Abstract
Background
Unravelling the serum proteome is the subject of intensified research. In this regard, two-dimensional electrophoresis coupled with MALDI MS analysis is still one of the most commonly used method. Despite some improvements, there is the need for better protocols to enable comprehensive identification of serum proteins.
Here we report a combination of two proteomic strategies, zoom in acidic and neutral part of 2-D gels and an application of two optimised matrix preparations for MALDI-MS analyses to simplify serum proteome mapping.
Results
Mouse serum proteins were separated by 2-D electrophoresis at the pH ranges 3–10 and 4–7, respectively. Then in gel tryptic digests were analysed by MALDI-MS. Notably, sample-matrix preparations consisted of either a thin-layer α-ciano-4-hydroxycinnamic acid (CHCA) matrix deposition or a matrix-layer 2,5-dihydroxybenzoic acid (DHB). This enabled an identification of 90 proteins. The herein reported method enhanced identification of proteins by 32% when compared with previously published studies of mouse serum proteins, using the same approaches. Furthermore, experimental improvements of matrix preparations enabled automatic identification of mouse proteins, even when one of the two matrices failed.
Conclusion
We report a simple and reliable protocol for serum proteome analysis that combines an optimized resolution of 2-D gels spots and improved sample-matrix preparations for MALDI-MS analysis. The protocol allowed automated data acquisition for both CHCA and DHB and simplified the MS data acquisition therefore avoiding time-consuming procedures. The simplicity and reliability of the developed protocol may be applied universally.
Background
From a disease diagnostic and drug monitoring point of view there is great interest in serum proteome mapping of humans and of laboratory animals. Indeed, various mouse strains and genetically engineered animals are considered to be good models for human diseases as they offer unprecedented opportunities for mechanistic studies with new experimental medicines. There is hope that serum proteomics enables an identification of biomarkers of disease and drug safety and serum proteins can be used for therapeutic monitoring. In the past 2-D maps for human serum have been reported [1-4]. And very recently a map for the C57BL6 mouse serum proteome was published [5].
In general, serum proteome profiling is challenging, because of interference by high-abundance proteins such as albumin, immunoglobulins, antitrypsin and transferrin, which typically constitute greater than 90% of total protein mass [1,2,6-9]. These abundant proteins may hinder the detection of low-abundance proteins that can be of specific interest in the search of biomarkers of disease. Additionally, protein biochips have been applied to proteomic studies with antibody microarrays offering new possibilities in the simultaneous identification of analytes from complex samples [10].
So far, only a handful of plasma proteins are routinely measured for diagnostic purposes, because an effective technology that rapidly detects and quantifies specific changes of proteins including low-abundance proteins of serum is not available. As summarized elsewhere [11], the most common methods for serum proteome studies include separation of proteins by gel electrophoresis, excision of spots from the gel, enzymatic digestion and analysis by mass spectrometry. In particular, pre-fractionation techniques such as serum albumin depletion are useful procedures in proteome profiling studies, but they may introduce bias as well. There is substantial run-to-run variation after albumin depletion with IgY immunoaffinity spin columns. Likewise, pre-fractionation increases the risk of depletion of low-abundance proteins as has been shown for paraneoplastic antigen MA I, coagulation factor VII precursor, prostate-specific antigen, as a result of multiple protein-protein interactions with IgG, transferrins, and/or gelsolin [11,12].
In this regard, MALDI-MS is considered to be one of the most powerful techniques for the analysis of proteins and peptides [13-15], but the sample matrix preparation greatly influences the quality of MALDI-MS spectra of peptides and therefore protein identification. Despite considerable knowledge in the use of MALDI-MS [15-18], sample-matrix preparations are basically empirical.
Here we report a protocol for serum proteome profiling based on zoom in gels in the acidic and neutral pH that enabled detection of many serum proteins. Moreover, the developed protocol allowed for an automated data acquisition, and the sample protocol was optimised by the use of two different matrix-sample preparations in sequence [19]. We thus applied tryptic in-gel digest matrix preparation to either α-ciano-4-hydroxycinnamic acid (CHCA) or 2, 5-dihydroxybenzoic acid (DHB) as recently reported by us [19].
Materials and methods
Serum sample preparation
C57/BL6 mice (healthy mice) were obtained from Harlan Winkelman (Borchen, Germany) and kept in an animal house with 12 hour of light and dark cycled. Food and water was given ad libitum. Blood serum was collected from vena cava and allowed to clot for 2 hours at room temperature. The clotted material was removed by centrifugation at 3000 rpm for 15 min. Hemolytic material was not observed. The sera obtained from the blood samples were frozen immediately without any further treatment in liquid nitrogen and stored at -80°C until further analysis. The protein concentration of serum was determined with the Bradford protein assay (Bio-Rad Protein Assay Dye Reagent Concentrate, Bio-Rad), using bovine gamma globulin as the standard. The protein concentration ranged from 80 to 90 μg/μl for wild type mouse serum samples.
Materials
IPG strips of pH 3 to 10 and 4 to 7 (ReadyStrip, 0.5 × 3 × 170 mm; BioRad, Germany), Bio-Lyte (pH 3 to 10), SDS, acrylamide, methylenebisacrylamide, TEMED, ammonium persulfate, DTT, urea, Tris, glycine, glycerol, and CHAPS were purchased from Bio-Rad. Alpha-cyano-4-hydroxycinnamic acid (CHCA) and 2,5 dihydroxybenzoic acid (DHB) from Bruker Daltonics. Methanol, ethanol, phosphoric acid, acetic acid, and formaldehyde were purchased from Merck (Darmstadt, Germany). Sequencing grade modified trypsin was obtained from Promega (Madison, WI, USA).
Coomassie Brilliant Blue G-250, TCA, iodoacetamide and other reagents were obtained from Sigma (St. Louis, MO, USA).
Two-dimensional electrophoresis
IEF was carried out using commercially available, dedicated apparatuses: IPGphor Protean IEF Cell (Bio-Rad). IPG strips were used according to manufacturer instructions [see also [20]]. About 500 μg of serum for gel were diluted to 350 μL with re-hydration solution (5 M urea, 2 M Thiourea, 2% CHAPS, 100 mM DTT, 0.5% v/v pH 3 to 10 IPG buffer, 40 mM Tris Base, 2% SB 3–10 and trace bromophenol blue), and applied to immobilized pH 3 to 10 nonlinear and pH 4 to 7 linear gradient strips by overnight re-hydration at 50 V. With Protean IEF Cell, focusing was done initially at 250 V for 15 min, then the voltage was increased to 10 000 V within 3 h, and maintained at 10 000 V for 7 h for a total of 70 kVh. All IEF steps were carried out at 20°C. After the first-dimensional IEF, IPG gel strips were placed in an equilibration solution (6 M urea, 2% SDS, 30% glycerol, 50 mM Tris-HCl, pH 8.8) containing 1% DTT for 10 min with shaking at 50 rpm on an orbital shaker. The gels were then transferred to the equilibration solution containing 2.5% iodoacetamide and shaken for a further 10 min before placing them on 12% polyacrylamide gel slab (185 × 200 × 1.0 mm).
Separation in the second dimension was carried out using Protean II electrophoresis equipment and Tris-glycine buffer (25 mM Tris, 192 mM glycine) containing 0.1% SDS, at a current setting of 5 mA/gel for the initial 1 h and 10 mA/gel thereafter. The second-dimensional SDS-PAGE was developed until the bromophenol blue dye marker had reached the bottom of the gel.
Protein visualization and image analysis
Following second-dimensional SDS-PAGE, analytical gels were fixed three times in 30% ethanol containing 2% phosphoric acid for 20 min and rinsed three times in 2% phosphoric acid. Gels were then equilibrated in a solution containing 18% ethanol, 2% phosphoric acid, and 15% ammonium sulfate for 30 min and Coomassie Brilliant Blue G-250 was added to a final concentration of 1%. Staining was carried out overnight. Protein patterns in the gels were recorded as digitalized images using a high-resolution scanner (Pharox FX, Bio-Rad). Gel image matching was done with PDQuest software (Version 8.01.40; Bio-Rad). Scanned gel images were processed to remove backgrounds, staining on the gel borders and to automatically detect spots. For all spot intensity calculations, normalized values were used. Normalization of spot intensity was done with Loess Regression Method and normalized spot intensities were expressed in ppm.
In-gel digestion
In-gel digestion of protein spots on Coomassie gels was carried out with 160 ng of Porcine Modified Trypsin (Sigma) in 10% ACN and 25 mM NH4HCO3 and performed essentially as described below.
Briefly, after the completion of staining, the gel were washed twice with water for 15 min, and then twice with water/ACN (1:1 v/v) for 15 min. The solvent volumes were about twice the gel volume. Liquid was removed, ACN was added to the gel pieces and the mixture was left for 5 min. Liquid was removed and the gel pieces were re-hydrated in 0.1 M NH4HCO3 for 5 min. ACN was added to give a 1:1 v/v mixture of 0.1 M NH4HCO3/ACN and the mixture was incubated for 15 min. All liquid was removed the digestion buffer containing 25 mM NH4HCO3 and 10 ng/μL of trypsin was added and all was incubated for 4 hours at 37°C. The supernatant was recovered and the extraction was carried out with 1% TFA/ACN (1:1 v/v).
MALDI-TOF-MS, MS/MS and database search
Tryptic peptides were spotted directly onto a 600 μm/384 well AnchorChip™ sample target (Bruker Daltonics). The matrix CHCA was saturated in 97% Acetone/0.1% TFA solution; DHB matrix was solved in 30% ACN/0.1% TFA solution (5 mg/ml). The matrix-analyte preparations were loaded onto the MALDI plate (AnchorChip™ 600 mm 384 well, Bruker Diagnostic) by the thin layer and the matrix layer (ML) method for CHCA and DHB respectively. A further re-crystallization approach was tested on peptide calibration standard preparation (Bruker Daltonics) and applied for the samples on the AnchorChip™ [19]. An external peptide calibration standard containing the following fragments was used to calibrate the instrument: angiotensin II ([M+H]+ 1046.54); angiotensin I ([M+H]+ 1296.680); substance P ([M+H]+ 1347.740); bombesin ([M+H]+ 1619.820); ACTH clip 1–17 ([M+H]+ 2093.090); ACTH clip 18–39 ([M+H]+ 2465.200); somatostatin 28 ([M+H]+ 1347.470) (Bruker Daltonics). Furthermore, the spectra were calibrated using trypsin autolysis products (m/zs 1045.564, 2211.108 and 2225.119) for three points internal calibration resulting in a mass accuracy of <50 ppm.
The MALDI mass spectra were obtained using an Ultraflex II TOF/TOF mass spectrometer equipped with a 384-sample scout source (Bruker Daltonics) and AutoXecute® software for automatic spectra acquisition (Bruker Daltonics) was used. Peptide masses were searched against SwissProt database employing Mascot (in-house MASCOT-server) for protein identification. Database searches were performed taking into account carbamidomethyl modification of cysteines and possible oxidation of methionine and allowing one missed cleavage. The mass accuracy required for PMF and MS/MS was basically chosen according to peptide mass-tolerance defined by Root Mean Square (RMS) error, since it defines the limit of peptide mass tolerance (Peptide tol +-) for respectively Mascot Peptide Mass Fingerprint and Mascot MS/MS Ion Search to obtain a significant score (p < 0.05) of matched peptides to select protein entry [21].
Peptides were identified using ProteinScape™ database (Protagen, Bruker, Germany) and Mascot search engine to cross-validate or consolidate the identification results through the complementary use of several software packages. We use ProteinScape™ ScoreBooster feature to improve database search results by automatic iterative recalibrations and background eliminations. Identified proteins were checked individually for further considerations.
The criteria used to accept identifications included the extent of sequence coverage, the number of matched peptides, the Mascot score, i.e. the Top Score obtained after Mascot has incorporated the Mowse score into a probabilistic framework. It is defined as -10*LOG10(P), where P is the absolute probability that the observed match is a random event [21]. In case of SwissProt as selected protein sequence database, protein scores greater than 53 were significant (p < 0.05). In addition we requested mouse protein as the top candidates in the first pass search when no restriction was applied to the species of origin. Moreover, when the identification was not possible to reach from PMF, MS/MS data were collected and used as result, if Significant hits were obtained by Probability Based Mowse Score (individual ion scores >25 that indicate identity or extensive homology-p < 0.05-) [21]. In general, false positive evaluation was done by software with randomized searches to remove redundant peptide and protein identification.
The new identifications were furthermore searched in PeptideMap free software (ProWL free software programs, National Resource for the Mass Spectrometric Analysis of Biological Macromolecules, the Rockefeller University) [22] and compared with Mascot results (for details, please refer to Additional files 1, 2).
Results and discussion
Two-dimensional electrophoresis of serum proteins
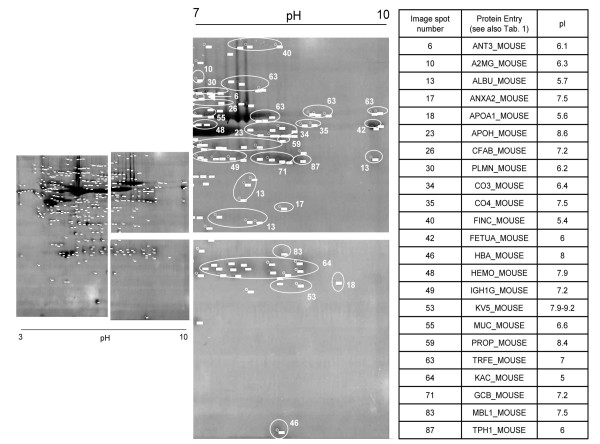
We found narrow 2-D gels to improve detection of low abundant proteins in sera, thereby avoiding any pre-fractionation. The 2-DE proteomics carries the advantage of visualizing changes in Mw and pI of a protein, which we find helpful in highlighting biologically significant processes. This electrophoresis technique has been applied successfully to identify oncoproteins in human serum and tissues [11,13,20,23]. Notably, mouse serum proteins were separated by 2-D electrophoresis (2-DE) and resolved in the first dimension in a broad pH range with IPGphor strips (pH 3 to 10 NL), and subsequently in a 12% gradient polyacrylamide SDS gel in the second dimension. On average, 350 spots could be detected and 77 unique mouse proteins were identified, of which more than 30% were in the basic region of the gel (Figure 1). Among them, we identified some proteins already known or evaluated as potential biomarkers for human malignancies. For instance, previous studies have shown the benefit of studying complement factors in human cancer, i.e. elevated CO3 levels identified in pancreatic cancer patients. Consequently, monitoring activity of CO3/CO4 is clinically relevant [24-26]. Moreover, heme in tissues and circulation may be come toxic due to oxygen radical formation. Hemopexin, a plasma protein binds heme with high affinity, and therefore limits its reactivity thereby facilitating its catabolism via receptor-mediated endocytosis. Furthermore, the heme-hemopexin complex is glycosilated at specific Hys residues. Obviously, monitoring the precursor at basic pH and its glycosilated form at acidic pH can be informative as biomarker of disease [27,28]. Likewise, we identified tryptophan 5-hydroxylase 1 (TPH1_MOUSE) in the serum proteome. This protein promotes the synthesis of 5-HT (serotonin). Intriguingly, a recent investigation has found its involvement in the regulation of the immunosystem and aberrant activity in cancers [29,30].
Figure 1.
Protein entries at basic region of 3–10 pH range. An amount of 23 proteins were identified at basic region of gels at pH range 3–10. As discussed in the text, most of those proteins could be relevant in biomarker discovery research because of their involvement in inflammation or in mechanisms that could bring toward the development of cancer.
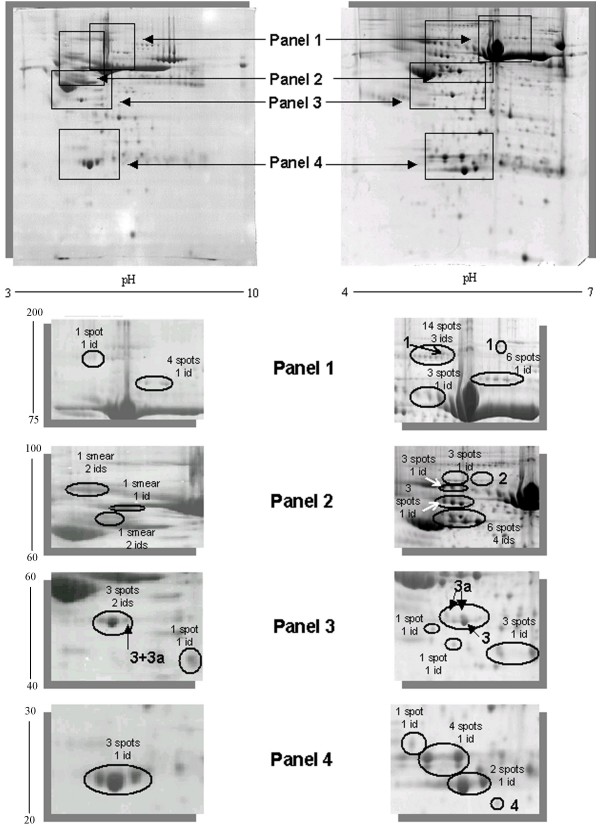
As shown in Figure 2 the sample complexity was further reduced by the use of IPG strips in the pH range of 4 to 7. The number of spots was increased by 2-fold. Approximately 660 spots were detected and 59 proteins were identified in this pH range. When compared with gels of the 3 to 10 pH-range, multiple isoforms of proteins can now be visualised and may have arisen from a combination of post-translational modifications as well as chemical modifications that occur during sample preparation (Figure 2) [20,31]. Apart from high abundant proteins, on average three spots for each protein at pH of 4 to 7 could be detected, when compared to one spot for each entry or smears observed with gels at pH of 3 to 10 (Figure 2). Altogether, the spot detection was improved and a total of 16 of these proteins were novel gene products resolved at pH 4 to 7 (Table 1, italic font). Notably, 4 of them had not been reported so far in mouse serum [5-9,32,33].
Figure 2.
Improved resolution with zoom-in 2-D gels. Details of the 2-D gel zoomed areas. We showed the improved separation and visualization of the mouse serum proteome. In fact, multiple isoforms for most identified proteins were found and other identified spots were detected only in 4–7 pH-range and not in 3–10 pH-range. Panel 1: 3–10 pH range (1 spot 1 id: mouse ceruloplasmin; 4 spots 1 id: mouse gelsolin) 4–7 pH range (14 spots 3 ids: mouse ceruloplasmin, mouse alpha-macroglobulin, mouse albumin; 6 spots 1 id: mouse gelsolin; 3 spots 1 id: mouse hemopexin).Panel 2: 3–10 pH range (1 smear 2 ids: mouse afamin and mouse hemopexin; 1 smear 1 id: mouse kininogen; 1 smear 2 ids: mouse antithrombin-III, mouse Alpha-2-HS-glycoprotein) 4–7 pH range (3 spots 1 id: mouse prothrombin; 3 spots 1 Id: mouse hemopexin; 3 spots 1 id: mouse kininogen; 6 spots 4 ids: mouse antithrombin-III, mouse Alpha-2-HS-glycoprotein, mouse vitamin D-binding protein and mouse fetuin-b).Panel 3: 3–10 pH range (3 spots 2 ids: mouse apolipoprotein A4 and mouse zinc-alpha-2-glycoprotein; 1 spot 1 id: mouse albumin) 4–7 pH range (1 spot 1 id: mouse serum paraoxonase/arylesterase 1; 1 spot 1 id: mouse H-2 class I histocompatibility antigen; 3 spots 1 id: mouse alpha-2-macroglobulin). Panel 4: 3–10 pH range (3 spots 1 id: mouse apolipoprotein A1) 4–7 pH range (1 spot 1 id: mouse mannose-binding protein2; 4 spots 1 id: mouse Ig kappa chain V-III region; 2 spots 1 id: mouse apolipoprotein A2). The spots 1 (alpha-2-macroglobulin); 2 (complement C1r-subcomponent); 3 and 3a (Apoliprotein A4 and Zinc-alpha-glycoprotein 2 respectively) and 4 (glutathione peroxidase 3) are examples discussed respectively in the text and in Figure 5.
Table 1.
Mouse serum protein map
| No. | SwissProt entry | SwissProt accession | Mw (KDa) | pI | Mascot score | sequence coverage (%) | msms (m/z) | matrix | Our Map 1 (matched peptides) | Ref Map 2 (matched peptides) | Ref Map 3 (matched peptides) | Ref Map 4 (matched peptides) | Ref Map 5 (matched peptides) | Ref Map 6 (matched peptides) |
| 1 | A1AG1 MOUSE | Q60590 | 24 | 5.6 | 124 | 32 | 1703.91 | CHCA DHB | 10 | not present | 4 | 12 | 5 (up regulated) | present |
| 2 | AMBP MOUSE | Q07456 | 40 | 6 | 38 | 4 | 1669.85 | CHCA | 1 | 3* | not present | not present | not present | not present |
| 3 | APOM MOUSE | Q9Z1R3 | 21.6 | 6 | 105 | 36 | 938.42 | CHCA DHB | 9 | not present | not present | not present | not present | present |
| 4 | MUP1 MOUSE | P11588 | 20.9 | 5 | 115 | 55 | DHB | 12 | 0 | 10 | not present | not present | not present | |
| MUP2 MOUSE | P11589 | 20.9 | 5 | 146 | 68 | DHB | 14 | 0 | 11 | 19 | not present | not present | ||
| MUP6 MOUSE | P02762 | 20.9 | 5 | 136 | 62 | DHB | 12 | 0 | 10 | not present | not present | not present | ||
| MUP8 MOUSE | P04938 | 17.7 | 5 | 141 | 74 | DHB | 12 | 0 | 10 | not present | not present | not present | ||
| 5 | A1AT1 MOUSE | P07758 | 46.1 | 5.4 | 145 | 41 |
981.56 1137.66 1232.70 2003.04 2327.09 2405.17 3498.80 |
CHCA DHB | 15 | 10* 2' |
13 | 12 | 20 (up regulated) | not present |
| A1AT2 MOUSE | P22599 | 46.1 | 5.3 | 104 | 36 |
2405.17 2003.04 2327.09 3498.80 |
CHCA DHB | 12 | 9' | not present | 7 | 3 (up regulated) | present | |
| A1AT3 MOUSE | Q00896 | 46 | 5.3 | 146 | 44 |
981.56 1137.66 2003.04 2327.09 2405.17 3498.80 |
CHCA DHB | 15 | not present | 16 | 15 | not present | not present | |
| A1AT4 MOUSE | Q00897 | 46.1 | 5.2 | 162 | 51 |
1232.74 2003.04 2327.09 3498.80 |
CHCA DHB | 17 | not present | not present | not present | not present | present | |
| A1AT5 MOUSE | Q00898 | 46 | 5.4 | 105 | 35 |
1232.71 2327.09 2405.17 |
CHCA DHB | 12 | not present | not present | not present | not present | present | |
| A1AT6 MOUSE | P81105 | 46 | 5.2 | 161 | 47 |
981.56 1137.66 1232.70 2003.04 2327.09 2405.17 3498.80 |
CHCA DHB | 16 | 2 | not present | 8 | not present | present | |
| 6 | ANT3 MOUSE | P32261 | 52.5 | 6.1 | 298 | 56 |
1198.70 1340.69 1359.67 1700.89 |
CHCA DHB | 29 | 6 | not present | not present | not present | present |
| 7 | A2AP MOUSE | Q61247 | 55.1 | 5.8 | 169 | 36 |
1591.80 1680.81 |
CHCA DHB | 15 | 3 | not present | not present | not present | present |
| 8 | CBG MOUSE | Q06770 | 44.9 | 5 | 148 | 32 | CHCA DHB | 10 | 3 | not present | not present | not present | present | |
| 9 | SPA3K MOUSE | P07759 | 47 | 5 | 113 | 29 | CHCA DHB | 12 | 12 | not present | 21 | 7 (up regulated) | present | |
| 10 | A2MG MOUSE | Q61838 | 167 | 6.3 | 153 | 19 | 2068.21 | CHCA DHB | 18 | 25 | 16 (immuno depletion) | not present | not present | present |
| 10a | A2MG MOUSE C-term | Q61839 | 28 | 7 | 62 | 6 |
1031.50 1111.58 1216.61 1787.96 |
CHCA DHB | 9 | ? | not present | 13 | 16 (up regulated) | present |
| 11 | ADIPO MOUSE | Q60994 | 26.9 | 5.3 | 63 | 19 | 1504.72 | DHB | 5 | not present | not present | not present | not present | present |
| 12 | AFAM MOUSE | O89020 | 71.5 | 5.5 | 155 | 31 | CHCA DHB | 15 | 1 | 9 | not present | not present | present | |
| 13 | ALBU MOUSE | P07724 | 70.7 | 5.7 | 426 | 68 |
1455.83 1479.70 1609.90 1882.96 1960.15 1981.98 |
CHCA DHB | 40 | 26* 46' |
17 | 48 | 12 (up regulated) | present |
| 14 | ALS MOUSE | P70389 | 67.7 | 6.1 | 97 | 28 | DHB | 13 | not present | not present | not present | not present | present | |
| 15 | CPN2 MOUSE | Q9DBB9 | 61.3 | 5.5 | 131 | 25 | CHCA | 10 | not present | not present | not present | not present | present | |
| 16 | ANGL6 MOUSE | Q8R0Z6 | 51.4 | 9.2 | 56 | 16 | DHB | 4 | not present | not present | not present | not present | not present | |
| 17 | ANXA2 MOUSE | P07356 | 38.8 | 7.5 | 82 | 18 | CHCA | 5 | not present | not present | not present | not present | not present | |
| 18 | APOA1 MOUSE | Q00623 | 30.5 | 5.6 | 207 | 49 |
1237.67 1318.61 1331.53 1340.77 |
CHCA DHB | 17 | 5* 18' |
13 | 18 | 22 | present |
| 19 | APOA2 MOUSE | P09813 | 11.3 | 6.6 | 85 | 14 |
1193.62 1831.98 |
CHCA DHB | 2 | 4' | 7 | 3 | 3 (up regulated) | present |
| 20 | APOA4 MOUSE | P06728 | 45 | 5.4 | 223 | 60 |
1131.66 1231.61 1461.75 2023.05 |
CHCA DHB | 21 | 2* 5' |
15 | 29 | 6 (up regulated) | present |
| 21 | APOE MOUSE | P08226 | 35.9 | 5.6 | 166 | 51 |
968.52 1075.60 1599.82 |
CHCA DHB | 21 | 5' | 7 | 17 | 16 (up regulated) | present |
| 22 | APOC3 MOUSE | P33622 | 10.9 | 4.6 | 140 | 19 |
1062.46 1078.45 1987.94 |
CHCA | 3 | not present | 3 | 3 | not present | present |
| 23 | APOH MOUSE | Q01339 | 39.9 | 8.6 | 276 | 62 |
1325.68 1544.85 2719.48 |
CHCA DHB | 22 | 7 | not present | 17 | 22 (up regulated) | present |
| 24 | CLUS MOUSE | Q06890 | 55.2 | 5.5 | 103 | 19 | CHCA DHB | 11 | 2* | 9 | 12 | not present | present | |
| 25 | C1R MOUSE | Q8CG16 | 81.5 | 5.4 | 78 | 17 | CHCA | 8 | not present | not present | not present | not present | not present | |
| 26 | CFAB MOUSE | P04186 | 86.3 | 7.2 | 269 | 37 | CHCA DHB | 23 | 3 | not present | not present | not present | present | |
| 27 | CFAI MOUSE | Q61129 | 69.5 | 7.4 | 103 | 21 | 1726.85 | DHB | 11 | 3 | not present | not present | not present | not present |
| 28 | HGFA MOUSE | Q9R098 | 72.9 | 6.6 | 58 | 13 | DHB | 6 | not present | not present | not present | not present | present | |
| 29 | HPT MOUSE | Q61646 | 39.2 | 5.9 | 146 | 49 |
980.49 1320.74 1373.61 |
DHB | 21 | not present | 11 | 5 | 14-3 (up and down regulated) | present |
| 29a | HPT MOUSE | Q61646 | 81 | 11 | 1679.78 | DHB | 6 | ? | ? | 14 | ? | not present | ||
| 30 | PLMN MOUSE | P20918 | 93.4 | 6.2 | 387 | 53 | 1138.46 | CHCA DHB | 35 | 8 | not present | 32 | 6 | present |
| 31 | CFAH MOUSE | P06909 | 144 | 6.6 | 269 | 31 | CHCA DHB | 29 | 6 | not present | 18 | not present | present | |
| 32 | CS1A MOUSE | Q8CG14 | 78.3 | 5 | 66 | 15 | DHB | 6 | not present | not present | not present | not present | not present | |
| 33 | F13B MOUSE | Q07968 | 78.3 | 5.6 | 148 | 26 | CHCA | 15 | not present | not present | not present | not present | present | |
| 34 | CO3 MOUSE | P01027 | 188 | 6.4 | 312 | 29 | 1886.93 | CHCA DHB | 40 | 23* 1' |
not present | not present | 17 (up regulated) | present |
| 35 | CO4 MOUSE | P01029 | 194 | 7.5 | 123 | 9 | CHCA | 14 | 3 | not present | not present | not present | present | |
| 36 | CO9 MOUSE | P06683 | 63.2 | 5.6 | 80 | 29 | DHB | 14 | 1 | not present | not present | not present | not present | |
| 37 | C1QB MOUSE | P14106 | 27 | 8.6 | 69 | 20 | CHCA | 5 | 1* | not present | not present | not present | present | |
| 38 | EGFR MOUSE | Q01279 | 138 | 6.5 | 171 | 16 | CHCA DHB | 15 | not present | not present | 18 | not present | present | |
| 39 | FIBB MOUSE | Q8K0E8 | 55.4 | 6.7 | 112 | 39 | CHCA DHB | 20 | not present | not present | not present | not present | present | |
| 40 | FINC MOUSE | P11276 | 276 | 5.4 | 90 | 8 | CHCA | 16 | 9* | not present | not present | not present | present | |
| 41 | FCN1 MOUSE | O70165 | 36.8 | 6 | 65 | 12 | DHB | 5 | not present | not present | 8 | not present | not present | |
| 42 | FETUA MOUSE | P29699 | 38.1 | 6 | 135 | 47 | 1653.75 2138 | CHCA DHB | 11 | 3 | 6 | 8 | not present | present |
| 43 | FETUB MOUSE | Q9QXC1 | 43.5 | 6.2 | 113 | 30 |
1382.89 1159.7 |
CHCA DHB | 14 | 1 | 5 | 13 | not present | present |
| 44 | GPX3 MOUSE | P46412 | 25.6 | 8.3 | 98 | 45 | 1955 | CHCA DHB | 12 | not present | not present | not present | not present | present |
| 45 | HA10 MOUSE | P01898 | 37.2 | 5.2 | 173 | 46 | 1671.86 | CHCA DHB | 16 | 3 | not present | not present | not present | not present |
| 46 | HBA MOUSE | P01942 | 15 | 8 | 79 | 31 |
1589.82 1819.93 |
CHCA | 5 | 2* 6' |
not present | not present | 8 | present |
| 47 | HBB1 MOUSE | P02088 | 16 | 7.3 | 68 | 73 | 1274.72 | CHCA DHB | 9 | not present | not present | not present | 7 (up regulated) | present |
| 48 | HEMO MOUSE | Q91X72 | 52 | 7.9 | 188 | 43 |
1100.47 1212.63 1504.76 1516.71 1727.77 2472.12 |
CHCA DHB | 21 | 14 | 8 | 25 | 3 (up regulated) | present |
| 49 | IGHG1 MOUSE | P01868 | 36.2 | 7.2 | 94 | 45 | DHB | 9 | 3 | not present | not present | not present | not present | |
| 50 | KNG1 MOUSE | O08677 | 74.1 | 6 | 164 | 29 |
1010.56 1060.56 1515.68 |
CHCA DHB | 18 | 9 | 12 | 24 | 5 (up regulated) | not present |
| 51 | KLKB1 MOUSE | P26262 | 73.4 | 8.4 | 105 | 22 | CHCA | 14 | 1* | 2 | not present | not present | present | |
| 52 | KV3A1/2/4 MOUSE | P01654 | 12 | 5 | 116 | 16 |
1616.89 1855.04 |
CHCA DHB | 2 | not present | not present | not present | not present | present |
| KV3AD MOUSE | P01665 | 12 | 4.9 | 54 | 44 |
1155.46 1616.89 1855.04 |
CHCA DHB | 4 | not present | not present | not present | not present | present | |
| 53 | KV5AB MOUSE | P01644 | 12 | 7.9 | 88 | 17 |
1028.56 1926.92 2455.35 |
CHCA DHB | 3 | not present | not present | not present | not present | not present |
| KV5J MOUSE | P01645 | 11.9 | 9.2 | 93 | 16 | 1926.81 | CHCA DHB | 1 | not present | not present | not present | not present | present | |
| 54 | KV3N MOUSE | P01666 | 12 | 4.5 | 58 | 53 | DHB | 3 | not present | not present | not present | not present | present | |
| 55 | MUC MOUSE | P01872 | 50 | 6.6 | 184 | 30 |
1330.78 1603.91 |
CHCA DHB | 14 | 4 | not present | not present | not present | not present |
| 56 | MUG1 MOUSE | P28665 (Q80XE6) | 166.4 | 6 | 168 | 20 | CHCA DHB | 24 | 12 | not present | not present | not present | present | |
| 57 | PHLD MOUSE | O70362 | 93.8 | 6.6 | 151 | 23 | CHCA DHB | 20 | 1 | not present | not present | not present | present | |
| 58 | PON1 MOUSE | P52430 | 34.6 | 5 | 117 | 32 | 1853.9 | CHCA DHB | 8 | 2 | not present | not present | not present | present |
| 59 | PROP MOUSE | P11680 | 50 | 8.4 | 87 | 21 | CHCA | 8 | not present | not present | not present | not present | present | |
| 60 | RETBP MOUSE | Q00724 | 23.5 | 5.7 | 138 | 59 |
1226.63 1360.58 1789.84 2079.88 |
CHCA DHB | 15 | 1 | not present | not present | not present | present |
| 61 | SAMP MOUSE | P12246 | 26.4 | 6 | 88 | 38 | 2133.03 | CHCA DHB | 8 | 1 | 8 | 8 | not present | present |
| 62 | THRB MOUSE | P19221 | 71.6 | 6 | 156 | 28 | 1189.56 | CHCA DHB | 19 | 4 | not present | 26 | 9 (up regulated) | present |
| 63 | TRFE MOUSE | Q921I1 | 78.8 | 7 | 308 | 51 |
1171.61 1419.86 1656.81 1990.82 2007.94 |
CHCA DHB | 38 | 39* 1' |
? | 26 | not present | present |
| 64 | KAC MOUSE | P01837 | 11.9 | 5 | 91 | 87 | 990.51 | CHCA DHB | 8 | 1 | not present | not present | not present | not present |
| 65 | ZA2G MOUSE | Q64726 | 35.4 | 5.8 | 173 | 55 |
1274.60 1318.81 1409.72 1610.74 |
CHCA DHB | 15 | 1 | not present | 19 | not present | present |
| 66 | VTDB MOUSE | P21614 | 55.1 | 5.4 | 266 | 44 |
1051.6 1303.77 2441.13 |
CHCA DHB | 24 | 3 | 12 | 31 | not present | present |
| 67 | TTHY MOUSE | P07309 | 15.9 | 5.8 | 122 | 67 |
1382.62 1554.89 2438.17 2517.22 |
CHCA DHB | 8 | 4* 1' |
10 | 9 | not present | present |
| 68 | GELS MOUSE | P13020 | 86.3 | 5.8 | 233 | 39 |
1254.75 1275.73 |
CHCA DHB | 22 | 6 | not present | 19 | not present | present |
| 69 | VTNC MOUSE | P29788 | 55.6 | 5.7 | 82 | 23 | CHCA DHB | 10 | 2 | not present | not present | not present | present | |
| 70 | GCAB MOUSE | P01864 | 37 | 8.5 | 31.5 | 5 | 1913.84 | DHB | 1 | 2 (membrane form) | not present | not present | not present | not present |
| 71 | GCB MOUSE | P01866 | 37.3 | 7.2 | 79 | 30 | 1778.87 | CHCA DHB | 8 | 3 (membrane form) | not present | not present | not present | not present |
| 72 | LAC1 MOUSE | P01843 | 11.7 | 5.9 | 70 | 61 | DHB | 4 | not present | not present | not present | not present | not present | |
| 73 | LAC2 MOUSE | P01844 | 11.4 | 5.9 | 70 | 89 | DHB | 5 | not present | not present | not present | not present | present | |
| 74 | ACTG MOUSE | P63260 | 42.1 | 5.3 | 188 | 53 |
1198.75 1790.93 1954.14 |
CHCA DHB | 16 | not present | not present | not present | not present | not present |
| ACTB MOUSE | P60710 | 42 | 5.3 | 171 | 49 |
1198.75 1790.93 1954.14 |
CHCA DHB | 15 | not present | not present | not present | not present | present | |
| 75 | APC MOUSE | Q61315 | 313 | 7.4 | 53 | 6 | CHCA | 15 | not present | not present | not present | not present | present | |
| 76 | CT160 MOUSE | Q8VCC6 | 22 | 9.5 | 56 | 20 | CHCA | 5 | not present | not present | not present | not present | not present | |
| 77 | CUL1 MOUSE | Q9WTX6 | 90.3 | 8.2 | 62 | 15 | CHCA | 7 | not present | not present | not present | not present | not present | |
| 78 | ESTN MOUSE | P23953 | 61.4 | 5.1 | 168 | 37 | 911.45 | CHCA DHB | 21 | 12 | 7 | not present | not present | present |
| 79 | ITIH2 MOUSE | Q61703 | 106 | 6.8 | 150 | 24 | 1337.73 | CHCA DHB | 14 | 2 | not present | not present | not present | present |
| 80 | ITIH4 MOUSE | 9055252 | 104.8 | 6 | 133 | 20 | CHCA | 17 | not present | not present | 18 | not present | not present | |
| 81 | K2C5 MOUSE | Q922U2 | 62 | 7.6 | 60 | 16 | DHB | 11 | not present | not present | not present | not present | not present | |
| 82 | LIFR MOUSE | P42703 | 123.8 | 5.7 | 151 | 19 | DHB | 15 | not present | not present | 22 | not present | present | |
| 83 | MBL1 MOUSE | P39039 | 25.8 | 7.5 | 100 | 27 | 1544.89 | CHCA | 9 | not present | not present | not present | 8 (up regulated) | present |
| 84 | MBL2 MOUSE | P41317 | 26.3 | 5 | 70 | 29 |
1323.60 1522.72 1766.83 |
CHCA DHB | 6 | 2 | not present | 8 | not present | not present |
| 85 | OST5 MOUSE | Q8BSL4 | 40.7 | 9.7 | 70 | 23 | CHCA | 6 | not present | not present | not present | not present | not present | |
| 86 | SYT2 MOUSE | P46097 | 47.7 | 8.2 | 61 | 19 | CHCA | 6 | not present | not present | not present | not present | present | |
| 87 | TPH1 MOUSE | P17532 | 51.9 | 6 | 59 | 17 | CHCA | 7 | not present | not present | not present | not present | not present | |
| 88 | KPYM MOUSE | P52480 | 58 | 7 | 53 | 30 | DHB | 12 | not present | not present | not present | not present | not present | |
| 89 | DYN2 MOUSE | P39054 | 98 | 7 | 68 | 12 | CHCA DHB | 9 | not present | not present | not present | not present | not present | |
| 90 | GAB2 MOUSE | Q9Z1S8 | 73.6 | 8.5 | 33 | 2 | 1794.8 | DHB | 1 | not present | not present | not present | not present | present |
Mouse serum proteins identified by us in serum from C57BL6 mice (Our Mapping 1), in comparison with previous mouse serum maps (Ref Map 2: [9], Ref Map 3: [8], Ref Map 4: [7,32], Ref Map 5: ref [33] and Ref Map 6: [6]). Exactly, 16 of them (italic font) were identified only by narrow IPGs of pH 4–7. In bold, we highlighted the proteins identified only with LIFT-MS/MS measurements. In case of TCA/methanol precipitation method (Ref Map 2: [9]) we specified where the proteins were identified. (n*: precipitated fraction; n': supernatant fraction-). In the case of MUPs, we marked with 0 the entries, because there are evidences that those proteins are present just in C57BL6 mice and not in BALA/cj inbred strain [32].
Improved MALDI TOF-MS analysis of serum proteins
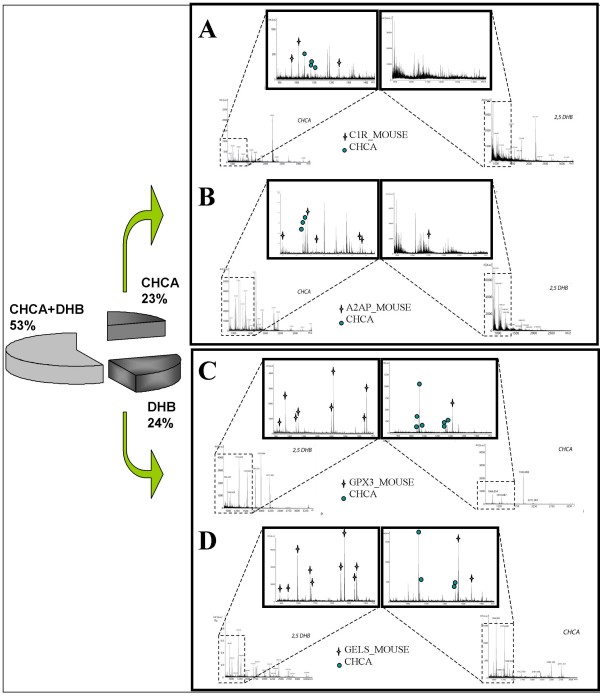
To increase the number of ionized tryptic-digested peptides, we took advantage of the properties of two commonly used matrices, CHCA and DHB [15,19]. To the best of our knowledge sample-matrix preparations with both matrices in sequence for serum proteome profiling has not been reported so far. These matrices differ considerably upon MALDI ionization. We combined a thin layer and the ML deposition on the AnchorChip™ to identify 2-D spots from mouse serum samples using a protocol recently described by us [19]. Briefly, we used a Bruker MALDI target plate, equipped with hydrophilic patches ("anchors") in hydrophobic surroundings (AnchorChip™, Bruker Daltonics), and achieved signal uniformity, high sensitivity and an improved signal-to-noise ratio which gave rise to less complex MS spectra of which an example is given in Figure 2. Moreover, the re-crystallization of the sample-matrix mixtures loaded on the target increased considerably the number of identifiable peptide ions in an automated MS and MS/MS spectra acquisition mode (see also Table 1). Striking differences in the MALDI MS spectra of peptides were observed when the two matrices were compared (Figure 3). We observed better ionization of peptides from the tryptic-digested proteins with DHB as compared with CHCA. Indeed with CHCA, we frequently observed peaks at m/z 1044, 1060, 1066, 1082, 1249 and 1271 (Figure 3, azure cycles). We used matrix ion suppression in deflection mode of m/z 800. These abundant matrix clusters display high s/n ratio and resulted in poor acquired PMF spectra [11,34]. Previous reports had already highlighted differences in PMF because of different behaviour of the two matrices [15,16,18]. In particular, Zhu and Papayannopoulos tested several matrices and reported that DHB gave the best results without interferences from matrix ion peaks. Furthermore, common interferences from matrix-adducts of CHCA were observed, in particular in the range of m/z 800–1100 of the MALDI spectra [35].
Figure 3.
Spectra comparisons between CHCA and DHB. (A, B) CHCA matrix vs DHB matrix. Considerably, in the case of DHB the peptide ions signals are less resolved than other signals in the spectrum, maybe connected to metastable decay of ions in the drift tube or "chemical noises" from matrix ions. On the other hand, CHCA was enabled to identify complement C1r-subcomponent (C1r_MOUSE) and alpha-2-antiplasmin (A2AP_MOUSE). Crosses represent matched peptides to the identification. (C, D) DHB matrix vs CHCA matrix. The spectra from DHB are notably rich of peptides ions fragments (crosses) which belong to the identification, i.e. glutatione peroxidase-secreted form (GPX3_MOUSE) and gelsolin (GELS_MOUSE). The blue circles on CHCA spectra, instead, represent matrix fragments which hide the peaks could be matched to the identifications. The pie chart represents our mouse proteome mapping, where both matrices have the almost same input in the identifications.
Nonetheless, there are obvious benefits in the use of the CHCA matrix in the MALDI MS analysis. This included a high uniform layer, which it forms, especially on the AnchorChip™ (data not shown). Its ability to induce desorption of ions at lower laser energies demonstrates transfer of sufficient energy for the pre-formation of ions. In some cases, metastable ions of matrix ion fragments were less formed when compared with DHB [36]. This enabled acquisition of less ambiguous spectra for the identification of proteins. The different behaviours of both matrices are the subject of several published studies. In particular, Luo et al. [37] reported loss of internal energy of ions generated by MALDI and the role of the two matrices in desorption and ionisation processes.
Indeed, in a complex mixture such as serum, it is not really clear why in some cases diagnostic PMFs are obtained with DHB rather than CHCA and vice versa. Some examples, depicted in Figure 3, clearly demonstrate that it is difficult to predict which matrix would deliver best PMFs. For that reason, we exploited both matrices in order to redress any drawbacks of one matrix by the use of the other one especially in automatic data acquisition procedures.
Mapping of the mouse serum
As shown in Table 1, we were able to identify 90 unique proteins, some of which were reported to be identifiable only after sample pre-treatment or sophisticated and time-consuming procedures. For instance, several members of complement factor family [CFAB_MOUSE, CFAI_MOUSE, CO3_MOUSE, CO4_MOUSE, CO9_MOUSE and C1QB_MOUSE], properdin [PROP_MOUSE] and mannose binding protein A [MBL1_MOUSE] were identified at the basic pH region of the 2-D gels. None of them were reported in previous mouse serum proteome profiling studies using 2-DE and MS analysis [7,8,32]. Indeed, when the serum was albumin-depleted, other high abundance proteins were lost as well. Furthermore, complement factor 3 and transthyretin were found in both fractions, albumin-rich and depleted (Table 1) [9]. This necessitated repetitive analyses. Indeed, complement factor 3 was shown to be up-regulated in human lung adenocarcinomas [33], as were increased serum levels of transthyretin and down-regulation of transferrin in human type-2 diabetes [38].
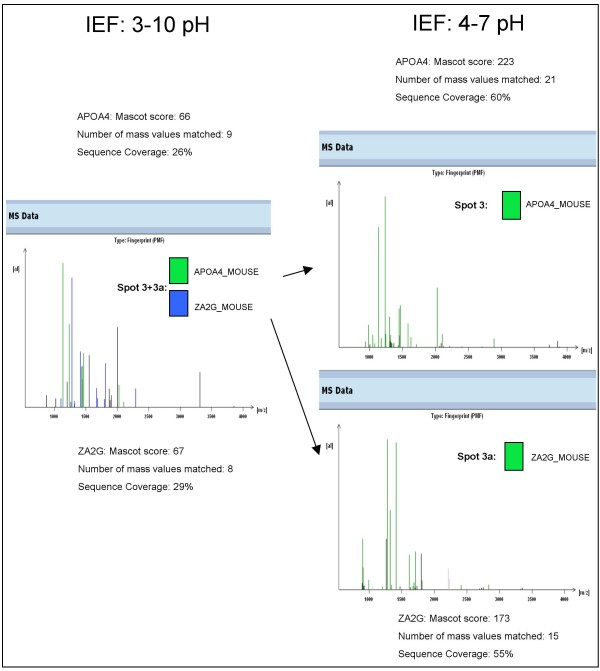
The application of our protocol enabled an identification of properdin [PROP_MOUSE] and adenomatous polyposis coli protein [APC_MOUSE]. Once again, in previous mouse serum maps these proteins were not identified [7-9,32,33] (Table 1). We were able to identify most of the proteins but some new proteins were identified as well (Table 1). Notably, with our optimized MALDI- matrix protocols, we were able to characterize the protein C1R complement [C1R_MOUSE] by the sequential use of DHB and CHCA (spot 2 in Figure 2, Figure 3). This is a serine protease that combines with C1q and C1s (which we had identified in our sample as well) to form C1, the first component of the classical pathway of the complement system. The spot corresponding to C1R is positional in the gel where the molecular weight and pH differs from the theoretical values, presumably because of glycosylation of this protein [39]. Post-translational modifications (PTMs) do change the chemical characteristics of the protein that runs in 2-D gels at more acidic and higher mass ranges. Indeed, we identified glutathione peroxidase 3 [GPX3_MOUSE] (pI 8.3) in 2-D gel at pH 4–7 (spot 4 in Figure 2, Figure 3). The gene product GPX3_MOUSE is a secreted protein that protects cells and enzymes from oxidative damage, by catalyzing the reduction of hydrogen peroxide, lipid peroxides and organic hydroperoxide. This protein was reported to be regulated in various malignancies [40].
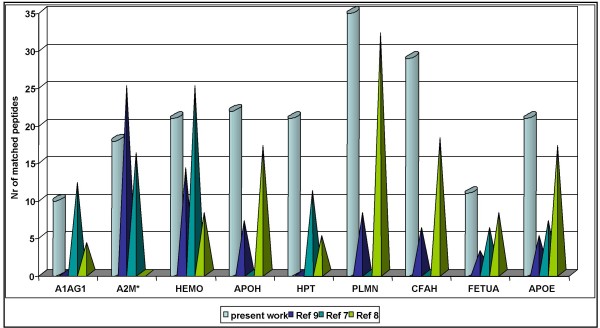
Without sample pre-treatments, we were able to identify proteins such as hemopexin [HEMO_MOUSE] and alpha-2-macroglobulin [A2MG_MOUSE]. Usually, an identification and quantification of these proteins requires time consuming procedures [9,32]. Here we describe a simple protocol that allowed facile identification of the same proteins even with a higher number of matching peptides. For instance, spot 1 (see panel 1 in Figure 2) was identified as A2MG_MOUSE with high sequence coverage. Previously, pre-fractionation of the serum was needed to enable its identification [32]. Likewise, with our protocol HEMO_MOUSE was identified with a Mascot score of 188 and 21 matched peptides, a result comparable to after immunodepletion (25 matched peptides), and pre-fractionation (14 matched peptides) but considerable better than with data from other mouse serum maps (8 matched peptides) [7-9]. In Figure 4, we display identified proteins where the number of matched peptides amongst different strategies was compared; in general, we obtain a better protein score and comparable or even higher number of matched peptides of the protein sequence, without any sample pre-treatment (cylinders in Figure 4, Table 1).
Figure 4.
Comparison of matched peptides. We have depicted here a comparison of some identification (x-axis) from our work (azure-cylinder) and three different mouse serum maps (prisms) [see ref [9,7,8]]. Note the number of matched peptides (y-axis) is higher or comparable with the pre-fractionation methods. Mouse protein identifications: A1AG1: alpha-1-acid glycoprotein, A2M: alpha-2-macroglobulin, HEMO: hemopexin, APOH: beta-2-glycoprotein 1, HPT: haptoglobin, PLMN: plasminogen, CFAH: complement factor H, FETUA: alpha-2-HS-glycoprotein-Fetuin-A, APOE: apoliproteinE.
When no significant PMF entries were obtained, we attempted identification by MS/MS spectra acquisition. For instance, we acquired the LIFT TOF-TOF spectrum of the peak yielded at m/z 1913.84 -the peptide sequence APQVYVLPPPAEEMTKK- with an ion score of 33 and error tolerance of 10 ppm. The peptide was then matched to Ig gamma-2A chain C [GCAB_MOUSE]. Additionally, we identified the secreted protein alpha-1-microglobulin (AMBP_MOUSE) by MS/MS with the parental ion at m/z 1669.85 and a peptide sequence of TIAACNLPIVQGPCR. We thus obtained the significant hit at ion score of 38 with 5 ppm as peptide mass tolerance; according to RMS error, and 0.5 Da as fragment mass tolerance [21].
Furthermore, we identified a number of proteins such as gamma actin [ACTG_MOUSE] which was believed to be primarily cytoskeletal and/or intracellular but was not reported so far for the mouse serum proteome. Three peaks belonging to ACTG_MOUSE were identified at m/z 1198.75 -peptide sequence AVFPSIVGRPR- 1790.93 -peptide sequence SYELPDGQVITIGNER- and 1954.14 -peptide sequence VAPEEHPVLLTEAPLNPK-, with an ion scores higher than 23. Further examples are listed in Table 1 (from number 74 to 90).
Taken collectively, our protocol enabled automated data acquisition and improved significantly the identification of proteins based on higher sequence coverage and the number of matched peptides. For instance, CFAH_MOUSE was identified with a Mascot score of 269, a 31% sequence coverage and 29 matched peptides, instead of 23% sequence coverage and 6 and 18 matched peptides as reported previously (Table 1). Similarly, the sequence coverage of apolipoprotein H (APOH_MOUSE) was increased by 17% with 5 additional peptides that could be mapped to this protein [7,9] (for sequence coverages, please refer to Additional file 3).
Newly identified proteins
Thirteen new mouse serum proteins were identified with our approach. Among them, some were recently confirmed by another group [5].
In their approach [5], however, matched peptides were less on average when compared to our data. Apart from this difference, a total of 6 new proteins are reported by us (italic font in Table 2).
Table 2.
New proteins, spectra interpretation and validation
| No | No Table 1 | Swiss Prot entry | Swiss Prot accession | putative PTMs | Subcellular location | Mw (KDa) | pI | RMS error (ppm) | matrix | Swiss Prot | NCBInr | MSDB | Nr of matched peptides (Mascot) | Nr of matched peptides (Peptide Map) |
| 1 | 16 | ANGL6 MOUSE | Q8R0Z6 | G | Secreted; highly expressed in the liver | 51.4 | 9 | 38 | DHB | 56 | 58 | 55 | 4 | 3 |
| 2 | 17 | ANXA2 MOUSE | P07356 | P | Secreted, extracellular space, extracellular matrix, basement membrane. Melanosome | 38.8 | 8 | 45 | CHCA | 82 | 81 | 82 | 5 | 3 |
| 3 | 25 | C1R MOUSE | Q8CG16 | G | Secreted | 81.5 | 5 | 15 | DHB CHCA | 78 | 78 | 78 | 8 | 5 |
| 4 | 32 | CS1A MOUSE | Q8CG14 | G | Predominantly expressed in liver | 78.3 | 5 | 11 | DHB | 66 | 66 | 66 | 6 | 5 |
| 5 | 72 | LAC1 MOUSE | P01843 | Secreted | 11.7 | 6 | 4 | DHB | 70 | 70 | 70 | 4 | 3 | |
| 6 | 74 | ACTG MOUSE | P63260 | P | Cytoplasm, cytoskeleton | 42.1 | 5 | 28 | DHB CHCA | 188 | 188 | 188 | 16 | 15 |
| 7 | 76 | CT160 MOUSE | Q8VCC6 | 22 | 10 | 35 | CHCA | 55 | 56 | 56 | 5 | 4 | ||
| 8 | 77 | CUL1 MOUSE | Q9WTX6 | G | Embryo fibroblasts and embryo preadipocytes | 90.3 | 8 | 49 | CHCA | 62 | 62 | 62 | 7 | 6 |
| 9 | 81 | K2C5 MOUSE | Q922U2 | P | Expressed in epidermis | 62 | 8 | 34 | DHB | 60 | 60 | 60 | 11 | 11 |
| 10 | 85 | OST5 MOUSE | Q8BSL4 | G | Golgi apparatus membrane; Single-pass type II membrane protein | 40.7 | 10 | 35 | CHCA | 70 | 70 | 70 | 6 | 5 |
| 11 | 87 | TPH1 MOUSE | P17532 | P | Cytoplasmatic enzyme, pineal gland | 51.9 | 6 | 30 | CHCA | 57 | 57 | 57 | 7 | 5 |
| 12 | 88 | KPYM MOUSE | P52480 | P | Liver, red cells, muscles, brain | 58 | 7 | 39 | DHB | 53 | 53 | 53 | 11 | 10 |
| 13 | 89 | DYN2 MOUSE | P39054 | P | Cytoplasm | 98 | 7 | 51 | DHB CHCA | 68 | 68 | 68 | 9 | 9 |
We listed here the 13 proteins which re not present in the previous mouse serum maps [6-9,32,33].
The SwissProt entries in Italic font are proteins not identified in mouse serum proteome so far [5-9,32,33]. RMS (Root Mean Square) error is the calculated error for set of matched mass values (in ppm) in Mascot Search (matrix science, LTD, UK). It is measured in ppm. RMS error defines the limit of peptide mass tolerance (Peptide tol +-) for Mascot Peptide Mass Fingerprint to obtain a significant score (p < 0.05) of matched peptides to select protein entry.
The results were first searched in SwissProt databank and furthermore in NCBInr and MSDB databanks. The same criteria were applied (such as: mus musculus for the taxonomy, < +/- 50 ppm as peptide tolerance and one missed cleavage allowed for trypsin enzyme). In bold we highlighted the significant score (p < 0.05) according to the chosen database (SwissProt >53, NCBInr and MSDB >61) [21,51].
We also specified if one matrix or the combination of them allowed the identification.
Post translation modifications (PTMs). P: phosphorylation; G: Glycosylation.
For instance, we identified members of complement cascade activation, a positive mediator for angiogenesis (ANGL6_MOUSE), proteins specifically regulated in several tumor cells such as [KPYM_MOUSE] and proteins playing an important role in protein degradation and protein ubiquitinylation, whose altered activity could allow for abnormal cell proliferation (CUL-1_MOUSE). Notably, some of these newly identified proteins may serve as cancer biomarkers [5,41-43].
In this regard, we identified heparan sulfate glucosamine 3-O-sulfotransferase 5 (OST-5_MOUSE), a sulfotransferase that catalyzes the transfer of a sulfo group from the sulfo donor, 3'-phosphoadenosine-5'-phosphosulfate (PAPS), to the 3-OH position of a glucosamine unit of the HS. It displays anticoagulant activity by interacting with antithrombin (AT) protein through sulfanation at the 3-OH position of HS-saccharide [44-46]. From recent studies, new biological functions of activated HS have emerged, for instance in regulation of cancer growth and inflammatory responses [44,47].
Likewise, our identification of dynamin-2 (DYN2_MOUSE) in serum may be suggestive for extracellular matrix degradation, in case of invasive tumor cells (metastasis). Extensive studies are already published on the "invasive feet" enrichments in dynamin and actin and their involvement in extracellular matrix degradation in hepatocellular carcinoma [48-50].
Conclusion
The combination of zoom in gels and the use of two different sample-matrix preparations in sequence improved protein identification of mouse serum proteins considerably and allowed for automated data acquisition as compared to previous methods using 2-DE and MALDI-MS (an example is given in Figure 4) [7,8]. Moreover, we obtained a higher number of matched peptides, as compared to sample pre-fractionation methods coupled with LC-MS/MS or other proteomic approaches (Table 1) [9,32,33]. Finally, we report an improved mouse serum proteome map and an identification of 13 proteins that were not reported in previous 2-DE studies. Even with the comprehensive study reported in [5], six of them are still novel (Table 2) [5-9,32,33]. We validated the newly identified proteins by repeating the searches against different databases (NCBInr and MSDB), as described above (Table 2) [51]. Moreover, the same spectra were further analysed with PeptideMap free software [22]. Altogether, identical results were obtained.
There is a need for a reliable characterization of serum proteomes to enable biomarker discovery. In our opinion, the strength of this work lies in the right combination of protocols and its simplification. We report a simple and reliable identification of mouse serum protein (for instance, see Figure 5) and as shown in Table 1, our work evidences an improved identification when compared with previous studies based on pre-treatments of serum or other sophisticated methods.
Figure 5.
Fast and reliable identification of mouse serum proteins. We have depicted here an example of improvement of data acquisition by the use narrow-pH IPG strips for the IEF. The data of score and matched peptides were chosen from the best outcome in MALDI-MS analysis by both matrices (CHCA and DHB) (ProteinScape™ database).
Taken collectively, we view our protocol to be useful in biomarker discovery.
List of abbreviations
ACN: acetonitril; DTT: dithiothreitol; IPG: immobilized pH gradient; MALDI-TOF-MS: Matrix Assisted Laser Desorption Ionization-Time of Flight-Mass Spectrometry; NH4HCO3: ammonium bicarbonate; SDS: Sodium Dodecyl Sulfate; TCA: trichloroacetic acid; TFA: trifluoracetic acid; TEMED: tetramethylenediamine.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MSR designed and carried out the 2-DE and MALDI MS analysis. MSR and JB were responsible for the design of the study and were involved in writing the manuscript. All authors have read and approved the final manuscript.
Supplementary Material
Validation of the new protein entries. We analysed the same spectra with Mascot search engine in ProteinScape™) and PeptideMap (PROWL free softwares). The ionized tryptic peptides resulted matched to the same protein sequences.
Mascot and PeptideMap. Single entries from both Mascot and PeptideMap for the new identifications.
Protein sequences. We listed all identified protein sequence, the PMF (MS analysis) and PFF (tandem-MS analysis). We chose representative tandem MS for every ion peak fitting with the protein sequence.
Acknowledgments
Acknowledgements
The authors thank Dott. Ignazio Garaguso for helpful discussions and support in MALDI MS analysis.
Contributor Information
Maria Stella Ritorto, Email: ritorto@item.fraunhofer.de.
Jürgen Borlak, Email: borlak@item.fraunhofer.de.
References
- Cho SY, Lee EY, Lee JS, Kim HY, Park JM, Kwon MS, Park YK, Lee HJ, Kang MJ, Kim JY, Yoo JS, Park SJ, Cho JW, Kim HS, Paik YK. Efficient prefractionation of low-abundance proteins in human plasma and construction of a two-dimensional map. Proteomics. 2005;5:3386–96. doi: 10.1002/pmic.200401310. [DOI] [PubMed] [Google Scholar]
- Molina H, Bunkenborg J, Reddy GH, Muthusamy B, Scheel PJ, Pandey A. A proteomic analysis of human hemodialysis fluid. Mol Cell Proteomics. 2005;4:637–50. doi: 10.1074/mcp.M500042-MCP200. [DOI] [PubMed] [Google Scholar]
- Anderson NL, Anderson NG. The human plasma proteome: history, character, and diagnostic prospects. Mol Cell Proteomics. 2002;1:845–867. doi: 10.1074/mcp.R200007-MCP200. [DOI] [PubMed] [Google Scholar]
- Anderson NL, Polanski M, Pieper R, Gatlin T, Tirumalai RS, Conrads TP, Veenstra TD, Adkins JN, Pounds JG, Fagan R, Lobley A. The human plasma proteome: a nonredundant list developed by combination of four separate sources. Mol Cell Proteomics. 2004;3:311–326. doi: 10.1074/mcp.M300127-MCP200. [DOI] [PubMed] [Google Scholar]
- Lai KK, Kolippakkam D, Beretta L. Comprehensive and quantitative proteome profiling of the mouse liver and plasma. Hepatology. 2008;3:1043–51. doi: 10.1002/hep.22123. [DOI] [PubMed] [Google Scholar]
- Hood BL, Zhou M, Chan KC, Lucas DA, Kim GJ, Issaq HJ, Veenstra TD, Conrads TP. Investigation of the mouse serum proteome. J Proteome Res. 2005;4:1561–1568. doi: 10.1021/pr050107r. [DOI] [PubMed] [Google Scholar]
- Duan X, Yarmush DM, Berthiaume F, Jayaraman A, Yarmush ML. Immunodepletion of albumin for two-dimensional gel detection of new mouse acute-phase protein and other plasma proteins. Proteomics. 2005;5:3991–4000. doi: 10.1002/pmic.200401257. [DOI] [PubMed] [Google Scholar]
- Wait R, Chiesa G, Parolini C, Miller I, Begum S, Brambilla D, Galluccio L, Ballerio R, Eberini I, Gianazza E. Reference maps of mouse serum acute-phase proteins: changes with LPS-induced inflammation and apolipoprotein A-I and A-II transgenes. Proteomics. 2005;5:4245–53. doi: 10.1002/pmic.200401292. [DOI] [PubMed] [Google Scholar]
- Chen YY, Lin SY, Yeh YY, Hsiao HH, Wu CY, Chen ST, Wang AH. A modified protein precipitation procedure for efficient removal of albumin from serum. Electrophoresis. 2005;26:2117–27. doi: 10.1002/elps.200410381. [DOI] [PubMed] [Google Scholar]
- Kiernan UA, Nedelkov D, Nelson RW. Multiplexed mass spectrometric immunoassay in biomarker research: a novel approach to the determination of a myocardial infarct. J Proteome Res. 2006;11:2928–34. doi: 10.1021/pr060062+. [DOI] [PubMed] [Google Scholar]
- Zhou M, Conrads TP, Veenstra TD. Proteomics approaches to biomarker detection. Brief Funct Genomic Proteomic. 2005;4:69–75. doi: 10.1093/bfgp/4.1.69. [DOI] [PubMed] [Google Scholar]
- Seam N, Gonzales DA, Kern SJ, Hortin GL, Hoehn GT, Suffredini AF. Quality control of serum albumin depletion for proteomic analysis. Clin Chem. 2007;53:1915–20. doi: 10.1373/clinchem.2007.091736. [DOI] [PubMed] [Google Scholar]
- Liao QL, Chen XD, Zhao L, Ding YQ. Comparative proteomics of the serum in patients with nasopharyngeal carcinoma: a study with two-dimensional electrophoresis and MALDI-TOF-MS. Nan Fang Yi Ke Da Xue Xue Bao. 2008;28:154–8. [PubMed] [Google Scholar]
- Mizukami M, Kanamoto T, Souchelnytskyi N, Kiuchi Y. Proteome profiling of embryo chick retina. Proteome Sci. 2008;6:3. doi: 10.1186/1477-5956-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonnet F, Lemaître G, Waksman G, Tortajada J. MALDI/MS peptide mass fingerprinting for proteome analysis: identification of hydrophobic proteins attached to eucaryote keratinocyte cytoplasmic membrane using different matrices in concert. Proteome Sci. 2003;1:2. doi: 10.1186/1477-5956-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padliya ND, Wood TD. A strategy to improve peptide mass fingerprinting matches through the optimization of matrix-assisted laser desorption/ionization matrix selection and formulation. Proteomics. 2004;4:466–73. doi: 10.1002/pmic.200300567. [DOI] [PubMed] [Google Scholar]
- Callesen AK, Mohammed S, Bunkenborg J, Kruse TA, Cold S, Mogensen O, Christensen R, Vach W, Jørgensen PE, Jensen ON. Serum protein profiling by miniaturized solid-phase extraction and matrix-assisted laser desorption/ionization mass spectrometry. Rapid Commun Mass Spectrom. 2005;19:1578–86. doi: 10.1002/rcm.1960. [DOI] [PubMed] [Google Scholar]
- Laugesen S, Roepstorff P. Combination of two matrices results in improved performance of MALDI MS for peptide mass mapping and protein analysis. J Am Soc Mass Spectrom. 2003;14:992–1002. doi: 10.1016/S1044-0305(03)00262-9. [DOI] [PubMed] [Google Scholar]
- Garaguso I, Borlak J. Matrix layer sample preparation: an improved MALDI-MS peptide analysis method for proteomic studies. Proteomics. 2008;8:2583–2595. doi: 10.1002/pmic.200701147. [DOI] [PubMed] [Google Scholar]
- Goerg A, Weiss W, Dunn MJ. Current two-dimensional electrophoresis technology for proteomics. Proteomics. 2004;4:3665–3685. doi: 10.1002/pmic.200401031. [DOI] [PubMed] [Google Scholar]
- Mascot Search http://www.matrixscience.com/search_form_select.html
- PeptideMap http://prowl.rockefeller.edu/prowl/peptidemap.html
- Wu RW, Wang FS, Ko JY, Wang CJ, Wu SL. Comparative serum proteome expression of osteonecrosis of the femoral head in adults. Bone. 2008;43:561–566. doi: 10.1016/j.bone.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Hanas JS, Hocker JR, Cheung JY, Larabee JL, Lerner MR, Lightfoot SA, Morgan DL, Denson KD, Prejeant KC, Gusev Y, Smith BJ, Hanas RJ, Postier RG, Brackett DJ. Biomarker identification in human pancreatic cancer sera. Pancreas. 2008;36:61–69. doi: 10.1097/mpa.0b013e3180d0a738. [DOI] [PubMed] [Google Scholar]
- Ali OS, Abo-Shadi MA, Hammad LN. The biological significance of serum complements C3 and C4 in HCV-related chronic liver diseases and hepatocellular carcinoma. Egypt J Immunol. 2005;12:91–99. [PubMed] [Google Scholar]
- Goldman R, Ressom HW, Abdel-Hamid M, Goldman L, Wang A, Varghese RS, An Y, Loffredo CA, Drake SK, Eissa SA, Gouda I, Ezzat S, Moiseiwitsch FS. Candidate markers for the detection of hepatocellular carcinoma in low-molecular weight fraction of serum. Carcinogenesis. 2007;10:2149–53. doi: 10.1093/carcin/bgm177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan WT, Muster P, Tatum F, Kao SM, Alam J, Smith A. Identification of the histidine residues of hemopexin that coordinate with heme-iron and of a receptor-binding region. J Biol Chem. 1993;268:6256–6262. [PubMed] [Google Scholar]
- Satoh T, Satoh H, Iwahara S, Hrkal Z, Peyton DH, Muller-Eberhard U. Roles of heme iron-coordinating histidine residues of human hemopexin expressed in baculovirus-infected insect cells. Proc Natl Acad Sci USA. 1994;18:8423–7. doi: 10.1073/pnas.91.18.8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybaczyk LA, Bashaw MJ, Pathak DR, Huang K. An indicator of cancer: downregulation of monoamine oxidase-A in multiple organs and species. BMC Genomics. 2008;9:134. doi: 10.1186/1471-2164-9-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich M. Depression and cancer: recent data on clinical issues, research challenges and treatment approaches. Curr Opin Oncol. 2008;20:353–359. doi: 10.1097/CCO.0b013e3282fc734b. [DOI] [PubMed] [Google Scholar]
- Nordgaard CL, Berg KM, Kapphahn RJ, Reilly C, Feng X, Olsen TW, Ferrington DA. Proteomics of the retinal pigment epithelium reveals altered protein expression at progressive stages of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2006;47:815–22. doi: 10.1167/iovs.05-0976. [DOI] [PubMed] [Google Scholar]
- Duan X, Yarmush DM, Berthiaume F, Jayaraman A, Yarmush ML. A mouse serum two-dimensional gel map: application to profiling burn injury and infection. Electrophoresis. 2004;25:3055–65. doi: 10.1002/elps.200406039. [DOI] [PubMed] [Google Scholar]
- Kuick R, Misek DE, Monsma DJ, Webb CP, Wang H, Peterson KJ, Pisano M, Omenn GS, Hanash SM. Discovery of cancer biomarkers through the use of mouse models. Cancer Lett. 2007;249:40–8. doi: 10.1016/j.canlet.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Smirnov IP, Zhu X, Taylor T, Huang Y, Ross P, Papayanopoulos IA, Martin SA, Pappin DJ. Suppression of alpha-cyano-4-hydroxycinnamic acid matrix clusters and reduction of chemical noise in MALDI-TOF mass spectrometry. Anal Chem. 2004;76:2958–65. doi: 10.1021/ac035331j. [DOI] [PubMed] [Google Scholar]
- Zhu X, Papayannopoulos IA. Improvement in the detection of low concentration protein digests on a MALDI TOF/TOF workstation by reducing alpha-cyano-4-hydroxycinnamic acid adduct ions. J Biomol Tech. 2003;14:298–307. [PMC free article] [PubMed] [Google Scholar]
- Krutchinsky AN, Chait BT. On the nature of the chemical noise in MALDI mass spectra. J Am Soc Mass Spectrom. 2002;13:129–34. doi: 10.1016/S1044-0305(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Luo G, Marginean I, Vertes A. Internal energy of ions generated by matrix-assisted laser desorption/ionization. Anal Chem. 2002;74:6185–90. doi: 10.1021/ac020339z. [DOI] [PubMed] [Google Scholar]
- Sundsten T, Eberhardson M, Göransson M, Bergsten P. The use of proteomics in identifying differentially expressed serum proteins in humans with type 2 diabetes. Proteome Sci. 2006;4:22. doi: 10.1186/1477-5956-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ExPASy Molecular Server http://www.expasy.org
- Yu YP, Yu G, Tseng G, Cieply K, Nelson J, Defrances M, Zarnegar R, Michalopoulos G, Luo JH. Glutathione peroxidase 3, deleted or methylated in prostate cancer, suppresses prostate cancer growth and metastasis. Cancer Res. 2007;67:8043–50. doi: 10.1158/0008-5472.CAN-07-0648. [DOI] [PubMed] [Google Scholar]
- Oike Y, Ito Y, Maekawa H, Morisada T, Kubota Y, Akao M, Urano T, Yasunaga K, Suda T. Angiopoietin-related growth factor (AGF) promotes angiogenesis. Blood. 2004;103:3760–5. doi: 10.1182/blood-2003-04-1272. [DOI] [PubMed] [Google Scholar]
- Roigas J, Deger S, Schroeder J, Wille A, Turk I, Brux B, Jung K, Schnorr D, Loening SA. Tumor type M2 pyruvate kinase expression in metastatic renal cell carcinoma. Urol Res. 2003;6:358–62. doi: 10.1007/s00240-003-0331-4. [DOI] [PubMed] [Google Scholar]
- Nalepa G, Wade Harper J. Therapeutic anti-cancer targets upstream of the proteasome. Cancer Treat Rev. 2003;1:49–57. doi: 10.1016/S0305-7372(03)00083-5. [DOI] [PubMed] [Google Scholar]
- Liu J, Pedersen LC. Anticoagulant heparan sulfate: structural specificity and biosynthesis. Appl Microbiol Biotechnol. 2007;2:263–72. doi: 10.1007/s00253-006-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan MB, Chen J, Krise JP, Liu J. The biosynthesis of anticoagulant heparan sulfate by the heparan sulfate 3-O-sulfotransferase isoform 5. Biochim Biophys Acta. 2004;1671:34–43. doi: 10.1016/j.bbagen.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Kamimura K, Rhodes JM, Ueda R, McNeely M, Shukla D, Kimata K, Spear PG, Shworak NW, Nakato H. Regulation of Notch signaling by Drosophila heparan sulfate 3-O sulfotransferase. J Cell Biol. 2004;166:1069–79. doi: 10.1083/jcb.200403077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Asada K, Fukutomi T, Okochi E, Yagi Y, Hasegawa T, Asahara T, Sugimura T, Ushijima T. Methylation-associated silencing of heparan sulfate D-glucosaminyl 3-O-sulfotransferase-2 (3-OST-2) in human breast, colon, lung and pancreatic cancers. Oncogene. 2003;22:274–80. doi: 10.1038/sj.onc.1206146. [DOI] [PubMed] [Google Scholar]
- Baldassarre M, Pompeo A, Beznoussenko G, Castaldi C, Cortellino S, McNiven MA, Luini A, Buccione R. Dynamin participates in focal extracellular matrix degradation by invasive cells. Mol Biol Cell. 2003;14:1074–84. doi: 10.1091/mbc.E02-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SY, Jeong MJ, Yoo J, Lee KI, Kwon BM, Lim DS, Lee CE, Park YM, Han MY. Grb2 dominantly associates with dynamin II in human hepatocellular carcinoma HepG2 cells. J Cell Biochem. 2001;84:150–155. doi: 10.1002/jcb.1275. [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhou K, Hao JJ, Liu J, Smith N, Zhan X. Regulation of cortactin/dynamin interaction by actin polymerization during the fission of clathrin-coated pits. J Cell Sci. 2005;118:807–17. doi: 10.1242/jcs.01668. [DOI] [PubMed] [Google Scholar]
- Matthiesen R. Methods, algorithms and tools in computational proteomics: a practical point of view. Proteomics. 2007;7:2815–32. doi: 10.1002/pmic.200700116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Validation of the new protein entries. We analysed the same spectra with Mascot search engine in ProteinScape™) and PeptideMap (PROWL free softwares). The ionized tryptic peptides resulted matched to the same protein sequences.
Mascot and PeptideMap. Single entries from both Mascot and PeptideMap for the new identifications.
Protein sequences. We listed all identified protein sequence, the PMF (MS analysis) and PFF (tandem-MS analysis). We chose representative tandem MS for every ion peak fitting with the protein sequence.