Abstract
Recent evidence suggests substantial overlap between mood and anxiety disorders, both in clinical presentation and associated features. A theoretical framework to account for this overlap focuses on negative affectivity, defined as the disposition to experience negative emotional states, including fear, sadness and guilt. This model has been successful in explaining the co-occurrence of depressive and anxiety disorders in humans. As a next step, development of an animal model focused on both depression- and anxiety-relevant behaviors may advance understanding of depression-anxiety symptom overlap, relations of these disorders with associated medical conditions and responses to treatment. The current study was designed to investigate inducible and quantifiable depression- and anxiety-like behaviors in prairie voles (Microtus ochrogaster). Adult, female prairie voles were exposed to 4 weeks of social pairing (control) or isolation, an established stressor for socially monogamous mammals (including humans). Operational measures of depression (sucrose intake and behaviors in the forced swim test), anxiety (behaviors in the elevated plus maze) and aggression (responses to an unrelated prairie vole pup) were investigated. Social isolation induced a progressive decline in sucrose intake and increased immobility time during the forced swim test. Social isolation also decreased the amount of time spent in the open arms of the elevated plus maze, and increased pup-directed attack behavior. The current findings suggest that isolation induces behaviors reflecting elevated negative affect. These results may provide a foundation for creating a rodent model to examine the mechanisms underlying comorbid mood and anxiety disorders.
Keywords: Affective disorders, Anhedonia, Anxiety disorders, Anxiogenic behaviors, Behavioral despair, Comorbidity, Depressive disorders, Elevated plus maze, Forced swimming, Social behavior
INTRODUCTION
The Diagnostic and Statistical Manual of Mental Disorders (DSM) [American Psychiatric Association 2000] contains separate major categories for mood versus anxiety disorders. However, converging clinical and experimental research indicates that there are notable similarities among these disorders in humans. A substantial literature addressing the co-occurrence of several of the DSM mood and anxiety disorders has accumulated during the past 20 years and represents a particular challenge to current diagnostic systems [Watson and Clark 1984; Clark, Watson, and Reynolds 1995; Widiger and Clark 2000]. These data find consistently that mood and anxiety disorders co-occur in approximately 50–60% of clinical cases, which is substantially higher than would be predicted from base rate data alone [Kessler, Nelson, McGonagle, Liu, Swartz, and Blazer 1996; Mineka, Watson, and Clark 1998]. Strong relations between mood and anxiety are reflected in several additional lines of research, including physiological characteristics (e.g., activation of the hypothalamic-pituitary-adrenal [HPA] axis, sympathoexcitation) [Leonard and Song 1996; Agelink, Majewski, Wurthmann, Postert, Linka, Rotterdam, and Klieser 2001; Young, Abelson, and Cameron 2004], association with other behavioral (e.g., personality disorders, substance abuse) and physiological disorders (e.g., cardiovascular disease) [Landry, Smith, and Steinberg 1991; Mineka, Watson, and Clark 1998; Konstam, Moser, and De Jong 2005; Haynes, Farrell, Singleton, Meltzer, Araya, Lewis, and Wiles 2005], central nervous system functions (e.g., serotonergic system) [File 1996; Overstreet, Commissaris, De La Garza II, File, Knapp, and Seiden 2003], behavioral responses to pharmacological intervention (e.g., serotonin reuptake inhibitors, partial serotonin agonists) [Stahl 1997; Kasper and Resinger 2001; Brawman-Mintzer 2001] and even shared genetic vulnerability [Kendler 1996; Middeldorp, Cath, Van Dyck, and Boomsma 2005]. To appreciate the strength of such relations, psychometric analyses often find that mood and anxiety assessment instruments lack adequate discriminant validity [Gotlib and Cane 1989; Clark and Watson 1991].
A theoretical framework to understand the co-occurrence of depression and anxiety was proposed by Clark and Watson [1991] initially as a tripartite model. Highlighting the influence of negative affect (the disposition to experience negative emotional states [Watson and Clark 1984]), this model outlined (a) the factor common to both sets of conditions and (b) factors relatively unique to each depression or anxiety. According to several factor analytic and psychometric studies with patient and non-patient samples, negative affect is common to both depressive and anxiety disorders [Watson and Clark 1984; Mineka, Watson, and Clark 1998; Brown, Chorpita, and Barlow 1998]. Low positive affect (i.e., anhedonia) is a specific defining feature of depression, and behavioral symptoms of sympathetic hyperarousal are specific to anxiety disorders. Mineka et al. [1998] offered a revision to this model, suggesting that each anxiety disorder is marked by its own relatively unique feature; this increased complexity of the model has encouraged research on the specific components that most clearly distinguish each disorder versus those that are common to many disorders. Frazer and Morilak [2005] have suggested that these formulations can provide a theoretical framework for understanding the neurobiological substrates of mood and anxiety symptoms as well as perhaps aid in understanding the mechanisms of antidepressant and anxiolytic treatments. Similarly, Suls and Bunde [2005] have suggested that generalized negative affect, rather than specific depressed or anxious states, may explain elevated disease risk (e.g., cardiovascular disease) in individuals with symptoms of depression or anxiety.
Evidence from human and non-human animal studies indicates that exposure to environmental and social stressors plays an etiological role in affective disorders [File 1996; Brugha, Bebbington, Stretch, MacCarthy, and Wyles 1997; Kalinichev, Easterling, Plotsky, and Holtzman 2002], and is associated with several mood- and anxiety-relevant physiological changes including neuroendocrine dysfunction [Nemeroff, Widerlöv, Bissette, Walléus, Karlsson, Eklund, Kilts, Loosen, and Vale 1984; Landgraf and Wigger 2003; Young, Abelson, and Cameron 2004], alterations in immune function [Leonard and Song 1996], dysregulated autonomic nervous system activity [Grippo, Moffitt, and Johnson 2002] and altered baroreceptor reflex control of the cardiovascular system [Watkins and Grossman 1999; Watkins, Blumenthal, and Carney 2002]. With attention to clinical and psychometric data, the study of analog depression and anxiety behaviors in non-human animals may increase our understanding of the mechanisms underlying mood and anxiety disorders. To this end, modeling valid behaviors relevant to both depression and anxiety (i.e., negative affect) may have utility for understanding shared signs and symptoms, physiological and central nervous system processes and efficacy and mechanisms of pharmacological treatments for mood and anxiety disorders.
The current study was designed to investigate behaviors relevant to depression and anxiety in prairie voles (Microtus ochrogaster) exposed to a chronic social stressor. Prairie voles are small rodents that demonstrate features of social monogamy similar to humans, including interactions with and reliance on the social environment [see Carter, DeVries, and Getz 1995], and therefore this species may provide a useful model system for studying the role of social experiences and neural mechanisms through which social interactions regulate behavior and physiology. For instance, our laboratory has demonstrated that female prairie voles display behavioral and neuroendocrine changes relevant to depression following exposure to a combination of chronic and acute social stressors [Grippo, Cushing, and Carter 2007]. The purpose of the present study was to examine the hypothesis that chronic social isolation, a stressor that is relevant to socially monogamous mammals (including humans), would induce in female prairie voles observable and quantifiable behaviors that are relevant to both mood and anxiety disorders.
In the present study, depression-like behaviors were evaluated using sucrose intake and the forced swim test; anxiety-like behaviors were evaluated using the elevated plus maze. These tests were chosen for their established validity in modeling behaviors relevant to depression or anxiety in rodents. Additionally, animals were presented with a social stimulus (exposure to an unrelated prairie vole pup), to determine whether this stimulus induced aggressive behaviors (this may represent a nonadaptive variant of the construct of maternal aggression that has been previously described in rodents [Gammie, Garland Jr., and Stevenson 2006; Lee and Gammie 2007]).
Female prairie voles were studied here for several reasons. First, affective disorders are more common in women than in men [American Psychiatric Association 2000], yet female rodents are an understudied group both in behavioral and physiological investigations of these disorders [see Introduction in Konkle, Baker, Kentner, Barbagallo, Merali, and Bielajew 2003]. Also, female prairie voles may be especially sensitive to the effects of social stressors [Cushing and Carter 2000; Grippo, Cushing, and Carter 2007]. Finally, female prairie voles do not show a spontaneous puberty or estrous cycle; in this species the ovaries remain comparatively inactive until the female has physical contact with a male [Carter, Witt, Schneider, Harris, and Volkening 1987], allowing for the use of reproductively intact animals without the need for controlling the estrous cycle.
MATERIALS AND METHODS
ANIMALS
Twenty-four adult (60–90 days) female prairie voles (40–50 grams) were used for the experimental procedures. Animals were descendants of a wild stock originally caught near Champaign, Illinois. Animals were maintained on a 14/10 h light/dark cycle (lights on at 0600 h), with a temperature of 25 ± 1° C and relative humidity of 21 ± 4 g/m3. All animals were allowed food (Purina rabbit chow; Purina, St. Louis, Missouri) and water ad libitum, unless otherwise specified. Offspring were housed with breeding pairs in large polycarbonate cages (25 × 45 × 60 cm) with cotton nesting material until 21 days of age, at which time they were removed and housed in same-sex sibling pairs in smaller cages (12 × 18 × 28 cm) until the commencement of the study. Only one animal from each sibling pair was studied here. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were preapproved by the University of Illinois at Chicago Institutional Animal Care and Use Committee.
SOCIAL ISOLATION
Animals were randomly divided into paired (control; n = 12) or isolated (n = 12) conditions. Animals were subjected to social isolation or pairing for 4 weeks. Isolation involved removing the experimental animal from the home cage and placing it into an individual cage (in a separate room, beyond smelling distance from its sibling). Paired (control) animals also were moved into new cages at the same time as the isolated animals, and then were continually housed with the same-sex siblings for the length of the respective isolation period. Throughout the isolation period, handling and cage changing procedures were matched between the two groups.
FLUID INTAKE
Voles were allowed ad libitum access to 1% sucrose, along with food and water, for 1 week before beginning experimental procedures to allow for adaptation to the sucrose taste. The consumption of water and 1% sucrose were measured as an operational index of anhedonia, defined specifically as reduced sucrose intake and sucrose preference relative to baseline values those of the control group [Willner, Muscat, and Papp 1992]. The fluid intake test was conducted during the light period (3–5 hours after light onset) using procedures similar to those described elsewhere [Grippo, Moffitt, and Johnson 2002]. Food and water were removed from the animal’s cage for a period of 20 hours prior to the fluid intake test. One hour before beginning the test, all animals were moved into clean, individual cages so as to ensure accurate fluid intake measurements of paired animals. Both groups (paired and isolated) were moved into clean cages for this 1-hour period to avoid potentially differential responses to a novel environment in the two groups. Tap water and 1% sucrose were placed on the cage in premeasured bottles, and fluid intake was monitored for 1 hour. Two baseline (i.e., pre-isolation) fluid intake tests were conducted, and the results were averaged. Fluid intake was measured following 2 and 4 weeks of social isolation in both groups (paired and isolated).
ELEVATED PLUS MAZE
Forty-eight hours following the final fluid intake test, all animals were tested in the elevated plus maze during the light period (3–5 hours after light onset), as a measure of anxiogenic behaviors using procedures similar to those described elsewhere [Pellow, Chopin, File, and Briley 1985]. The maze consisted of two open arms of clear Plexiglas, 49.5 × 10 cm, and two closed arms of black Plexiglas with an open roof, 49.5 × 10 × 30.5 cm. The center of the maze was a 10 × 10 cm section of clear Plexiglas. The arms were arranged such that the two open arms were opposite each other, and the maze was elevated to a height of 57 cm. The animal was placed in the center of the maze and allowed to explore it for a total of 5 minutes. Behavior was recorded using a video camera. All animals were returned to the home cage immediately following the test. The following behaviors were recorded by two trained, experimentally blind raters: (a) duration of time spent in the open arms; (b) duration of time spent in the closed arms; (c) duration of time spent in the center section; (d) total entries into the open arms; (e) total entries into the closed arms, and (f) percent of total arm time (excluding the center section) spent in the open arms. The animal was defined to be in one of the three sections of the maze (open, closed, center) when all 4 paws were in the respective section.
FORCED SWIM TEST
Forty-eight hours following the elevated plus maze, half of the animals from each group (n = 6 isolated and n = 6 paired) were tested in the forced swim test during the light period (3–5 hours after light onset), over a 2-day period, using the modified forced swim test procedures described by Cryan and colleagues [see Cryan, Valentino, and Lucki 2005]. A clear, cylindrical Plexiglas tank (height 46 cm; diameter 20 cm) was filled to a depth of 18 cm with tap water (23–25° C). Animals were placed individually into the tank for a total of 15 minutes. Twenty-four hours later, animals were again placed into the tank for a total of 5 minutes. Behaviors were recorded using a video camera. The tank was rinsed thoroughly and filled with clean water prior to testing each animal. The following behaviors were recorded by two trained, experimentally blind raters: (a) swimming, defined as movements of the forelimbs and hindlimbs without breaking the surface of the water; (b) struggling, defined as the forelimbs breaking the surface of water; (c) climbing, a variant of struggling, defined as attempts to climb the walls of the tank; and (d) immobility, defined as no limb or body movements (floating) or using the limbs to keep afloat without corresponding trunk movements.
PUP EXPOSURE
Forty-eight hours following the elevated plus maze, the remaining half of the animals in each group (n = 6 isolated and n = 6 paired) were exposed to an unrelated pup during the light period (3–5 hours after light onset). Each animal was placed into a clean testing cage (12×18×28 cm) and allowed to adapt to the cage for 2 hours. The unrelated and unfamiliar pup (1–2 days of age) was placed into the cage, and the behavior of the experimental animal was recorded using a video camera. The pup remained in the cage for a total of 5 minutes, unless the animal attacked the pup, at which time the test was aborted and the pup was immediately removed from the cage and euthanized according to approved procedures. All animals were returned to the home cage following the test. Each pup was used only once, and those pups that were not attacked were returned to the home cage following the test. The following behaviors were recorded by two trained, experimentally blind raters: (a) latency to approach the pup; (b) latency to attack the pup (if an attack occurred); (c) duration of time spent ignoring the pup; (d) duration of time spent approaching the pup; and (e) proportion of animals in each group that attacked the pup.
DATA ANALYSIS
The data are presented as means ± (or +) standard deviation (SD) or standard error of the mean (SEM; figures only). All data were analyzed using mixed-design analyses of variance (ANOVA) and/or a priori Student’s t-tests. The number of animals in each group that attacked the pup during the pup exposure test was analyzed using a test for a significant difference between two proportions (z test). Sucrose preference was calculated with the following formula: [% preference = (sucrose intake/total fluid intake)*100]. A probability value of p < 0.05 was considered to be statistically significant. A Bonferroni correction was used for all multiple comparisons involving t-tests; in all instances of multiple comparisons, the adjusted probability value, depending on the number of comparisons made, was used to determine whether the result was statistically significant (the probably values of 0.05 is reported in the text for accuracy).
RESULTS
FLUID INTAKE AND BODY WEIGHT
Social isolation reduced sucrose intake and sucrose preference as compared with baseline and control conditions (social pairing), indicative of anhedonia. Figure 1 displays absolute fluid intake and sucrose preference relative to total fluid intake, in the paired and isolated groups following the isolation period. Mixed-design ANOVAs were performed on absolute water and sucrose intake, total fluid intake and sucrose preference. The ANOVA performed on water intake yielded no significant effects (p > 0.05 for main effects and interaction), indicating that there was no difference in water intake between the paired and isolated groups; no follow-up tests were performed.
Figure 1.
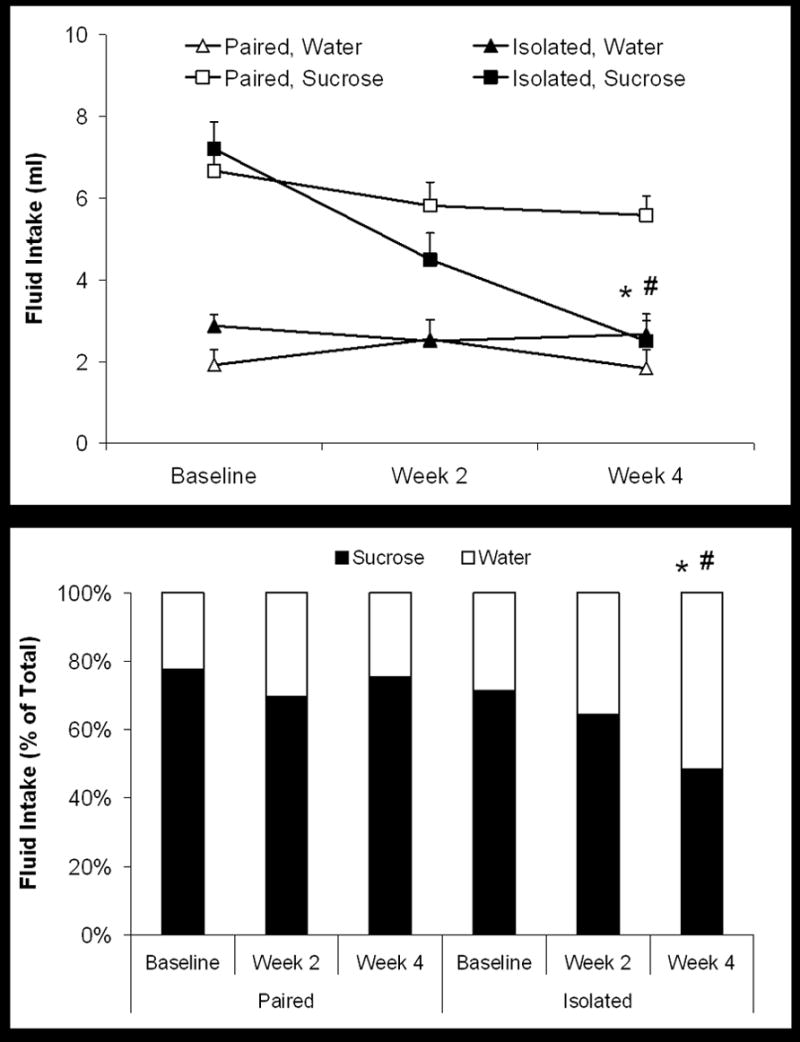
Mean (+ SEM) fluid intake (top panel) and mean sucrose preference (bottom panel) during a 1-hour fluid intake test in paired and isolated prairie voles at baseline and following 2 and 4 weeks of isolation. Social isolation reduced both absolute sucrose intake and sucrose preference compared with paired conditions (Student’s t-tests with a Bonferroni correction; *p < 0.05 vs. respective paired value; #p < 0.05 vs. respective baseline value).
The ANOVA performed on sucrose intake yielded a significant main effect of group [F(1,22) = 5.14, p < 0.05], time [F(2,44) = 15.92, p < 0.05], and a group by time interaction [F(2,44) = 6.13, p < 0.05]. The groups did not differ in baseline sucrose consumption (p > 0.05). Following 4 weeks of social isolation, the isolated group drank significantly less sucrose compared to its respective baseline consumption [t(11) = 6.43, p < 0.05] and that of the paired group [t(22) = 4.50, p < 0.05]. Conversely, the paired group did not exhibit a significant difference in its sucrose consumption between baseline and following the isolation period (p > 0.05).
The ANOVA performed on total fluid intake yielded a significant main effect of time [F(2,44) = 14.15, p < 0.05] and a group by time interaction [F(2,44) = 5.64, p < 0.05]. Paired and isolated animals did not differ in total fluid intake at baseline (p > 0.05). Following 4 weeks of isolation, the isolated group drank significantly less total fluid versus its respective baseline intake [t(11) = 5.49, p < 0.05] and the intake of the paired group [t(21) = 2.59, p < 0.05]. Total fluid intake in the paired group did not differ significantly between baseline and following 4 weeks of isolation (p > 0.05). The reduction in total fluid intake in the isolated group was due entirely to a reduction in sucrose consumption, without any corresponding reduction in water consumption (see above paragraphs).
The ANOVA performed on sucrose preference yielded a significant main effect of group [F(1,22) = 5.84, p < 0.05], and a group by time interaction [F(2,44) = 3.30, p < 0.05]. The baseline sucrose preference was not different between paired and isolated groups (p > 0.05). Following 4 weeks of social isolation, however, the isolated group showed a significantly reduced sucrose preference compared with both its respective baseline preference [t(11) = 3.17, p < 0.05] and the preference of the paired group [t(22) = 3.01, p < 0.05]. Sucrose consumption following the isolation period in the paired group was not significantly different from this group’s respective baseline preference (p > 0.05).
Social isolation did not significantly affect body weight. The body weights in paired and isolated groups were (a) baseline: 37 ± 7 g and 34 ± 9 g, respectively; (b) week 2 isolation: 39 ± 7 g and 35 ± 10 g, respectively; and (c) week 4 isolation: 40 ± 7 g and 35 ± 9 g, respectively. The ANOVA did not yield any significant effects (p > 0.05 for both main effects and interaction); no follow-up tests were conducted.
ELEVATED PLUS MAZE
Social isolation induced anxiogenic behaviors in the elevated plus maze, versus social pairing. Behaviors during the elevated plus maze were statistically analyzed in all animals, with the exception of one isolated animal that fell off the maze onto the floor (this test was immediately aborted). Figure 2 displays the amount of time spent in the open, closed and center sections (Panel A) and the percent of total arm time spent in the open arms of the maze (Panel B) in paired and isolated groups following 4 weeks of social isolation. The isolated group spent significantly less time in the open arms (t(21) = 1.81, p < 0.05], and significantly more time in the closed arms [t(21) = 2.20, p < 0.05], versus the paired group, consistent with anxiogenic behaviors. The amount of time spent in the center of the maze did not differ between the two groups (p > 0.05). The percentage of total arm time spent in the open arms was significantly reduced in the isolated group, versus the paired group [t(21) = 2.37, p < 0.05]. The isolated group made significantly fewer open arm entries than the paired group [3.1 ± 1.5 entries and 4.5 ± 1.9 entries in isolated and paired groups, respectively; t(21) = 2.00, p < 0.05], consistent with an anxiogenic response. However the number of closed arm entries did not differ between isolated and paired animals (6.7 ± 4.8 entries and 7.0 ± 3.3 entries in isolated and paired groups, respectively; p > 0.05), indicating no significant difference in generalized locomotor activity between the two groups.
Figure 2.
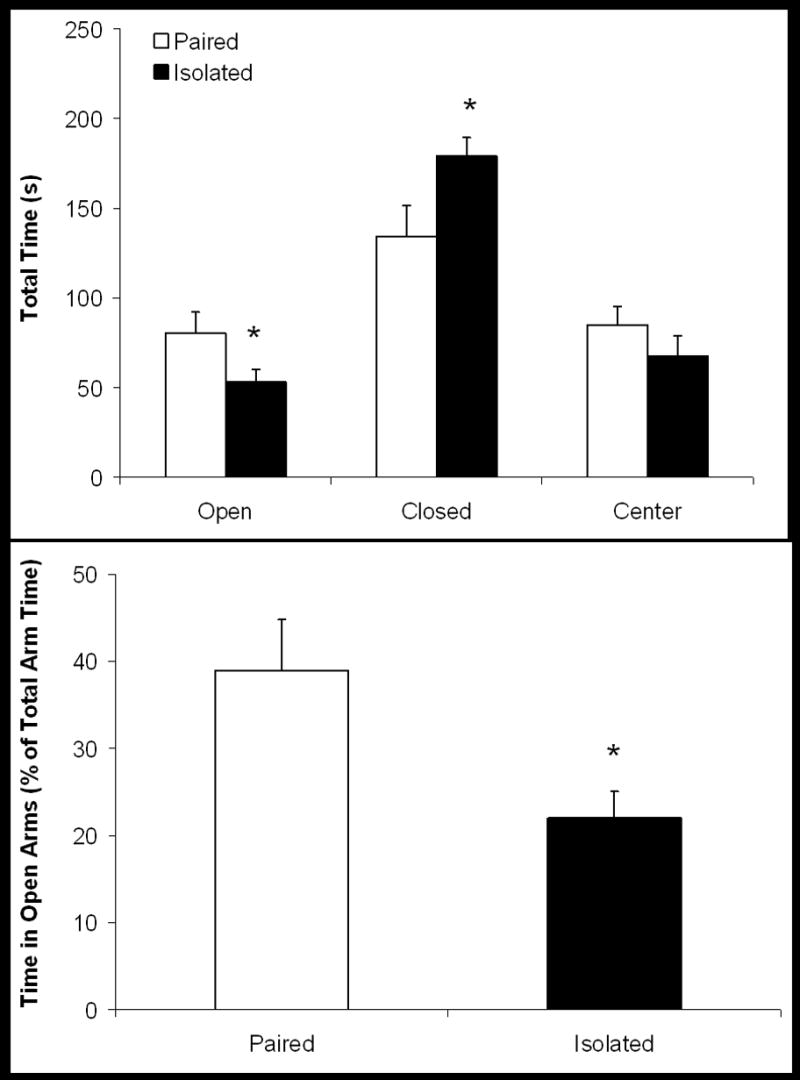
Mean (+ SEM) amount of time spent in the open, closed and center sections (top panel), and mean (+ SEM) percent of total arm time (excluding the center section) spent in the open arms (bottom panel), during a 5-minute elevated plus maze test in paired and isolated prairie voles following 4 weeks of social isolation. Socially isolated prairie voles spent significantly less time in the open arms and significantly more time in the closed arms of the maze versus paired animals (Student’s t-tests; *p < 0.05 vs. respective paired value).
FORCED SWIM TEST
On the first day of swimming (15 minute test), there were no significant differences between paired and isolated groups in struggling, climbing, swimming or immobility time (p > 0.05 for all comparisons; data not shown). On the second day of swimming (5 minute test), social isolation increased immobility time, versus social pairing [t(10) = 1.93, p < 0.05], possibly indicative of behavioral despair. The amount of time spent struggling, climbing and swimming was not different between the groups (p > 0.05 for all comparisons). Figure 3 displays the behaviors of paired and isolated animals during the 5-minute forced swim test.
Figure 3.
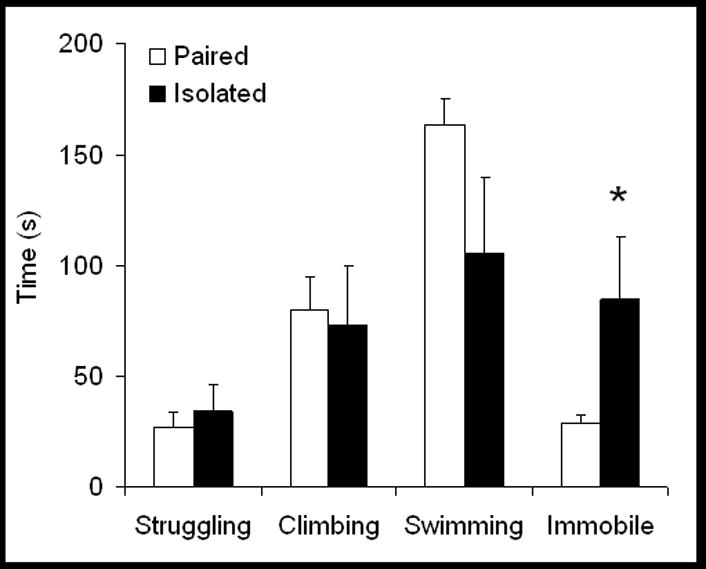
Mean (+ SEM) amount of time spent struggling, climbing, swimming and immobile during a 5-minute forced swim test in paired and isolated prairie voles following 4 weeks of isolation. Socially isolated prairie voles displayed significantly more immobility than did paired animals (Student’s t-tests; *p < 0.05 vs. respective paired value).
PUP EXPOSURE
Social isolation increased aggression when animals were exposed to an unrelated and unfamiliar prairie vole pup, versus social pairing. Table 1 displays the social behaviors of paired and isolated animals during this test. Isolated animals were significantly more likely to attack the pup versus paired animals (50% of paired animals attacked the pup, versus 100% of isolated animals attacked the pup; z = 2.00, p < 0.05). The latency to attack the pup was analyzed in relevant animals (n = 3 paired and 6 isolated). The paired group spent significantly more time than the isolated group ignoring the pup [t(10) = 1.93, p < 0.05]. The two groups did not differ in latency to approach the pup, latency to attack the pup or amount of time spent approaching the pup (p > 0.05 for all comparisons).
Table 1.
Pup-directed behaviors of paired (n = 6) and isolated (n = 6) prairie voles following 4 weeks of social isolation
| Attacks (% of Each Group) | Latency to Approach (s) | Latency to Attack (s) | Duration of Time Ignoring Pup (s) | Duration of Time Approaching Pup (s) | |
|---|---|---|---|---|---|
| Paired | 50 | 59.3 ± 94.5 | 107.4 ± 121.2^ | 95.7 ± 114.1 | 40.6 ± 51.7 |
| Isolated | 100* | 25.4 ± 29.6 | 44.4 ± 42.4 | 5.7 ± 8.4* | 15.1 ± 11.8 |
Note: Data (excluding first column) are shown as means ± SD. Student’s t-tests;
p < 0.05 vs. respective paired value.
Value includes data only from those animals that attacked the pup.
DISCUSSION AND CONCLUSIONS
There is increasing evidence that the social environment may play a role in the development of depressive and anxiety disorders [Post 1992; File 1996; Kiecolt-Glaser and Newton 2001; Cacioppo, Hawkley, Crawford, Ernst, Burleson, Kowalewski, Malarkey, Van Cauter, and Berntson 2002; Heinrichs, Baumgartner, Kirschbaum, and Ehlert 2003; Steptoe, Owen, Kunz-Ebrecht, and Brydon 2004]. The current findings provide preliminary evidence that social isolation induces depression-like behaviors in the form of anhedonia and behavioral despair [Porsolt, Bertin, and Jalfre 1977; Porsolt, Anton, Blavet, and Jalfre 1978], and anxiety-like behaviors in the form of altered elevated plus maze responses, using behavioral tests that are considered to be reliable and valid in rodents. Isolation also was associated with increased aggression toward an unfamiliar prairie vole pup. The present results may provide a foundation for the development of a novel rodent model system in prairie voles for the investigation of potential underlying neural substrates of co-occurring depression and anxiety (negative affect).
The operational measures employed in the current study were chosen for their established validity, reliability and utility in rodent models. Sucrose or saccharin preference represents a well-studied, reliable, and face- and construct-valid behavioral sign of depression in rodents, and is sensitive to traditional pharmacological antidepressants (i.e., high predictive validity) [Pucilowski, Overstreet, Rezvani, and Janowsky 1993; Willner 1997; Willner 2005; Grippo, Beltz, Weiss, and Johnson 2006]. The reduction of sucrose intake and preference in isolated prairie voles represents a specific hedonic deficit; similar to previous reports from other rodent species [Willner, Moreau, Nielsen, Papp, and Sluzewska 1996; Grippo, Moffitt, and Johnson 2002; Grippo, Beltz, Weiss, and Johnson 2006], the decreased responsiveness to sucrose was not due to a decrease in body weight in isolated animals, and water intake was unaffected by the isolation procedure indicating the lack of a generalized deficit in consummatory behavior. Additionally, the forced swim test was designed initially as a pharmacological model of depression with predictive validity [see Porsolt, Le Pichon, and Jalfre 1977], however, more recently the face and construct validity of this test in rodents have been described [Willner 1984; Cryan, Valentino, and Lucki 2005]. Isolated prairie voles showed predictable behavioral despair, as indexed by increased immobility time during the forced swim test.
For the study of anxiety-relevant behaviors, the elevated plus maze has been employed to screen for anxiolytic drugs in rodents and is sensitive to traditional pharmacological anxiolytic agents (i.e., high predictive validity) [Pellow and File 1986; Lister 1987], but also is considered to possess construct and ethological validity [File 1990; Carobrez and Bertoglio 2005]. In the current study, isolated prairie voles exhibited specific anxiogenic behaviors in the elevated plus maze (fewer open arm entries and less total time spent in open arms), without any change in generalized locomotor activity (total closed arm entries did not differ between paired and isolated groups). Finally, while social behaviors have been studied in the context of anxiety [File, Ouagazzal, Gonzalez, and Overstreet 1999; Razzoli, Roncari, Guidi, Carboni, Arban, Gerrard, and Bacchi 2006], exposure to an unrelated pup in prairie voles has received less attention. A recent study from Kim et al. [2003] suggests that acute exposure to a pup may facilitate social behavior in a pair-bonding test in adult female prairie voles. It is possible that aggression toward an unfamiliar pup represents a nonadaptive variant of maternal aggression which has been previously described in rodents [Gammie, Garland Jr., and Stevenson 2006; Lee and Gammie 2007]. This behavior may involve the brain γ-aminobutyric acid (GABA) system [Lee and Gammie 2007], which also has been linked to anxiety disorders and the mechanisms of anxiolytic drugs [Mohler 2006; Strous, Maayan, and Weizman 2006]. Further attention should be devoted to this paradigm as a potential operational measure related to aggression or anxiety-like behaviors. When considering the development of an animal model focused on the comorbidity or shared mechanisms of depression and anxiety, additional operational measures, such as responses to intracranial stimulation, exploratory behavior in the open field and fear-potentiated startle, might also be useful to investigate.
The present findings are consistent with those of Bosch et al. [2004], who showed altered behaviors (including less struggling) in the forced swim test in male prairie voles that were isolated acutely (3 days) from a female social partner. These results also are consistent with previous findings from our laboratory showing depression-like behaviors and neuroendocrine disturbances in female prairie voles exposed to 2 months of social isolation [Grippo, Cushing, and Carter 2007]. However, some responses to social isolation in mice and rats are unlike those described here; differences between prairie voles and other rodents in response to manipulations of the social environment may be partially dependent on the social structure of these different rodent species. For instance, in male NIH Swiss mice, acute social isolation (2 or 5 days) reduced immobility time in the forced swim test, and chronic social isolation (10–20 days) increased the proportion of entries made onto the open arms of the elevated plus maze [De Vry 1995]. Similarly, Kim and Kirkpatrick [1996] review some ambiguities in behavioral and physiological responses to social isolation in rats, and conclude that variability in results may be due to the duration of isolation, the particular strain of rat utilized, differential handling, or environmental differences between isolated and socially-housed animals.
The current study may be limited by species differences between prairie voles and humans. Although the lack of spontaneous puberty and estrous cycle in female prairie voles [Carter, Witt, Schneider, Harris, and Volkening 1987] provides an internal control for potential confounding female-specific hormonal influences on the dependent variables in question, it may limit the translation of results to human conditions during which the menstrual cycle cannot be controlled (for instance, naturalistic settings in regularly cycling, pre- or perimenopausal human females). Future research, focused on the effects of social isolation in mediating behavior in reproductively-primed prairie voles, can therefore extend the current findings.
The present results provide preliminary evidence for the role of negative social manipulations in mediating behavioral signs of both depression and anxiety. Future research is required to determine whether specific antidepressant and anxiolytic drugs are effective in preventing or reversing the behavioral alterations reported here. Knowledge relating to the interrelations among depression- and anxiety-like behaviors in isolated prairie voles may provide a model for examining the effectiveness of novel behavioral or pharmacological treatments. Several recent studies relevant to the mechanisms underlying both anxiety and depression have focused on serotonin or its receptors. For example, Frazer and Morilak [2005] suggest that alleviation of symptoms of negative affect, such as those reflecting agitation, distractibility, fear, anger, irritability, hostility or aggression, may be related to both the antidepressant and anxiolytic properties of serotonin reuptake inhibitors. Similarly, Overstreet and colleagues [2003] have reviewed the involvement of serotonin type 1A (5-HT1A) receptors in affective disorders. Oxytocin also may have antidepressant or anxiolytic effects [Arletti and Bertolini 1987; Uvnäs-Moberg, Ahlenius, Hillegaart, and Alster 1994; Zhang, Raap, Garcia, Serres, Ma, Battaglia, and Van de Kar 2000], and has been associated with social behavior [Knox and Uvnäs-Moberg 1998; Carter 1998]. Our laboratory has observed increases in both central and peripheral measures of oxytocin in isolated female prairie voles [Grippo, Cushing, and Carter 2007]. Oxytocin also is regulated by serotonin, and serotonin reuptake inhibitors may influence peripheral oxytocin release [Li, Levy, Cabrera, Brownfield, Battaglia, and Van de Kar 1993; Uvnäs-Moberg, Björkstrand, Hillegaart, and Ahlenius 1999]. This model offers the opportunity to examine these mechanisms, and their possible interactions, in the context of neural systems that may be involved in both anxiety and depression.
This model also presents an opportunity to determine whether factors that have been implicated in anxiety or depression are correlational or causal. Social isolation is associated with exaggerated neuroendocrine responses to an acute social stressor (resident-intruder test) [Grippo, Cushing, and Carter 2007] and reduced neurogenesis in the hypothalamus [Fowler, Liu, Ouimet, and Wang 2002] of adult prairie voles. Both high levels of adrenal hormones [Holsboer 1995; Engelmann, Landgraf, and Wotjak 2004] and reductions in neurogenesis [Nestler, Barrot, DiLeone, Eisch, Gold, and Monteggia 2002; Hoshaw, Malberg, and Lucki 2005] have been proposed as possible causal factors in depression. In addition, activation of the HPA axis, indexed by increased corticotropin-releasing hormone (CRF), adrenocorticotropic hormone or corticosterone, could play a major role in both anxiety and depression.
To conclude, these finding provide a first step in the development of a rodent model focused on negative social environmental manipulations in mediating signs of depression and anxiety. It will be necessary to conduct studies to determine more specifically the validity and reliability of the behaviors shown here, and the utility of this model for investigating shared mechanisms and responses to novel treatments. In combination with clinical, psychometric and experimental data in humans, the study of behaviors and associated features related to negative affect using an animal model may enhance our understanding of the mechanisms that underlie the comorbidity of mood and anxiety disorders.
Acknowledgments
This research was funded by National Institute of Mental Health MH 73233 (AJG) and MH 72935 (CSC), and National Institute of Child Health and Human Development HD 38490 (CSC). The authors are grateful to Dr. Rochelle Cohen, Ms. Rayna Brooks, Mr. Jonathan Huang, Ms. Suzanne Rothman, Ms. Lisa Sanzenbacher and Mr. Maulin Shah for assistance.
References
- Agelink MW, Majewski T, Wurthmann C, Postert T, Linka T, Rotterdam S, Klieser E. Autonomic neurocardiac function in patients with major depression and effects of antihypertensive treatment with nefazodone. J Affect Disord. 2001;62:187–198. doi: 10.1016/s0165-0327(99)00202-5. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 2000. text revision. [Google Scholar]
- Arletti R, Bertolini A. Oxytocin acts as an antidepressant in two animal models of depression. Life Sci. 1987;41:1725–1730. doi: 10.1016/0024-3205(87)90600-x. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Nair HP, Neumann ID, Young LJ. Pair-bonded prairie voles display depression-like behavior after separation 2004 Abstract Viewer/Itinerary Planner. Washington DC: Society for Neuroscience; 2004. Program No. 762.12 (Online) [Google Scholar]
- Brawman-Mintzer O. Pharmacologic treatment of generalized anxiety disorder. Psychiatr Clin North Am. 2001;24:119–137. doi: 10.1016/s0193-953x(05)70209-4. [DOI] [PubMed] [Google Scholar]
- Brown TA, Chorpita BF, Barlow DH. Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. J Abnorm Psychol. 1998;107:179–192. doi: 10.1037//0021-843x.107.2.179. [DOI] [PubMed] [Google Scholar]
- Brugha TS, Bebbington PE, Stretch DD, MacCarthy B, Wyles T. Predicting the short-term outcome of first episodes and recurrences of clinical depression: a prospective study of life events, difficulties, and social support networks. J Clin Psychiatry. 1997;58:298–306. doi: 10.4088/jcp.v58n0703. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Hawkley LC, Crawford LE, Ernst JM, Burleson MH, Kowalewski RB, Malarkey WB, Van Cauter E, Berntson GG. Loneliness and health: potential mechanisms. Psychosom Med. 2002;64:407–417. doi: 10.1097/00006842-200205000-00005. [DOI] [PubMed] [Google Scholar]
- Carobrez AP, Bertoglio LJ. Ethological and temporal analyses of anxiety-like behavior: the elevated plus maze 20 years on. Neurosci Biobehav Rev. 2005;29:1193–1205. doi: 10.1016/j.neubiorev.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Carter CS, Witt DM, Schneider J, Harris ZL, Volkening D. Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster) Horm Behav. 1987;21:74–82. doi: 10.1016/0018-506x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J Abnorm Psychol. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Clark LA, Watson D, Reynolds S. Diagnosis and classification of psychopathology: challenges to the current system and future directions. Annu Rev Psychol. 1995;46:121–153. doi: 10.1146/annurev.ps.46.020195.001005. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Cushing BS, Carter CS. Peripheral pulses of oxytocin increase partner preferences in female, but not male, prairie voles. Horm Behav. 2000;37:49–56. doi: 10.1006/hbeh.1999.1558. [DOI] [PubMed] [Google Scholar]
- De Vry J. 5-HT(1A) receptor agonists: recent developments and controversial issues. Psychopharmacology. 1995;121:1–26. doi: 10.1007/BF02245588. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Landgraf R, Wotjak CT. The hypothalamic-neurohypophysial system regulates the hypothalamic-pituitary-adrenal axis under stress: an old concept revisited. Front Neuroendocrinol. 2004;25:132–149. doi: 10.1016/j.yfrne.2004.09.001. [DOI] [PubMed] [Google Scholar]
- File SE. New strategies in the search for anxiolytics. Drug Des Deliv. 1990;5:195–201. [PubMed] [Google Scholar]
- File SE. Recent developments in anxiety, stress, and depression. Pharmacol Biochem Behav. 1996;54:3–12. doi: 10.1016/0091-3057(95)02175-2. [DOI] [PubMed] [Google Scholar]
- File SE, Ouagazzal AM, Gonzalez LE, Overstreet DH. Chronic fluoxetine in tests of anxiety in rat lines selectively bred for differential 5-HT1A receptor function. Pharmacol Biochem Behav. 1999;62:695–701. doi: 10.1016/s0091-3057(98)00208-1. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- Frazer A, Morilak DA. What should animal models of depression model? Neurosci Biobehav Rev. 2005;29:515–523. doi: 10.1016/j.neubiorev.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Garland T, Jr, Stevenson SA. Artificial selection for increased maternal defense behavior in mice. Behavior Gen. 2006;36:713–722. doi: 10.1007/s10519-006-9071-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Cane DB. Self-report assessment of depression and anxiety. In: Kendall PC, Watson D, editors. Anxiety and depression: distinctive and overlapping features. San Diego: Academic Press; 1989. pp. 131–169. [Google Scholar]
- Grippo AJ, Beltz TG, Weiss RM, Johnson AK. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol Psychiatry. 2006;59:309–316. doi: 10.1016/j.biopsych.2005.07.010. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom Med. 2007;69:149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1333–R1341. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- Haynes JC, Farrell M, Singleton N, Meltzer H, Araya R, Lewis G, Wiles NJ. Alcohol consumption as a risk factor for anxiety and depression: results from the longitudinal follow-up of the National Psychiatric Morbidity Survey. Br J Psychiatry. 2005;187:544–551. doi: 10.1192/bjp.187.6.544. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to psychosocial stress. Biol Psychiatry. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Holsboer F. Neuroendocrinology of mood disorders. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the fourth generation of progress. New York: Raven Press; 1995. pp. 957–969. [Google Scholar]
- Hoshaw BA, Malberg JE, Lucki I. Central administration of IGF-I and BDNF leads to long-lasting antidepressant-like effect. Brain Res. 2005;1037:204–208. doi: 10.1016/j.brainres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG. Long-lasting changes in stress-induced corticosterone response and anxiety-like behaviors as a consequence of neonatal maternal separation in Long-Evans rats. Pharmacol Biochem Behav. 2002;73:131–140. doi: 10.1016/s0091-3057(02)00781-5. [DOI] [PubMed] [Google Scholar]
- Kasper S, Resinger E. Panic disorder: the place of benzodiazepines and selective serotonin reuptake inhibitors. Eur Neuropsychopharmacol. 2001;11:307–321. doi: 10.1016/s0924-977x(01)00100-6. [DOI] [PubMed] [Google Scholar]
- Kendler KS. Major depression and generalised anxiety disorder: same genes, (partly) different environments- revisited. Br J Psychiatry. 1996;168:68–75. [PubMed] [Google Scholar]
- Kessler RC, Nelson CB, McGonagle KA, Liu J, Swartz M, Blazer DG. Comorbidity of DSM-III-R major depressive disorder in the general population: results from the US national comorbidity survey. Br J Psychiatry. 1996;30(Suppl):17–30. [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychol Bull. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kim AJ, Bales KL, Carter CS. Pup exposure influences the development of a partner preference in prairie voles. Horm Behav. 2003;44:58. [Google Scholar]
- Kim JW, Kirkpatrick B. Social isolation in animal models of relevance to neuropsychiatric disorders. Biol Psychiatry. 1996;40:918–922. doi: 10.1016/0006-3223(95)00546-3. [DOI] [PubMed] [Google Scholar]
- Knox SS, Uvnäs-Moberg K. Social isolation and cardiovascular disease: an atherosclerotic pathway? Psychoneuroendocrinology. 1998;23:877–890. doi: 10.1016/s0306-4530(98)00061-4. [DOI] [PubMed] [Google Scholar]
- Konkle AT, Baker SL, Kentner AC, Barbagallo LS, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–238. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Konstam V, Moser DK, De Jong MJ. Depression and anxiety in heart failure. J Cardiac Failure. 2005;11:455–463. doi: 10.1016/j.cardfail.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Wigger A. Born to be anxious: neuroendocrine and genetic correlates of trait anxiety in HAB rats. Stress. 2003;6:111–119. doi: 10.1080/1025389031000104193. [DOI] [PubMed] [Google Scholar]
- Landry MJ, Smith DE, Steinberg JR. Anxiety, depression, and substance use disorders: diagnosis, treatment, and prescribing practices. J Psychoactive Drugs. 1991;23:397–416. doi: 10.1080/02791072.1991.10471611. [DOI] [PubMed] [Google Scholar]
- Lee G, Gammie SC. GABA enhancement of maternal defense in mice: possible neural correlates. Pharmacol Biochem Behav. 2007;86:176–187. doi: 10.1016/j.pbb.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE, Song C. Stress and the immune system in the etiology of anxiety and depression. Pharmacol Biochem Behav. 1996;54:299–303. doi: 10.1016/0091-3057(95)02158-2. [DOI] [PubMed] [Google Scholar]
- Li Q, Levy AD, Cabrera TM, Brownfield MS, Battaglia G, Van de Kar LD. Long-term fluoxetine, but not desipramine, inhibits the ACTH and oxytocin responses to the 5-HT1A agonist, 8-OH-DPAT, in male rats. Brain Res. 1993;630:148–156. doi: 10.1016/0006-8993(93)90652-4. [DOI] [PubMed] [Google Scholar]
- Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- Middeldorp CM, Cath DC, Van Dyck R, Boomsma DI. The co-morbidity of anxiety and depression in the perspective of genetic epidemiology. A review of twin and family studies. Psychol Med. 2005;35:611–624. doi: 10.1017/s003329170400412x. [DOI] [PubMed] [Google Scholar]
- Mineka S, Watson D, Clark LA. Comorbidity of anxiety and unipolar mood disorders. Annu Rev Psychol. 1998;49:377–412. doi: 10.1146/annurev.psych.49.1.377. [DOI] [PubMed] [Google Scholar]
- Mohler H. GABAA receptors in central nervous system disease: anxiety, epilepsy, and insomnia. J Recep Signal Transduction Res. 2006;26:731–740. doi: 10.1080/10799890600920035. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlöv E, Bissette G, Walléus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34:13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Commissaris RC, De La Garza R, II, File SE, Knapp DJ, Seiden LS. Involvement of 5-HT(1A) receptors in animal tests of anxiety and depression: evidence from genetic models. Stress. 2003;6:101–110. doi: 10.1080/1025389031000111311. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm enteries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatment. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioural despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodynam. 1977;229:327–336. [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Post RM. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am J Psychiatry. 1992;149:999–1010. doi: 10.1176/ajp.149.8.999. [DOI] [PubMed] [Google Scholar]
- Pucilowski O, Overstreet DH, Rezvani AH, Janowsky DS. Chronic mild stress-induced anhedonia: greater effect in a genetic rat model of depression. Physiol Behav. 1993;54:1215–1220. doi: 10.1016/0031-9384(93)90351-f. [DOI] [PubMed] [Google Scholar]
- Razzoli M, Roncari E, Guidi A, Carboni L, Arban R, Gerrard P, Bacchi F. Conditioning properties of social subordination in rats: behavioral and biochemical correlates of anxiety. Horm Behav. 2006;50:245–251. doi: 10.1016/j.yhbeh.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Stahl SM. Mixed depression and anxiety: serotonin(1A) receptors as a common pharmacologic link. J Clin Psychiatry. 1997;58(Suppl 8):20–26. [PubMed] [Google Scholar]
- Steptoe A, Owen N, Kunz-Ebrecht SR, Brydon L. Loneliness and neuroendocrine, cardiovascular, and inflammatory stress responses in middle-aged men and women. Psychoneuroendocrinology. 2004;29:593–611. doi: 10.1016/S0306-4530(03)00086-6. [DOI] [PubMed] [Google Scholar]
- Strous RD, Maayan R, Weizman A. The relevance of neurosteroids to clinical psychiatry: from the laboratory to the bedside. Eur Neuropsychopharmacol. 2006;16:155–169. doi: 10.1016/j.euroneuro.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Suls J, Bunde J. Anger, anxiety and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K, Ahlenius S, Hillegaart V, Alster P. High doses of oxytocin cause sedation and low doses cause an anxiolytic-like effect in male rats. Pharmacol Biochem Behav. 1994;49:101–106. doi: 10.1016/0091-3057(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K, Björkstrand E, Hillegaart V, Ahlenius S. Oxytocin as a possible mediator of SSRI-induced antidepressant effects. Psychopharmacology. 1999;142:95–101. doi: 10.1007/s002130050867. [DOI] [PubMed] [Google Scholar]
- Watkins LL, Blumenthal JA, Carney RM. Association of anxiety with reduced baroreflex cardiac control in patients after acute myocardial infarction. Am Heart J. 2002;143:460–466. doi: 10.1067/mhj.2002.120404. [DOI] [PubMed] [Google Scholar]
- Watkins LL, Grossman P. Association of depressive symptoms with reduced baroreflex cardiac control in coronary artery disease. Am Heart J. 1999;137:453–457. doi: 10.1016/s0002-8703(99)70491-6. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark LA. Negative affectivity: the disposition to experience aversive emotional states. Psychol Bull. 1984;96:465–490. [PubMed] [Google Scholar]
- Widiger TA, Clark LA. Toward DSM-V and the classification of psychopathology. Psychol Bull. 2000;126:946–963. doi: 10.1037/0033-2909.126.6.946. [DOI] [PubMed] [Google Scholar]
- Willner P. The validity of animal models of depression. Psychopharmacology. 1984;83:1–16. doi: 10.1007/BF00427414. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- Willner P, Moreau J-L, Nielsen CK, Papp M, Sluzewska A. Decreased hedonic responsiveness following chronic mild stress is not secondary to loss of body weight. Physiol Behav. 1996;60:129–134. doi: 10.1016/0031-9384(95)02256-2. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson JL, Cameron OG. Effect of comorbid anxiety disorders on the hypothalamic-pituitary-adrenal axis response to a social stressor in major depression. Biol Psychiatry. 2004;56:113–120. doi: 10.1016/j.biopsych.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Raap DK, Garcia F, Serres F, Ma Q, Battaglia G, Van de Kar LD. Long-term fluoxetine produces behavior anxiolytic effects without inhibiting neuroendocrine responses to conditioned stress in rats. Brain Res. 2000;855:58–66. doi: 10.1016/s0006-8993(99)02289-1. [DOI] [PubMed] [Google Scholar]


