Abstract
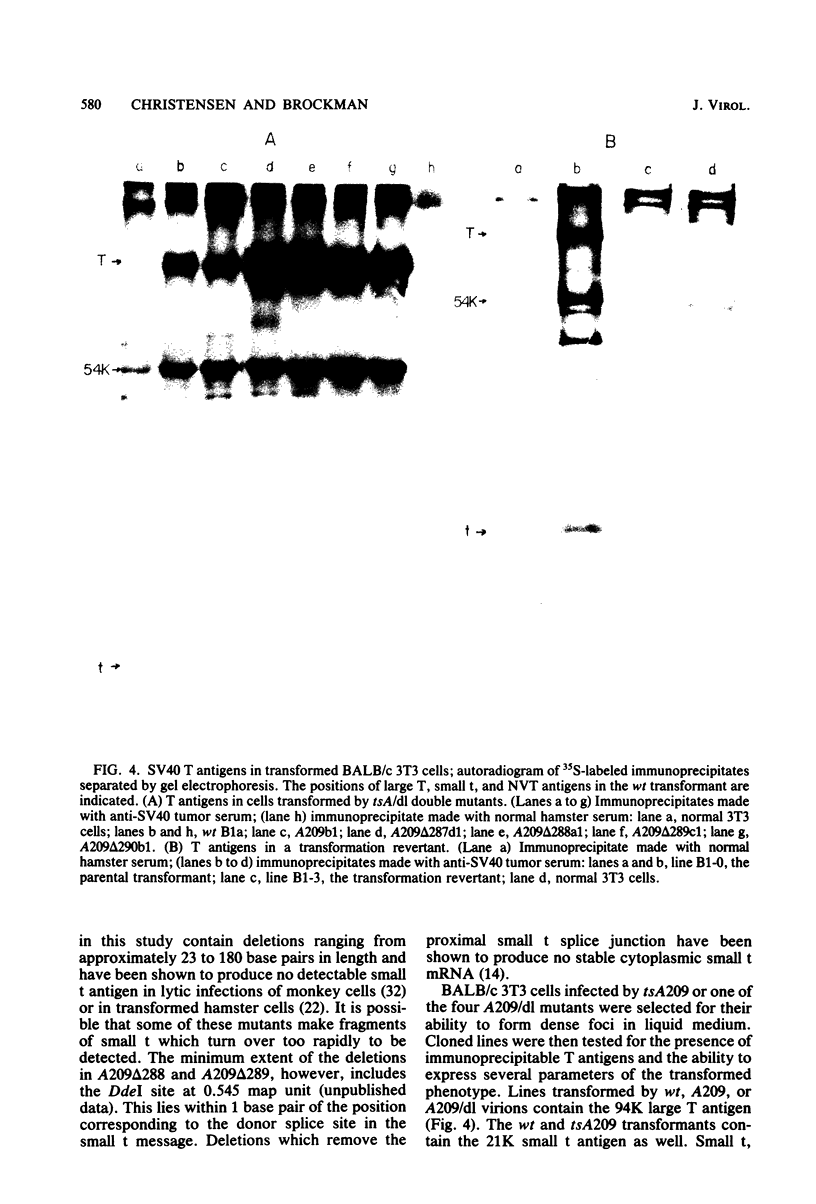
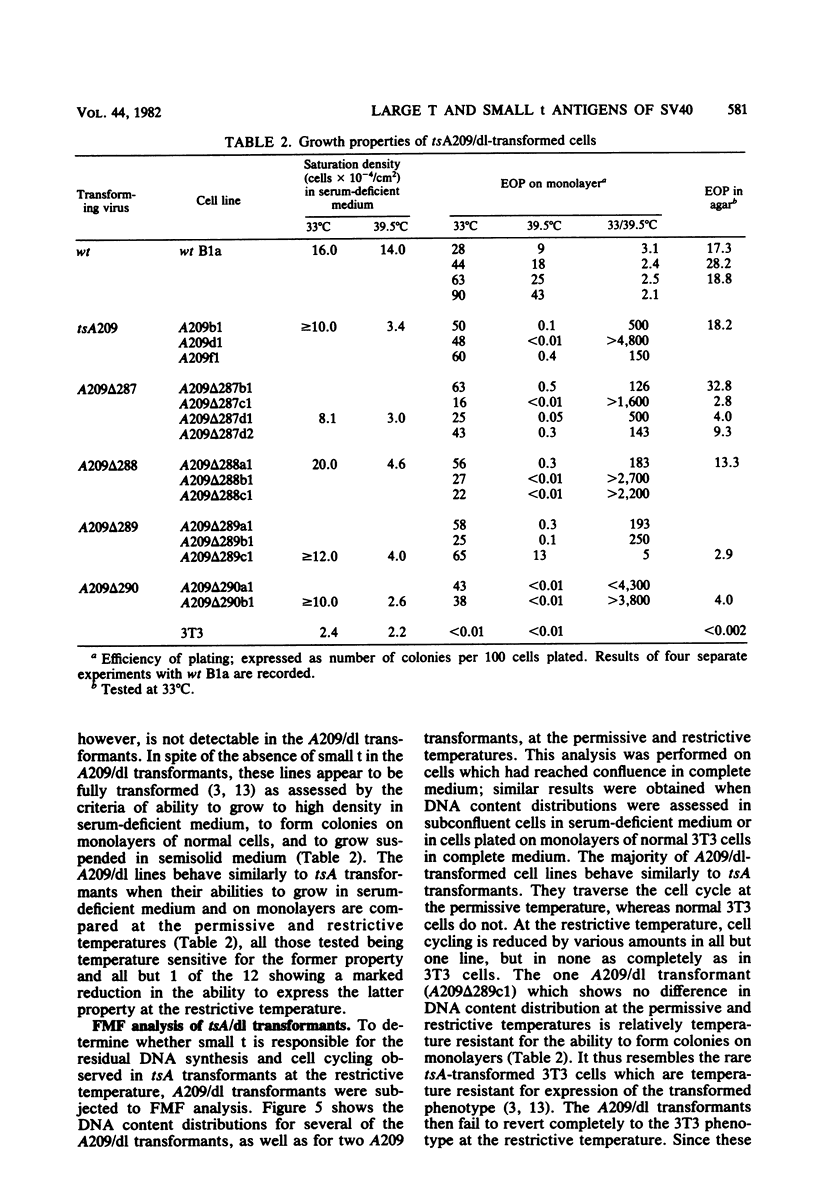
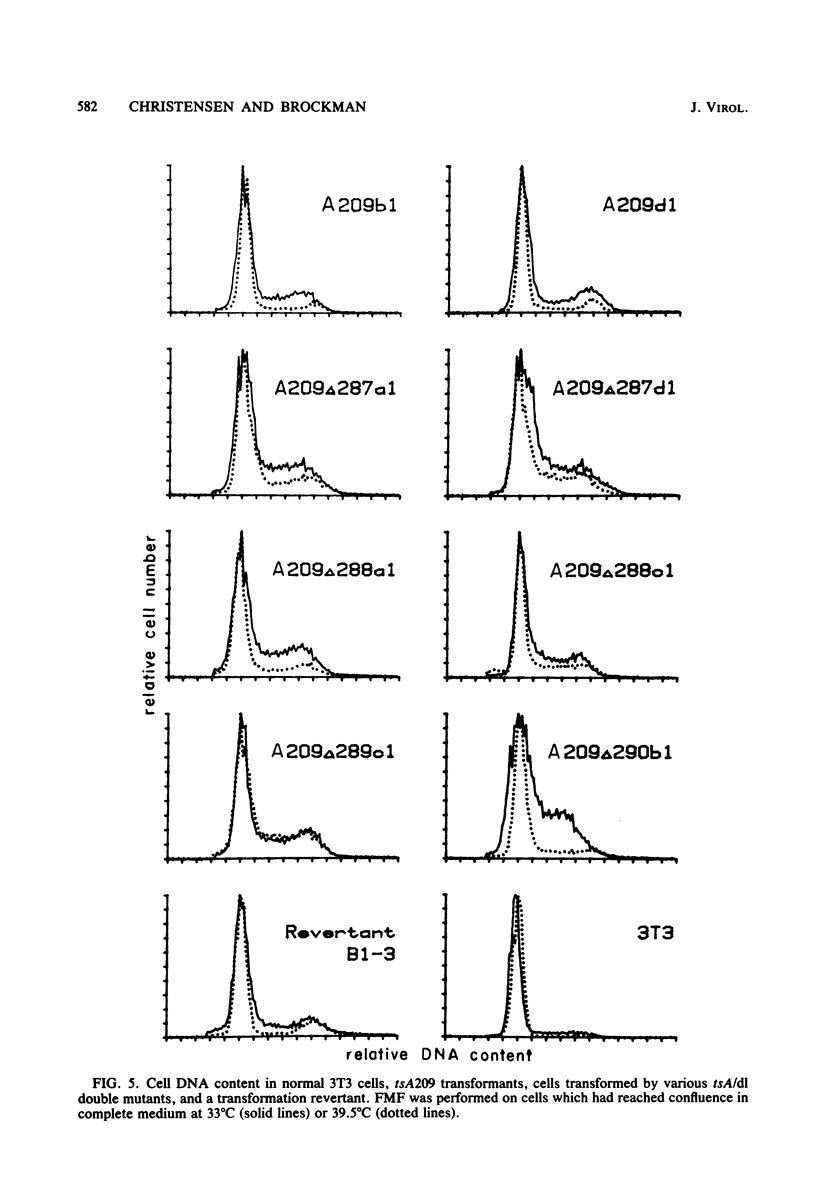
The roles of the large T and small t antigens of simian virus 40 in cellular DNA synthesis and cell division were analyzed in BALB/c 3T3 mouse cells transformed by wild-type, temperature-sensitive A (tsA), or tsA-deletion (tsA/dl) double mutants. Assessment of DNA replication and cell cycle distribution by radioautography of [3H]thymidine-labeled nuclei and by flow microfluorimetry indicate that tsA transformants do not synthesize DNA or divide at the restrictive temperature to the same extent as they do at the permissive temperature or as wild-type transformants do at the restrictive temperature. This confirms earlier studies suggesting that large T induces DNA synthesis and mitosis in transformed cells. Inhibition of replication in tsA transformants at the restrictive temperature, however, is not complete. Some residual cell division does occur but is in large part offset by cell detachment and death. This failure to revert completely to the parental 3T3 phenotype, as indicated by residual cell cycling at the restrictive temperature, was also observed in cells transformed by tsA/dl double mutants which, in addition to producing a ts large T, make no small t protein. Small t, therefore, does not appear to be responsible for the residual cell cycling and plays no demonstrable role in the induction of DNA synthesis or cell division in stably transformed BALB/c 3T3 cells. Comparison of cell cycling in tsA and tsA/dl transformants, normal 3T3 cells, and a transformation revertant suggests that the failure of tsA transformants to revert completely may be due to leakiness of the tsA mutation as well as to a permanent cellular alteration induced during viral transformation. Finally, analysis of cells transformed by tsA/dl double mutants indicates that small t is not required for full expression of growth properties characteristic of transformed cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bender M. A., Brockman W. W. Rearrangement of integrated viral DNA sequences in mouse cells transformed by simian virus 40. J Virol. 1981 Jun;38(3):872–879. doi: 10.1128/jvi.38.3.872-879.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouck N., Beales N., Shenk T., Berg P., di Mayorca G. New region of the simian virus 40 genome required for efficient viral transformation. Proc Natl Acad Sci U S A. 1978 May;75(5):2473–2477. doi: 10.1073/pnas.75.5.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W. Transformation of BALB/c-3T3 cells by tsA mutants of simian virus 40: temperature sensitivity of the transformed phenotype and retransofrmation by wild-type virus. J Virol. 1978 Mar;25(3):860–870. doi: 10.1128/jvi.25.3.860-870.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butel J. S., Brugge J. S., Noonan C. A. Transformation of primate and rodent cells by temperature-sensitive mutants of SV40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):25–36. doi: 10.1101/sqb.1974.039.01.006. [DOI] [PubMed] [Google Scholar]
- Butel J. S., Soule H. R. Role of the simian virus 40 gene A product in regulation of DNA synthesis in transformed cells. J Virol. 1978 Jun;26(3):584–594. doi: 10.1128/jvi.26.3.584-594.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. DNA infectivity and the induction of host DNA synthesis with temperature-sensitive mutants of simian virus 40. J Virol. 1975 Jan;15(1):145–150. doi: 10.1128/jvi.15.1.145-150.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo A. B., Jay G., Appella E., Dubois G. C., Law L. W., Old L. J. Detection of a transformation-related antigen in chemically induced sarcomas and other transformed cells of the mouse. Proc Natl Acad Sci U S A. 1979 May;76(5):2420–2424. doi: 10.1073/pnas.76.5.2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- Frisque R. J., Rifkin D. B., Topp W. C. Requirement for the large T and small T proteins of SV40 in the maintenance of the transformed state. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):325–331. doi: 10.1101/sqb.1980.044.01.037. [DOI] [PubMed] [Google Scholar]
- Hiscott J. B., Defendi V. Simian virus 40 gene A regulation of cellular DNA synthesis. I. In permissive cells. J Virol. 1979 May;30(2):590–599. doi: 10.1128/jvi.30.2.590-599.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscott J. B., Defendi V. Simian virus 40 gene A regulation of cellular DNA synthesis. II. In nonpermissive cells. J Virol. 1981 Feb;37(2):802–812. doi: 10.1128/jvi.37.2.802-812.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson M. A., Hunter T., Eckhart W. Characterization of T antigens in polyoma-infected and transformed cells. Cell. 1978 Sep;15(1):65–77. doi: 10.1016/0092-8674(78)90083-1. [DOI] [PubMed] [Google Scholar]
- Kelley S., Bender M. A., Brockman W. W. Transformation of BALB/c-3T3 cells by tsA mutants of simian virus 40: effect of transformation technique on the transformed phenotype. J Virol. 1980 Jan;33(1):550–552. doi: 10.1128/jvi.33.1.550-552.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Gruss P., Dhar R., Lai C. J. Processing and expression of early SV40 mRNA: a role for RNA conformation in splicing. Cell. 1979 Sep;18(1):85–92. doi: 10.1016/0092-8674(79)90356-8. [DOI] [PubMed] [Google Scholar]
- Kimura G., Dulbecco R. A temperature-sensitive mutant of simian virus 40 affecting transforming ability. Virology. 1973 Apr;52(2):529–534. doi: 10.1016/0042-6822(73)90348-6. [DOI] [PubMed] [Google Scholar]
- Kimura G., Itagaki A. Initiation and maintenance of cell transformation by simian virus 40: a viral genetic property. Proc Natl Acad Sci U S A. 1975 Feb;72(2):673–677. doi: 10.1073/pnas.72.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Mapping temperature-sensitive mutants of simian virus 40: rescue of mutants by fragments of viral DNA. Virology. 1974 Aug;60(2):466–475. doi: 10.1016/0042-6822(74)90340-7. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Burger M. M. A working hypothesis explaining the maintenance of the transformed state by SV40 and polyoma. J Theor Biol. 1972 Dec;37(3):436–446. doi: 10.1016/0022-5193(72)90084-7. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Maltzman W., Levine A. J. The SV40 A gene product is required for the production of a 54,000 MW cellular tumor antigen. Virology. 1979 Oct 30;98(2):308–318. doi: 10.1016/0042-6822(79)90554-3. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Chou J. Y. Simian virus 40 functions required for the establishment and maintenance of malignant transformation. J Virol. 1975 Mar;15(3):599–612. doi: 10.1128/jvi.15.3.599-612.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Oppenheim A. Initiation points for DNA replication in nontransformed and simian virus 40-transformed Chinese hamster lung cells. Cell. 1977 Aug;11(4):859–869. doi: 10.1016/0092-8674(77)90297-5. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Edwards C. A. Roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells: studies with simian virus 40 double mutants. J Virol. 1979 Sep;31(3):596–607. doi: 10.1128/jvi.31.3.596-607.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P., Edwards C. A., Vembu D. The roles of the simian virus 40 tumor antigens in transformation of Chinese hamster lung cells. Cell. 1979 Jul;17(3):635–643. doi: 10.1016/0092-8674(79)90271-x. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Stein S. Resting state in normal and simian virus 40 transformed Chinese hamster lung cells. Proc Natl Acad Sci U S A. 1976 May;73(5):1655–1659. doi: 10.1073/pnas.73.5.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Weber K. Simian virus 40 gene A function and maintenance of transformation. J Virol. 1975 Mar;15(3):636–644. doi: 10.1128/jvi.15.3.636-644.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C. C., Lehman J. M. Simian virus 40 A gene function: DNA content analysis of Chinese hamster cells transformed by an early temperature-sensitive virus mutant. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4389–4393. doi: 10.1073/pnas.75.9.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson C. C., Swartzendruber D. E., Lehman J. M. Replication of Chinese hamster embryo cells transformed by temperature-sensitive T-antigen mutants of simian virus 40. J Virol. 1980 Jul;35(1):246–248. doi: 10.1128/jvi.35.1.246-248.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundell K., Cox J. Simian virus 40 t antigen affects the sensitivity of cellular DNA synthesis to theophylline. J Virol. 1979 Apr;30(1):394–396. doi: 10.1128/jvi.30.1.394-396.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Benjamin T. L. Cellular alterations dependent upon the polyoma virus Hr-t function: separation of mitogenic from transforming capacities. Cell. 1978 Jul;14(3):587–599. doi: 10.1016/0092-8674(78)90244-1. [DOI] [PubMed] [Google Scholar]
- Scott W. A., Brockman W. W., Nathans D. Biological activities of deletion mutants of simian virus 40. Virology. 1976 Dec;75(2):319–334. doi: 10.1016/0042-6822(76)90031-3. [DOI] [PubMed] [Google Scholar]
- Seif R., Martin R. G. Simian virus 40 small t antigen is not required for the maintenance of transformation but may act as a promoter (cocarcinogen) during establishment of transformation in resting rat cells. J Virol. 1979 Dec;32(3):979–988. doi: 10.1128/jvi.32.3.979-988.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow V. P., Persico-Dilauro M., Edwards C. A., Martin R. G. The isolation of SV40 tsA/deletion, double mutants and the induction of host DNA synthesis. Virology. 1980 Feb;101(1):250–260. doi: 10.1016/0042-6822(80)90500-0. [DOI] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. J., Topp W. C., Hanich R., Sambrook J. F. Mutants of SV40 with an altered small t protein are reduced in their ability to transform cells. Cell. 1978 May;14(1):79–88. doi: 10.1016/0092-8674(78)90303-3. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P. Function of simian virus 40 gene A in transforming infection. J Virol. 1975 Mar;15(3):613–618. doi: 10.1128/jvi.15.3.613-618.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P. Simian virus 40 deoxyribonucleic acid synthesis: the viral replicon. J Virol. 1972 Oct;10(4):591–598. doi: 10.1128/jvi.10.4.591-598.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenen D. G., Martin R. G., Anderson J., Livingston D. M. Biological and biochemical studies of cells transformed by simian virus 40 temperature-sensitive gene A mutants and A mutant revertants. J Virol. 1977 Apr;22(1):210–218. doi: 10.1128/jvi.22.1.210-218.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]