Abstract
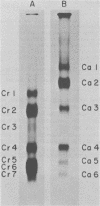
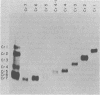
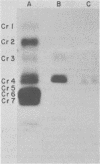
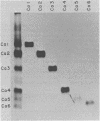
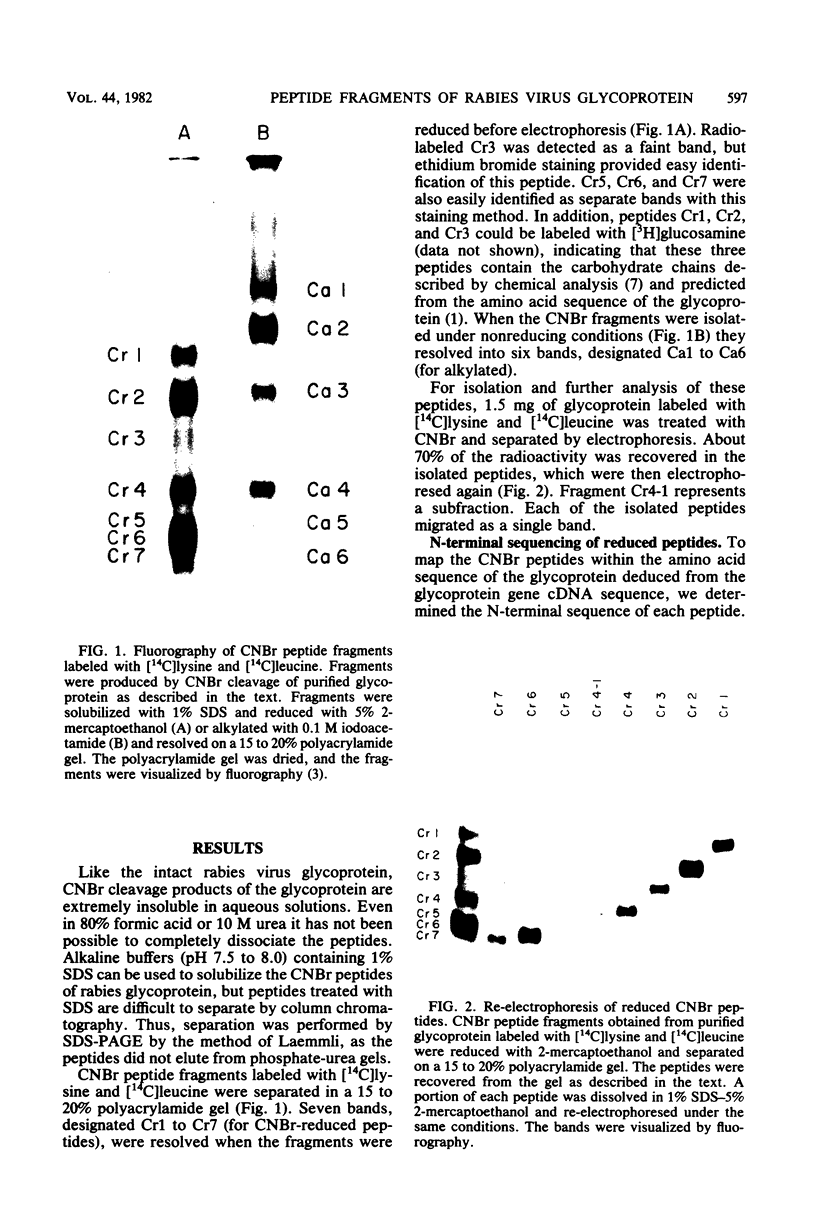
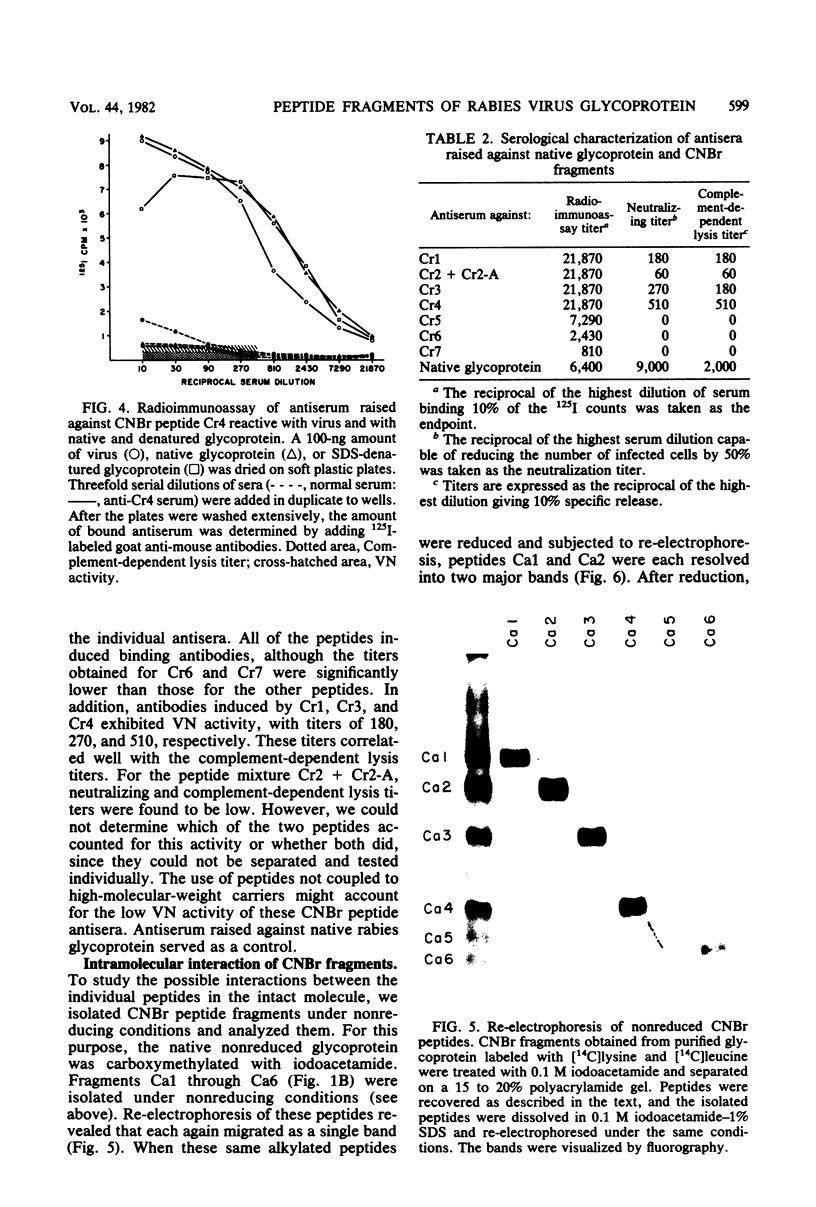
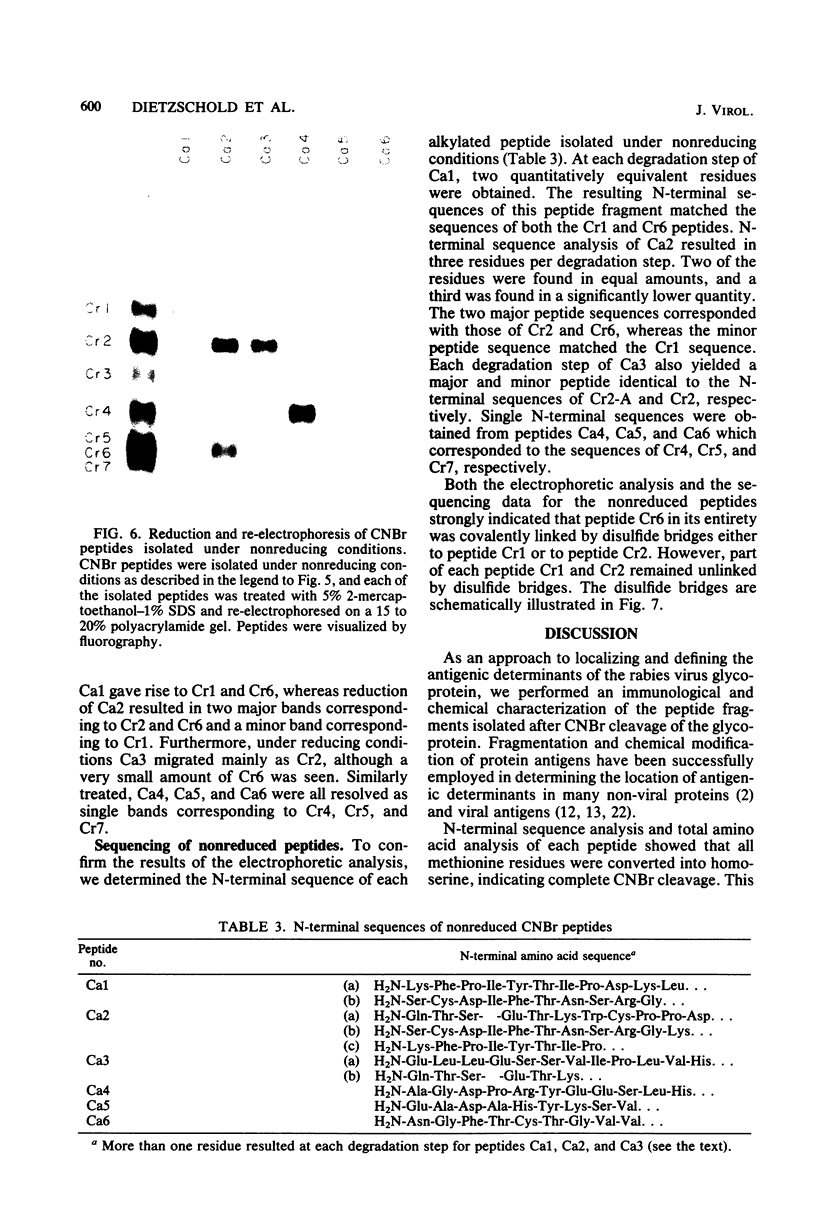
After rabies virus glycoprotein was treated with CNBr, the peptide mixture was fractionated by preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis. CNBr-cleaved peptide fragments were resolved into seven peptide bands under reducing conditions and six peptide bands under nonreducing conditions. The isolated nonreduced polypeptides were further analyzed by electrophoresis under reducing conditions. The N-terminal amino acid sequences were determined for the peptides in each of the isolated bands. The sequence data identified eight CNBr peptides and allowed the peptide fragments to be ordered within the deduced amino acid sequence of the glycoprotein. Analysis of the nonreduced CNBr peptides revealed two conformations of the glycoprotein. Two CNBr peptide fragments were specifically immunoprecipitated with a hyperimmune anti-rabies glycoprotein serum. These two and one other CNBr peptide induced the production of rabies virus-neutralizing antibodies, indicating the existence of at least three distinct antigenic sites on the rabies virus glycoprotein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anilionis A., Wunner W. H., Curtis P. J. Structure of the glycoprotein gene in rabies virus. Nature. 1981 Nov 19;294(5838):275–278. doi: 10.1038/294275a0. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z., Kazim A. L. First consequences of the determination of the entire antigenic structure of sperm-whale myoglobin. Adv Exp Med Biol. 1978;98:19–40. doi: 10.1007/978-1-4615-8858-0_3. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Clark H. F. Rabies serogroup viruses in neuroblastoma cells: propagation, "autointerference," and apparently random back-mutation of attenuated viruses to the virulent state. Infect Immun. 1980 Mar;27(3):1012–1022. doi: 10.1128/iai.27.3.1012-1022.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coslett G. D., Holloway B. P., Obijeski J. F. The structural proteins of rabies virus and evidence for their synthesis from separate monocistronic RNA species. J Gen Virol. 1980 Jul;49(1):161–180. doi: 10.1099/0022-1317-49-1-161. [DOI] [PubMed] [Google Scholar]
- Cox J. H., Dietzschold B., Schneider L. G. Rabies virus glycoprotein. II. Biological and serological characterization. Infect Immun. 1977 Jun;16(3):754–759. doi: 10.1128/iai.16.3.754-759.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzschold B., Cox J. H., Schneider L. G. Rabies virus strains: a comparison study by polypeptide analysis of vaccine strains with different pathogenic patterns. Virology. 1979 Oct 15;98(1):63–75. doi: 10.1016/0042-6822(79)90525-7. [DOI] [PubMed] [Google Scholar]
- Dietzschold B. Oligosaccharides of the glycoprotein of rabies virus. J Virol. 1977 Aug;23(2):286–293. doi: 10.1128/jvi.23.2.286-293.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamand A., Wiktor T. J., Koprowski H. Use of hybridoma monoclonal antibodies in the detection of antigenic differences between rabies and rabies-related virus proteins. II. The glycoprotein. J Gen Virol. 1980 May;48(1):105–109. doi: 10.1099/0022-1317-48-1-105. [DOI] [PubMed] [Google Scholar]
- Foster J. A., Bruenger E., Hu C. L., Albertson K., Franzblau C. A new, improved technique for automated sequencing of non-polar peptides. Biochem Biophys Res Commun. 1973 Jul 2;53(1):70–74. doi: 10.1016/0006-291x(73)91402-2. [DOI] [PubMed] [Google Scholar]
- Gerber G. E., Anderegg R. J., Herlihy W. C., Gray C. P., Biemann K., Khorana H. G. Partial primary structure of bacteriorhodopsin: sequencing methods for membrane proteins. Proc Natl Acad Sci U S A. 1979 Jan;76(1):227–231. doi: 10.1073/pnas.76.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. C., Russell R. J., Ward C. W., Dopheide T. A. Antigenic determinants of influenza virus hemagglutinin. I. Cyanogen bromide peptides derived from A/MEMPHIS/72 hemagglutinin possess antigenic activity. Virology. 1978 Aug;89(1):199–205. doi: 10.1016/0042-6822(78)90052-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai C. Y., Dietzschold B. Amino acid composition and terminal sequence analysis of the rabies virus glycoprotein: identification of the reading frame on the cDNA sequence. Biochem Biophys Res Commun. 1981 Nov 30;103(2):536–542. doi: 10.1016/0006-291x(81)90485-x. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Host cell- and virus strain-dependent differences in oligosaccharides of hemagglutinin glycoproteins of influenza A viruses. Virology. 1979 May;95(1):8–23. doi: 10.1016/0042-6822(79)90397-0. [DOI] [PubMed] [Google Scholar]
- Rosner M. R., Grinna L. S., Robbins P. W. Differences in glycosylation patterns of closely related murine leukemia viruses. Proc Natl Acad Sci U S A. 1980 Jan;77(1):67–71. doi: 10.1073/pnas.77.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Young H. A., Scolnick E. M. p21 of Kirsten murine sarcoma virus is thermolabile in a viral mutant temperature sensitive for the maintenance of transformation. J Virol. 1979 Aug;31(2):546–546. doi: 10.1128/jvi.31.2.546-546.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Yager P. A., Baer G. M. A rapid tissue culture test for determining rabies neutralizing antibody. Monogr Ser World Health Organ. 1973;(23):354–357. [PubMed] [Google Scholar]
- Somack R. Complete phenylthiohydantoin amino acid analysis by high-performance liquid chromatography on ULTRASPHERE-octadecyltrimethyloxysilane. Anal Biochem. 1980 May 15;104(2):464–468. doi: 10.1016/0003-2697(80)90100-1. [DOI] [PubMed] [Google Scholar]
- Versteegen R. J., Oroszlan S. Effect of chemical modification and fragmentation on antigenic determinants of internal protein p30 and surface glycoprotein gp70 of type C retroviruses. J Virol. 1980 Mar;33(3):983–992. doi: 10.1128/jvi.33.3.983-992.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A., Scherrer K. A rapid and sensitive method for detection of proteins in polyacrylamide SDS gels: staining with ethidium bromide. Mol Biol Rep. 1979 Dec 31;5(4):209–214. doi: 10.1007/BF00782890. [DOI] [PubMed] [Google Scholar]
- Wiktor T. J., Dietzschold B., Leamnson R. N., Koprowski H. Induction and biological properties of defective interfering particles of rabies virus. J Virol. 1977 Feb;21(2):626–635. doi: 10.1128/jvi.21.2.626-635.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]