2+. The blocking α2β1 mAb BHA2.1 abolished the stimulated adhesion by JBS2, with or without Mn2+. In comparison, KX2C2(I−) did not bind collagen or laminin either constitutively or when stimulated by JBS2 or JBS2 in the presence of Mn2+ (Figure 2, D and E). This observation is consistent with studies demonstrating a critical role of I-domains in the ligand-binding properties of integrins (Diamond et al., 1993; Kamata et al., 1994; Lee et al., 1995). The mock-transfectant KpF lacking α2β1 expression yielded results similar to KX2C2(I−) cells (our unpublished observation). Adhesion of all three transfectants to fibronectin were comparable, and JBS2 did not stimulate increased adhesion to fibronectin (Figure 2, C and F). However, JBS2 did stimulate adhesion of KX2C2 pretreated with cycloheximide (10 μM), indicating that the effect of JBS2 on cell adhesion to collagen did not involve an induction of de novo protein synthesis (our unpublished observation).
Figure 2.
Effect of stimulatory mAb JBS2 on the adhesion of KX2C2 transfectant cells to collagen, laminin, and fibronectin. Adhesion of K562 transfectant cells, KX2C2 (A–C) and KX2C2(I−) (D–F) to collagen (A and D), laminin (B and E), and fibronectin (C and F) was measured in the presence of stimulatory mAb JBS2 (20 μg/ml) alone or in combination with the blocking mAb BHA2.1 (5 μg/ml), in the presence or absence of 10 mM Mn2+. Results were compared with adhesion in the presence of cells alone or Nmlg.
Integrin α2β1 Can Mediate K562 Cell Migration on Collagen and Laminin
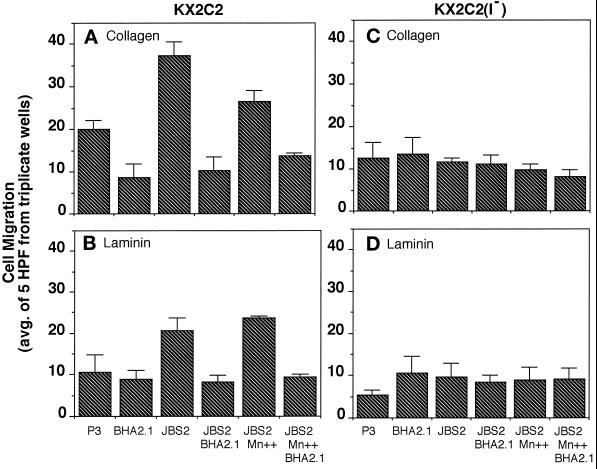
It has been suggested that integrins mediate cell migration at optimal levels of interaction with ECM proteins (Grzesiak et al., 1992; DiMilla et al., 1993; Keely et al., 1995a). To determine the role of α2β1 in random cell movement upon increases in its adhesive function, migration of KX2C2 cells on collagen and laminin was examined. KX2C2(I−) cells expressing α2β1 lacking α2 I-domain were used as control. Cell migration was estimated by the average of cell counts from five random high-power fields (400×) of ECM-coated filters under light microscopy. Results presented are the average (±SD) of triplicate wells from a representative experiment that has been repeated for a minimum of three times.
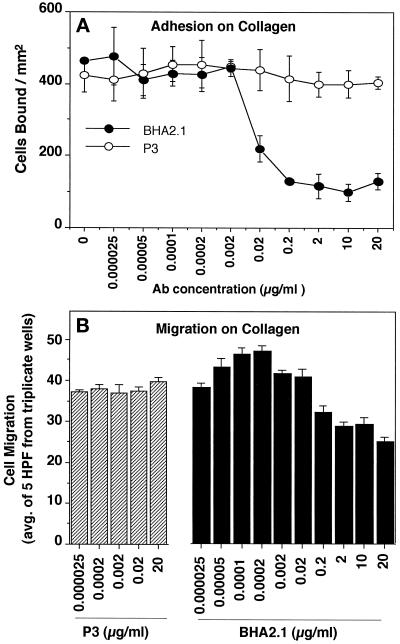
In the presence of normal mouse mAb P3, KX2C2 cells were significantly more migratory on collagen than KX2C2(I−) cells, which produce a nonfunctional α2β1 molecule (p < 0.01; Figure 3, A and C). The blocking mAb BHA2.1 at 20 μg/ml abolished the difference in their migratory activities on collagen. Therefore, although expression of α2β1 did not result in stable adhesion of KX2C2, as detected by static cell adhesion assays, it interacted with collagen and allowed increased KX2C2 cell movement. JBS2, which stimulated adhesion to collagen, further stimulated KX2C2 migration on this matrix protein (p < 0.001; Figure 3A). Whereas adhesion to collagen was further increased by the combination of JBS2 and 10 mM Mn2+, cell migration was decreased (p < 0.05). Blocking mAb BHA2.1 at 20 μg/ml inhibited migration in the presence of P3, JBS2, or JBS2 in combination with Mn2+, which indicated these effects were specifically due to α2β1 expression. KX2C2(I−), expressing the I-domain deletion variant of α2β1, exhibited only a basal level of migration after treatment of these cells with P3, JBS2 with or without Mn2+, or BHA2.1. Results are therefore consistent with the biphasic relationship between cell adhesion and migration (Grzesiak et al., 1992; Wu et al., 1994; Palecek et al., 1997). Thus, up to a point, there is a progressive increase in migratory function with cell adhesion; however, combined stimulation by JBS2 and Mn2+ may go beyond the optimal level of adhesion for migratory function.
Figure 3.
α2β1-mediated K562 transfectant cell migration on collagen and laminin upon stimulation by JBS2. Migration of KX2C2 (A and B) and KX2C2(I−) (C and D) transfectant cells were carried out on polycarbonate filters (10-μm pore size) coated with collagen (A and C) or laminin (B and D) at 10 μg/ml in the presence of α2β1 stimulatory mAb JBS2 or in combination with 10 mM Mn2+. The role of α2β1 was determined using blocking mAb BHA2.1, with the isotype matched mAb P3 as control. All mAbs were used at 20 μg/ml. Values were enumerated from the average (±SD) of cell counts from five random high-power fields (400×) from a minimum of three independent experiments.
As shown in Figure 3B, both KX2C2 and KX2C2(I−) migrated on laminin to similar extents in the presence of P3 or the blocking mAb BHA2.1. Therefore, in contrast to what was observed on collagen, α2β1 did not have a constitutive migratory function on laminin. Although JBS2 did not stimulate detectable adhesion of KX2C2 to laminin (Figure 2B), it stimulated migration on laminin (p < 0.001; Figure 3B). While JBS2 stimulation in the presence of 10 mM Mn2+ resulted in a decrease in the migration of KX2C2 on collagen, Mn2+ had no effect on the stimulated cell movement on laminin. BHA2.1 abolished the migration stimulated by JBS2 alone or in combination with Mn2+. The basal levels of KX2C2(I−) cell movement on collagen and laminin were not affected by JBS2 alone, JBS2 in combination with Mn2+, or with blocking mAb BHA2.1 (Figure 3, C and D).
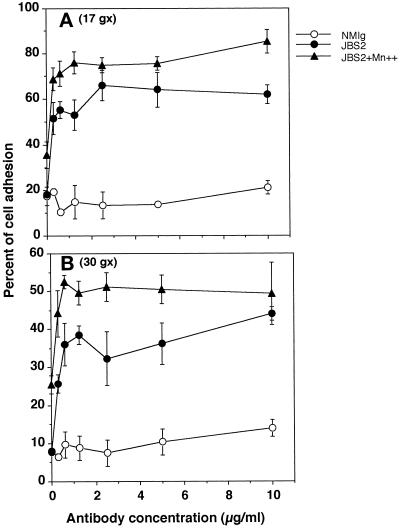
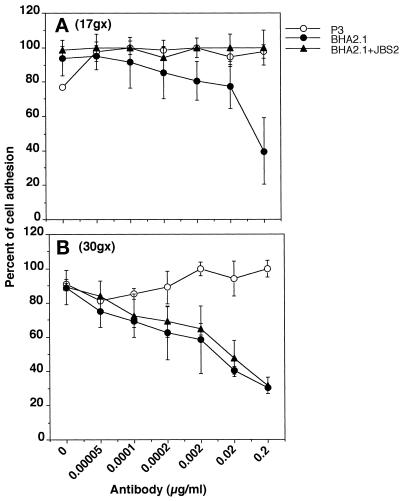
To determine whether the observed modulation of KX2C2 cell adhesion and migration on collagen (Figures 2 and 3) was due to an increase in the adhesive strength by JBS2 stimulation, adherent cells were removed at defined centrifugation force (g; Figure 4). At both 17 × g and 30 × g, the presence of JBS2 increased the percentage of KX2C2 cells adherent on collagen, in comparison with control mAb. The percentage of adherent cells increased further when Mn2+ was added. For both JBS2-treated and JBS2- plus Mn2+-treated cells, more cells remained adherent at 17 × g than at 30 × g.
Figure 4.
Determination of adhesive strength of KX2C2 cells bound onto collagen. KX2C2 cells were labeled with BCECF fluorescence label, treated with various concentrations of NmIg, JBS2, or JBS2 with Mn2+ at 20 μg/ml, and allowed to adhere to collagen-coated wells for 45 min at 37°C. Nonadherent cells were removed by centrifugation at 17 × g (A) or 30 × g (B). Adherent cells were expressed as the percentage of bound cells based on the fluorescence of 5 × 104 labeled cells after a subtraction of background fluorescence.
Modulation of α2β1 Adhesive Function Can Affect RD Cell Movement
In a previous study, α2β1 expression was shown to enhance RD cell adhesion and did not have a detectable effect on cell movement when compared with control RDpF transfectant (Chan et al., 1992). This may be related to the biphasic cell movement with respect to adhesive function. Thus, RDpF exhibited basal adhesion and migration on collagen. An expression of α2β1 resulted in increases in adhesion to the point that interaction was already beyond the optimal level, resulting in a reduction in cell movement to the same basal level of movement observed with RDpF cells. To determine whether RDX2C2 cell movement on collagen can be regulated by modulation of its adhesive function using blocking mAb BHA2.1, RDX2C2 cell movement was characterized in the presence of various concentrations of BHA2.1. As shown in Figure 5A, results from a static adhesion assay showed that the total number of adherent RDX2C2 remained relatively constant between 0 and 0.0002 μg/ml BHA2.1. Within this range of concentration of BHA2.1, RDX2C2 cell movement increased with increasing concentration of BHA2.1 (Figure 4B). At concentrations greater than 0.002 μg/ml, RDX2C2 were inhibited from adhering and migrating on collagen. Control mAb P3 at the corresponding concentrations had no effect on adhesion and migration. A similar modulation of cell movement by various concentrations of BHA2.1 was also observed when cell migration was characterized by the method of wounding assay using collagen-coated glass slips (our unpublished observation). To determine whether the observed increases in RDX2C2 cell movement on collagen was due to a reduction in the adhesive strength by BHA2.1, adherent cells were removed at defined centrifugation force (g; Figure 6). The percentage of RDX2C2 remaining adherent decreased with increasing BHA2.1; as expected, a greater percentage of cells were removed at 30 × g than when at 17 × g (p < 0.05). In addition, at 17 × g, JBS2 (20 μg/ml) increased the adhesive strength and neutralized the effect of BHA2.1 blocking; the percentages of RDX2C2 remained adherent were similar to those observed in the presence of control mAb P3. At 30 × g, JBS2 was no longer able to compensate for the reduction in the adhesive strength by BHA2.1. In the presence of control mAb P3, the percentage of adherent RDX2C2 cells remained relatively constant at 17 × g and 30 × g. When the concentration of BHA2.1 was at zero, the percentage of RDX2C2 cells adherent on collagen was not significantly different after treatment with control antibody or JBS2 alone. This result indicated that α2β1 expressed on RDX2C2 cells was already fully functional, and JBS2 stimulatory effect could be detected only when α2β1 function was not fully active or partially blocked.
Figure 5.
Adhesion and migration of RD transfectant cells at various concentrations of blocking α2β1 mAb BHA2.1. Adhesion (A) and migration (B) of RD transfectant cells expressing α2β1 (RDX2C2) on collagen substrate (10 μg/ml) in the presence of BHA2.1 were compared using isotype-matched control mAb P3 at the indicated concentrations.
Figure 6.
Determination of adhesive strength of RDX2C2 cells bound onto collagen. RDX2C2 cells were labeled with BCECF fluorescence label, treated with various concentrations of mAb P3, BHA2.1, or BHA2.1 with JBS2, at a constant concentration of 20 μg/ml, and allowed to adhere to collagen-coated wells for 45 min at 37°C. Nonadherent cells were removed by centrifugation at 17 × g (A) or 30 × g (B). Cell adhesion was expressed as the percentage of bound cells based on the fluorescence of 5 × 104 labeled cells after a subtraction of background fluorescence.
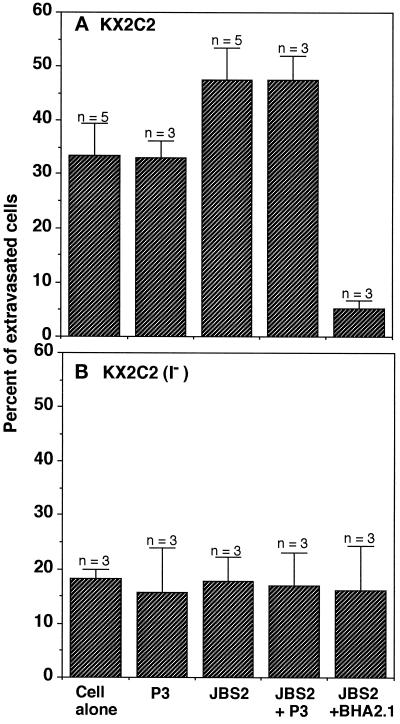
Integrin α2β1-mediated Cell Movement Can Be Modulated In Vivo
To determine whether the modulation of α2β1 adhesion and migratory function observed in vitro can be demonstrated in vivo, KX2C2 and KX2C2(I−) cell movement was further examined by IVVM. KX2C2 cells completed extravasation within 12 h after cell injection (our unpublished observation). Thus, to determine whether the α2β1 migratory function in postextravasation can be modulated in vivo, combinations of JBS2, BHA2.1, and P3 were injected (i.v.) at 100 μg per mouse 12 h after cell injection. The percentage of extravasated KX2C2 cells that reached the liver subcapsular region was then determined by IVVM at 15 h after cell injection. As shown in Figure 7A, an injection of JBS2 with or without the control P3 increased the percentage of KX2C2 cells that reached the liver subcapsular space in comparison with either the cells alone or treated with the control mAb P3 (p < 0.01). In addition, injection of BHA2.1 abolished the migratory function of α2β1 in vivo. Therefore, results suggest that the modulation of α2β1 function by JBS2 in vitro can also be demonstrated in vivo. In addition, the ability of KX2C2(I−) cells, expressing the nonfunctional α2β1, to migrate to the liver subcapsular region was also examined. KX2C2(I−) cells alone or treated with control mAb, JBS2, JBS2 and control mAb, or JBS2 and BHA2.1 had approximately the same low basal percentage of cells reaching the subcapsular region of the liver (Figure 7B). Thus, expression of a functional α2β1 on KX2C2 was required for JBS2 modulation of KX2C2 cell migration to the liver subcapsular region.
Figure 7.
Modulation of migratory α2β1 function in vivo. At 12 h after mesenteric vein injection of K562 transfectant cells KX2C2 (A) or KX2C2(I−) (B) into nu/nu mice, the mice received an injection of mAb JBS2 alone (100 μg/mouse) or in combination with either blocking α2β1 mAb BHA2.1 or control mAb P3 (i.v.) at 100 μg/mouse. Control mice were not injected or injected with control P3 mAb alone (200 μg/mouse). IVVM was then carried out 3 h after mAb injection to determine the percentages of extravasated cells that reached the subcapsular region of the liver.
DISCUSSION
It is well established that β1 integrins have major roles in the process of extravasation (Lawrence and Springer, 1991; von Andrian et al., 1991; Springer, 1994; Alon et al., 1995; Berlin et al., 1995). However, at present, little is known regarding their involvement in the movement of cells after extravasation into tissues. To reach the nonvascularized subcapsular region of the liver, cells in the circulation are required to extravasate and migrate through the parenchyma. In a recent study, we have demonstrated that expression of α2β1 reduced the ability of RD cells to migrate through liver parenchyma, and RDX2C2 cells became arrested after extravasation with the appearance of wrapping around the sinusoids (Hangan et al., 1996). Moreover, an injection of blocking mAb BHA2.1 restored RDX2C2 cell movement in liver parenchyma. Although this observation indicated a critical role of α2β1 in affecting cell movement, it was not clear whether α2β1 expression could in fact directly cause cell movement in vivo. We report herein that α2β1 integrin can provide migratory function to human erythroleukemia K562 cells after extravasation into the liver. Thus, significantly more KX2C2 cells, expressing wild-type α2β1, migrated through the parenchyma and entered the subcapsular region than did KX2C2(I−), which expressed the nonfunctional α2β1 variant lacking the I-domain. Therefore, the observed α2β1 integrin migratory function in vivo could be stimulated or inhibited by α2β1-specific mAbs JBS2 and BHA2.1, respectively.
Thus, with results from the previous study (Hangan et al., 1996), it is apparent that α2β1 expression enhanced K562 but reduced RD cells movement into the subcapsular region of the liver after extravasation. These in vivo observations correlated with in vitro results showing differential α2β1 function between K562 and RD transfectant cells. Thus, expression of α2β1 enhanced RD cell adhesion to collagen and laminin; however, on K562 cells α2β1-mediated adhesion to either matrix protein was not detectable in static cell adhesion assays involving simple washing steps. These results are also consistent with studies demonstrating that α2β1 function may vary among cell types (Elices and Hemler, 1989; Languino et al., 1989; Kirchhofer et al., 1990; Lotz et al., 1990). Thus, on platelets and fibroblast cell lines, α2β1 bound only collagen but not laminin, whereas on endothelial cells, α2β1 bound both collagen and laminin. Although integrin function in cell movement may be modulated by local production of cytokines and chemokines (Luscinskas et al., 1994; Issekutz, 1995; Campbell et al., 1996; Carr et al., 1996; Lloyd et al., 1996; Weber et al., 1996), it is apparent that the cell type dependence of integrin function initially demonstrated in vitro may also have a major role in determining cell movement in vivo.
At present, the exact mechanism that explains the different ligand-binding properties of α2β1, as demonstrated in static cell adhesion washing assays, is still unclear. However, binding of stimulatory β1-specific mAb Ts2/16 induced a functional transition of KX2C2 so that these cells now bind both collagen and laminin (Chan and Hemler, 1993). In comparison, results from the present study detected JBS2 stimulation of α2β1 binding to collagen but not laminin. In addition, we have consistently observed that Ts2/16 stimulated more KX2C2 adhesion to collagen than JBS2 (our unpublished observation). Stimulation of α2β1 function by JBS2 and Ts2/16 likely involved an induction of conformational changes that favor ligand binding (Arroyo et al., 1993; Chan and Hemler, 1993; Stupack et al., 1994). In addition, α2β1 appears to interact with collagen more readily than with laminin as supported by our observation that KX2C2 exhibited constitutive migratory function on collagen but not laminin and binding of JBS2 stimulated adhesion of collagen but not laminin. Although JBS2 did not stimulate a detectable increase in the binding of laminin, α2β1-mediated KX2C2 cell movement on laminin was enhanced. A preferential ligand interaction has also been demonstrated in studies of the other β1 integrins; for example, α4β1 binds vascular cell adhesion molecule-1 (VCAM-1) more readily than fibronectin, and α3β1 binds epiligrin/kalinin more readily than the other ECM proteins (Masumoto and Hemler, 1993; Weitzman et al., 1993; Delwel et al., 1994). In addition, based on static cell adhesion washing assays, α2β1 expressed on K562 cells had been described as “nonfunctional” because no stable adhesion of KX2C2 to collagen or laminin was detectable (Elices and Hemler, 1989; Chan and Hemler, 1993). Results from the present study demonstrated that KX2C2 cells were constitutively more migratory on collagen when compared with KX2C2(I−). Thus, it is apparent that α2β1 mediated a low level of interaction of K562 cells with collagen that enhanced cell movement.
It is well established that the inserted (I)-domains are critical for integrin function (Diamond et al., 1993; Kamata et al., 1994; Lee et al., 1995). The I-domain in α2 integrin subunit has been shown to be important for interaction with collagen (Kamata et al., 1994). We have previously expressed the α2β1 variant lacking α2 I-domain on RD cells and showed that α2 I-domain was also important for interaction with laminin (Hangan et al., 1996). We show herein that α2 I-domain was critical for α2β1 response to stimulation by JBS2. However, results from these studies cannot exclude the possibility of a global structural disruption of α2β1 as a result of the removal of α2 I-domain. This is, however, unlikely since binding of HAS4 to the previously described RDX2C2(I−) (Hangan et al., 1996) and in the present study KX2C2(I−) cells was still detectable, indicating that at least certain epitopes of α2β1 are conserved after the deletion of α2 I-domain. The integrin α2β1 function was not restored by a substitution with the I-domain of αL integrin subunit (our unpublished observation). Mutation of only two amino acids (Asp-151 and Asp-254) within α2 I-domain was sufficient to abolish α2β1 binding of collagen (Kamata et al., 1994) and recombinant α2 I-domain has been shown to bind collagen (Tuckwell et al., 1995).
The migratory function of α2β1 appeared to be, at least in part, regulated by its adhesive properties for matrix protein ligands. Results from the present study of α2β1 migratory function is consistent with the concept that the adhesive strength of cell–matrix interactions affects cell movement in a biphasic manner; cell movement occurs when interaction with matrix proteins is optimal (DiMilla et al., 1993; Huttenlocher et al., 1996). The biphasic cell movement upon modulation of β1 integrin adhesive function has been demonstrated in vitro using echistatin, an integrin-binding competitor, and divalent cations (Grzesiak et al., 1992; Wu et al., 1994). More recently, the speed of α5β1- and αIIbβ3-mediated cell movement has been defined in terms of the concentration of the substrate, the level of integrin expression, and the extent of ligand binding (Palecek et al., 1997). As shown in the present study with KX2C2, stimulation of α2β1 by mAb JBS2 resulted in increases in both cell adhesion and movement on collagen; in the presence of Mn2+, adhesion was further increased, whereas α2β1-mediated migration was reduced. The removal of adherent KX2C2 cells from collagen at the different g forces revealed that JBS2 stimulation increased the adhesive strength to be closer to the optimal level required for migration of KX2C2 on collagen; addition of Mn2+ further increased adhesive strength beyond this optimal level and thus reduced cell movement. This observation may explain the variable adhesive and migratory function of α2β1 reported for the different cells. With adhesive strength beyond optimal interaction, α2β1 expression on mammary carcinoma Mm5 mT resulted in increases in adhesion but a reduction in cell movement (Zutter et al., 1995). In the present study, RDX2C2 interaction with collagen was beyond the optimal level for cell movement. Decreases in adherence allowed RDX2C2 cells to approach optimal interaction with collagen and thus an increase in cell movement. In addition to our in vitro studies, we have also demonstrated for the first time that a α2β1-modulated increase in adhesion can also affect cell movement in vivo. Thus, JBS2 stimulation of α2β1 adhesion was associated with an increase in KX2C2 cell migration to the subcapsular region of the liver.
Cell migration in vitro involves protrusion of the leading edge of the cell that forms an adhesive complex with the matrix. Subsequently, the cell releases the adhesive bonds at the rear of the cell, which allows the cell to move over the substrate (Stossel, 1993; Lee et al., 1994). The pseudopodial-like projections, termed invadopodia, may contain membrane-bound proteinases to facilitate cell movement by localized degradation of the matrix components (Kelly et al., 1994; Monsky et al., 1994). The β1 integrins may also play a role in this process. α6β1 integrin, as a receptor for laminin, has been shown to be involved in the formation of invadopodia with localized ECM degredation activities (Nakahara et al., 1996). In vivo we also find that extravasated RD and mammary carcinoma cells frequently send out pseudopodial projections before they migrate (Morris et al., 1994; Hangan et al., 1996). Thus, in the liver, these cells first send these projections to the subcapsular region and then the body of the cell follows. RD transfectant cells expressing α2β1 fail to migrate after extravasation; they generally wrap around blood vessels and remain firmly attached there (Hangan et al., 1996). Our in vivo results and our in vitro findings suggest that limited adhesion with the extracellular matrix via β1 integrins may allow the pseudopodial projections to form and cell migration to occur. However, if the adhesive forces become too strong, then cells will tend to wrap around structures such as the basement membrane of blood vessels and prevent further cell movement. Thus, the ability of α2β1 to confer stationary or motile phenotypes between different cell types may have determining effects on the distribution of cells in tissues after extravasation. The presence of cytokines and growth factors at sites where cancer cells eventually reside may have direct impact on the outcome of tumor metastasis. It has been shown that the metastatic potential of melanoma, osteosarcoma, rhabdomyosarcoma, and lung tumors correlated with increases in α2β1 expression; whereas, decreases in α2β1 expression has been associated with epithelial malignant transformation (Dedhar and Saulnier, 1990; Chan et al., 1991; Klein et al., 1991; Mortarini et al., 1991; Pignatelli et al., 1991; Danen et al., 1993; Chen et al., 1994; Santala et al., 1994; Zutter et al., 1995). Our current work and our recent studies of RD cells may explain why both an enhancing and inhibitory effect of α2β1 on tumor metastasis have been reported. Thus, the extent of the interaction between α2β1 and matrix proteins may confer differing migratory and adhesive properties among different tumor cell types; these differences, in turn, can cause them to reside in different tissue sites with various degrees of permissiveness to tumor foci formation. However, a complete understanding of cell movement in vivo will require addition determination of the role of the other adhesion molecules and their regulation by localized production of chemotactic and growth factors.
Table 1.
Functional relationship between α2β1-mediated adhesion and migration of KX2C2 cells on collagen and laminin
| Collagen
|
Laminin
|
|||
|---|---|---|---|---|
| Adhesion | Migration | Adhesion | Migration | |
| Control Ab | Not detectablea | + | Not detectable | Not detectable |
| JBS2 | + | +++ | Not detectable | + |
| JBS2 + Mn2+ | +++ | ++ | Not detectable | + |
Ab, antibody.
Not detectable as determined by static cell adhesion assay involving simple washing steps.
ACKNOWLEDGMENTS
This work was supported by the Medical Research Council and Natural Sciences and Engineering Research Council of Canada (to B.M.C.C.) and the Cancer Research Society and Academic Development Fund, University of Western Ontario (to V.L.M.).
REFERENCES
- Adams JC, Watt FM. Changes in keratinocyte adhesion during terminal differentiation: reduction in fibronectin binding precedes α5β1 integrin loss from the cell surface. Cell. 1990;63:425–435. doi: 10.1016/0092-8674(90)90175-e. [DOI] [PubMed] [Google Scholar]
- Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo AG, Garcia-Pardo A, Sanchez-Madrid F. A high affinity conformational state on VLA integrin heterodimers induced by an anti-β1 chain monoclonal antibody. J Biol Chem. 1993;268:9863–9868. [PubMed] [Google Scholar]
- Arroyo AG, Sanchez-Mateos P, Campanero MR, Martin-Padura I, Dejana E, Sanchez-Madrid F. Regulation of the VLA integrin-ligand interactions through the β1 subunit. J Cell Biol. 1992;117:659–670. doi: 10.1083/jcb.117.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer JS, Varner J, Schreiner C, Kornberg L, Nicholas R, Juliano RL. Functional role of the cytoplasmic domain of the integrin alpha 5 subunit. J Cell Biol. 1993;122:209–221. doi: 10.1083/jcb.122.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdichevsky F, Gilbert C, Shearer M, Papadimitriou TJ. Collagen-induced rapid morphogenesis of human mammary epithelial cells: the role of the α2β1 integrin. J Cell Sci. 1992;102:437–446. doi: 10.1242/jcs.102.3.437. [DOI] [PubMed] [Google Scholar]
- Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hassien SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- Briesewitz R, Kern A, Marcantonio EE. Assembly and function of integrin receptors is dependent on opposing α and β cytoplasmic domains. Mol Biol Cell. 1995;6:997–1010. doi: 10.1091/mbc.6.8.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger SR, Zutter MM, Sturgill-Koszycki S, Santoro SA. Induced cell surface expression of functional α2β1 integrin during megakaryocytic differentiation of K562 leukemic cells. Exp Cell Res. 1992;202:28–35. doi: 10.1016/0014-4827(92)90400-3. [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Qin S, Bacon KB, Mackay CR, Butcher EC. Biology of chemokine and classical chemoattractant receptors: differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J Cell Biol. 1996;134:255–266. doi: 10.1083/jcb.134.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MW, Alon R, Springer TA. The C-C chemokine MCP-1 modulates the avidity of β1 but not β2 integrins on T lymphocytes. Immunity. 1996;4:179–187. doi: 10.1016/s1074-7613(00)80682-2. [DOI] [PubMed] [Google Scholar]
- Chan BMC, Elices MJ, Murphy E, Hemler ME. Adhesion to vascular cell adhesion molecule 1 and fibronectin comparison of α4β1 (VLA-4) and α4β7 on the human B cell line JY. J Biol Chem. 1992;267:8366–8370. [PubMed] [Google Scholar]
- Chan BMC, Hemler ME. Multiple functional forms of the integrin VLA-2 derived from a single alpha 2 cDNA clone: interconversion of forms induced by an anti-beta 1 antibody. J Cell Biol. 1993;120:537–543. doi: 10.1083/jcb.120.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan BMC, Kassner PD, Schiro JA, Byers HR, Kupper TS, Hemler ME. Distinct cellular functions mediated by different VLA integrin α subunit cytoplasmic domains. Cell. 1992;68:1051–1060. doi: 10.1016/0092-8674(92)90077-p. [DOI] [PubMed] [Google Scholar]
- Chan BMC, Matsuura N, Takada Y, Zetter BR, Hemler ME. In vitro and in vivo consequences of VLA-2 expression on rhabdomyosarcoma cells. Science. 1991;251:1600–1602. doi: 10.1126/science.2011740. [DOI] [PubMed] [Google Scholar]
- Chen F-A, Alosco T, Crpy BA, Narumi K, Percy DH, Bankert RB. Clones of tumor cells derived from a single primary human lung tumor reveal different patterns of β-1 integrin expression. Cell Adhes Commun. 1994;2:345–357. doi: 10.3109/15419069409014209. [DOI] [PubMed] [Google Scholar]
- Chou DH-I, Lee W, McCulloch CAG. TNF-α inactivation of collagen receptors. Implications for fibroblast function and fibrosis. J Immunol. 1996;156:4354–4362. [PubMed] [Google Scholar]
- Danen EHJ, van Muijen GNP, van de Wiel-van Kemenade E, Jansen KFJ, Ruiter DJ, Figdor CG. Regulation of integrin-mediated adhesion to laminin and collagen in human melanocytes and in non-metastatic and highly metastatic human melanoma cells. Int J Cancer. 1993;54:315–321. doi: 10.1002/ijc.2910540225. [DOI] [PubMed] [Google Scholar]
- Dedhar S, Saulnier R. Alterations in integrin receptor expression on chemically transformed human cells: specific enhancement of laminin and collagen receptor complexes. J Cell Biol. 1990;110:481–489. doi: 10.1083/jcb.110.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwel GO, de Melker AA, Hogervorst F, Jaspars LH, Fles DLA, Kuikman I, Lindblom A, Paulsson M, Timpl R, Sonnenberg A. Distinct and overlapping ligand specificities of the α3Aβ1 and α6Aβ1 integrins: recognition of laminin isoforms. Mol Biol Cell. 1994;5:203–215. doi: 10.1091/mbc.5.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Garcia-Aguilar J, Bickford JK, Corbi AL, Springer TA. The I Domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120:1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMilla PA, Stone JA, Quinn JA, Albelda SM, Lauffenburger DA. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J Cell Biol. 1993;122:729–737. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices MJ, Hemler ME. The human integrin VLA-2 is a collagen receptor on some cells and a collagen/laminin receptor on others. Proc Natl Acad Sci USA. 1989;86:9906–9910. doi: 10.1073/pnas.86.24.9906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etoh T, Thomas L, Pastel-Levy C, Colvin RB, Mihm MCJ, Byers HR. Role of integrin α2β1 (VLA-2) in the migration of human melanoma cells on laminin and type IV collagen. J Invest Dermatol. 1993;100:640–647. doi: 10.1111/1523-1747.ep12472299. [DOI] [PubMed] [Google Scholar]
- Faull RJ, Kovach NL, Harlan JM, Ginsberg MH. Stimulation of integrin-mediated adhesion of T lymphocytes and monocytes: two mechanisms with divergent biological consequences. J Exp Med. 1994;179:1307–1316. doi: 10.1084/jem.179.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzesiak JJ, Davis GE, Kirchhofer D, Pierschbacher MD. Regulation of α2β1-mediated fibroblast migration on type I collagen by shifts in the concentrations of extracellular Mg2+ and Ca2+ J Cell Biol. 1992;117:1109–1117. doi: 10.1083/jcb.117.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangan D, Uniyal S, Morris VL, MacDonald IC, von Ballestrem CG, Chau T, Schmidt EE, Chambers AF, Groom AV, Chan BMC. Integrin VLA-2 (α2β1) function in post-extravasation movement of human rhabdomyosarcoma RD cells in the liver. Cancer Res. 1996;56:3142–3149. [PubMed] [Google Scholar]
- Hauzenberger D, Klominek J, Sundqvist K-G. Functional specialization of fibronectin-binding beta-1 integrins in T lymphocyte migration. J Immunol. 1994;153:960–971. [PubMed] [Google Scholar]
- Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Cell Biol. 1996;134:1551–1562. doi: 10.1083/jcb.134.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Issekutz TB. In vivo blood monocyte migration to acute inflammatory reactions, IL-1α, TNF-α, IFN-γ, and C5a utilizes LFA-1, Mac-1, and VLA-4: the relative importance of each integrin. J Immunol. 1995;154:6533–6540. [PubMed] [Google Scholar]
- Kamata T, Puzon W, Takada Y. Identification of putative ligand binding sites within I domain of integrin α2β1 (VLA-2, CD49b/CD29) J Biol Chem. 1994;269:9659–9663. [PubMed] [Google Scholar]
- Kassner PD, Alon R, Springer TA, Hemler ME. Specialized functional properties of the integrin α4 cytoplasmic domain. Mol Biol Cell. 1995;6:661–674. doi: 10.1091/mbc.6.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi S, Bergelson JM, Finberg RW, Hemler ME. Integrin α2 cytoplasmic domain deletion effects: loss of adhesive activity parallels ligand-independent recruitment into focal adhesions. Mol Biol Cell. 1994;5:977–988. doi: 10.1091/mbc.5.9.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi S, Hemler ME. Role of the α subunit cytoplasmic domain in regulation of adhesive activity mediated by the integrin VLA-2. J Biol Chem. 1993;268:16279–16285. [PubMed] [Google Scholar]
- Kearney JF, Radbruch A, Liesegang B, Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979;123:1548–1550. [PubMed] [Google Scholar]
- Keely PJ, Fong AM, Zutter MM, Santoro SA. Alteration of collagen-dependent adhesion, motility, and morphogenesis by the expression of antisense α2 integrin mRNA in mammary cells. J Cell Sci. 1995a;108:595–607. doi: 10.1242/jcs.108.2.595. [DOI] [PubMed] [Google Scholar]
- Keely PJ, Wu JE, Santoro SA. The spatial and temporal expression of the α2β1 integrin and its ligands, collagen I, collagen V, and laminin, suggest important roles in mouse mammary morphogenesis. Differentiation. 1995b;59:1–13. doi: 10.1046/j.1432-0436.1995.5910001.x. [DOI] [PubMed] [Google Scholar]
- Kelly T, Mueller SC, Yeh Y, Chen W-T. Invadopodia promote proteolysis of a wide variety of extracellular matrix proteins. J Cell Physiol. 1994;158:299–308. doi: 10.1002/jcp.1041580212. [DOI] [PubMed] [Google Scholar]
- Kirchhofer D, Languino LR, Ruoslahti E, Pierschbacher MD. α2β1 integrins from different cell types show different binding specificities. J Biol Chem. 1990;265:615–618. [PubMed] [Google Scholar]
- Klein CE, Dressel D, Steinmayer T, Mauch C, Eckes B, Krieg T, Bankert RB, Weber L. Integrin α2β1 is upregulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J Cell Biol. 1991;115:1427–1436. doi: 10.1083/jcb.115.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson JR, Iida J, Fields GB, McCarthy JB. CD44/chondoitin sulfate proteoglycan and α2β1 integrin mediate human melanoma cell migration on type IV collagen and invasion of basement membranes. Mol Biol Cell. 1996;7:383–396. doi: 10.1091/mbc.7.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach NL, Carlos TM, Yee E, Harlan JM. A monoclonal antibody to β1 integrin (CD29) stimulates VLA-dependent adherence of leukocytes to human umbilical vein endothelial cells and matrix components. J Cell Biol. 1992;116:499–509. doi: 10.1083/jcb.116.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpers TW, Mul EPJ, Blom M, Kovach NL, Gaeta FCA, Tollefson V, Elices MJ, Harlan JM. Freezing adhesion molecules in a state of high-avidity binding blocks eosinophil migration. J Exp Med. 1993;178:279–284. doi: 10.1084/jem.178.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languino LR, Gehlsen KR, Wayner E, Carter WG, Engvall E, Ruoslahti E. Endothelial cells use α2β1 integrin as a laminin receptor. J Cell Biol. 1989;109:2455–2462. doi: 10.1083/jcb.109.5.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Lawrence MB, Springer TA. Leukocyte rolling on selectin at physiologic flow rates: distribution from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Lee J, Leonard M, Oliver T, Ishihara A, Jacobson K. Traction forces generated by locomoting leukocytes. J Cell Biol. 1994;127:1957–1964. doi: 10.1083/jcb.127.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-O, Rieu P, Arnaout MA. Crystal structure of the A domain from the α subunit of integrin CR3 (CD11b/CD18) Cell. 1995;80:631–638. doi: 10.1016/0092-8674(95)90517-0. [DOI] [PubMed] [Google Scholar]
- Lloyd AR, Oppenheim JJ, Kelvin DJ, Taub DD. Chemokines regulate T cell adherence to recombinant adhesion molecules and extracellular matrix proteins. J Immunol. 1996;156:932–938. [PubMed] [Google Scholar]
- Lotz MM, Korzelius CA, Mercurio AM. Human colon carcinoma cells use multiple receptors to adhere to laminin: involvement of α6β4 and α2β1 integrins. Cell Regul. 1990;1:249–257. doi: 10.1091/mbc.1.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscinskas FW, Kansas GS, Ding P, Pizcueta P, Schleiffenbaum BE, Tedder TF, Gimbrone MA. Monocyte rolling, arrest and spreading on IL-4-activated vascular endothelium under flow is mediated via sequential action of L-selectin, β1-integrins, and β2-integrins. J Cell Biol. 1994;125:1417–1427. doi: 10.1083/jcb.125.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto A, Hemler ME. Mutation of putative divalent cation sites in the α4 subunit of the integrin VLA-4: distinct effects on adhesion to CS1/fibronectin, VCAM-1, and invasin. J Cell Biol. 1993;123:245–253. doi: 10.1083/jcb.123.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsky WL, Lin C-Y, Aoyama A, Kelly T, Akiyama SK, Mueller SC, Chen W-T. A potential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. Cancer Res. 1994;54:5702–5710. [PubMed] [Google Scholar]
- Morris VL, Koop S, MacDonald IC, Schmidt EE, Grattan M, Percy DX, Chambers AF, Groom AC. Mammary carcinoma cell lines of high and low metastatic potential differ not in extravasation but in subsequent migration and growth. Clin Exp Metastasis. 1994;12:357–367. doi: 10.1007/BF01755879. [DOI] [PubMed] [Google Scholar]
- Mortarini R, Anichini A, Parmiani G. Heterogeneity for integrin expression and cytokine mediated VLA modulation can influence the adhesion of human melanoma cells to extracellular proteins. Int J Cancer. 1991;47:551–559. doi: 10.1002/ijc.2910470413. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Nomizu M, Akiyama SK, Yamada Y, Yeh Y, Chen W-T. A mechanism for regulation of melanoma invasion. J Biol Chem. 1996;271:27221–27224. doi: 10.1074/jbc.271.44.27221. [DOI] [PubMed] [Google Scholar]
- Ohashi P, Mak TW, Van der Elsen P, Yanagi Y, Yoshikai Y, Calman AF, Terhorst C, Stobo JD, Weiss A. Reconstitution of an active surface T3/T-cell antigen receptor by DNA transfer. Nature. 1985;316:606–609. doi: 10.1038/316606a0. [DOI] [PubMed] [Google Scholar]
- Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Pignatelli M, Hanby AM, Stamp GW. Low expression of β1, α2 and α3 subunits of VLA integrins in malignant mammary tumours. J Pathol. 1991;165:25–32. doi: 10.1002/path.1711650106. [DOI] [PubMed] [Google Scholar]
- Pignatelli M, Smith MEF, Bodmer WF. Low expression of collagen receptors in moderate and poorly differentiated colorectal adenocarcinomas. Br J Cancer. 1990;61:636–638. doi: 10.1038/bjc.1990.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Mateos P, Arroyo AG, Balboa MA, Sanchez-Madrid F. Post-receptor occupancy events in leukocytes during β1 integrin-ligand interactions. Eur J Immunol. 1993;23:2642–2648. doi: 10.1002/eji.1830231038. [DOI] [PubMed] [Google Scholar]
- Santala P, Larjava H, Nissinen L, Riikonen T, Maatta A, Heino J. Suppressed collagen gene expression and induction of α2β1 integrin-type collagen receptor in tumorigenic derivatives of human osteogenic sarcoma (HOS) cell line. J Biol Chem. 1994;269:1276–1283. [PubMed] [Google Scholar]
- Scharffetter-Kochanek K, Klein CE, Heinen G, Mauch C, Schaefer T, Adelmann-Grill BC, Goerz G, Fusenig NE, Krieg TM, Plewig G. Migration of a human keratinocyte cell line (HACAT) to interstitial collagen type I is mediated by the α2β1-integrin receptor. J Invest Dermatol. 1992;98:3–11. doi: 10.1111/1523-1747.ep12493266. [DOI] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Stossel TP. On the crawling of animal cells. Science. 1993;260:1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Stupack D, Shen C, Wilkins JA. Control of lymphocyte integrin function: evidence for multiple contributing factors. Cell Immunol. 1994;155:237–245. doi: 10.1006/cimm.1994.1116. [DOI] [PubMed] [Google Scholar]
- Tenchini ML, Adams JC, Gilbert C, Steel J, Hudson DL, Malcovati M, Watt FM. Evidence against a major role for integrins in a calcium-dependent intercellular adhesion of epidermal keratinocytes. Cell Adhes Commun. 1993;1:55–66. doi: 10.3109/15419069309095681. [DOI] [PubMed] [Google Scholar]
- Tuckwell D, Calderwood DA, Green LJ, Humphries MJ. Integrin α2 I-domain is a binding site for collagens. J Cell Sci. 1995;108:1629–1637. doi: 10.1242/jcs.108.4.1629. [DOI] [PubMed] [Google Scholar]
- van de Wiel-van Kemenade E, van Kooyk Y, de Boer AJ, Huijbens RJF, Weder P, van de Kasteele W, Melief CJM, Figdor CG. Adhesion of T and B lymphocytes to extracellular matrix and endothelial cells can be regulated through the β subunit of VLA. J Cell Biol. 1992;117:461–470. doi: 10.1083/jcb.117.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors K-E, Butcher EC. Two-step model of leukocyte-endothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte β2 integrins in vivo. Proc Natl Acad Sci USA. 1991;88:7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Alon R, Moser B, Springer TA. Sequential regulation of α4β1 and α5β1 integrin avidity by CC chemokines in monocytes: implications for transendothelial chemotaxis. J Cell Biol. 1996;134:1063–1073. doi: 10.1083/jcb.134.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman JB, Pasqualini R, Takada Y, Hemler ME. The function and distinctive regulation of the integrin VLA-3 in cell adhesion, spreading, and homotypic cell aggregation. J Biol Chem. 1993;268:8651–8657. [PubMed] [Google Scholar]
- Wu P, Hoying JB, Williams SK, Kozikowski BA, Lauffenburger DA. Integrin-binding peptide in solution inhibits or enhances endothelial cell migration, predictably from cell adhesion. Ann Biomed Eng. 1994;22:144–152. doi: 10.1007/BF02390372. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Kennedy DW, Yamada SS, Gralnick H, Chen W-T, Akiyama SK. Monoclonal antibody and synthetic peptide inhibitors of human tumor cell migration. Cancer Res. 1990;50:4485–4496. [PubMed] [Google Scholar]
- Zutter MM, Mazoujian G, Santoro SA. Decreased expression of integrin adhesive protein receptors in adenocarcinoma of the breast. Am J Pathol. 1990;137:863–870. [PMC free article] [PubMed] [Google Scholar]
- Zutter MM, Santoro SA, Staatz WD, Tsung YL. Re-expression of the α2β1 integrin abrogates the malignant phenotype of breast carcinoma cells. Proc Natl Acad Sci USA. 1995;92:7411–7415. doi: 10.1073/pnas.92.16.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]