Abstract
Identifying the mechanisms that drive suprachiasmatic nucleus (SCN) neurons to fire action potentials with a higher frequency during the day than during the night is an important goal of circadian neurobiology. Selective chemical tools with defined mechanisms of action on specific ion channels are required for successful completion of these studies. The transient K+ current (IA) plays an active role in neuronal action potential firing and may contribute to modulating the circadian firing frequency. Tetraethylammonium (TEA) is frequently used to inhibit delayed rectifier K+ currents (IDR) during electrophysiological recordings of IA. Depolarizing voltage-clamped hamster SCN neurons from a hyperpolarized holding potential activated both IA and IDR. Holding the membrane potential at depolarized values inactivated IA and only the IDR was activated during a voltage step. The identity of IA was confirmed by applying 4-aminopyridine (5 mM), which significantly inhibited IA. Reducing the TEA concentration from 40 mM to 1 mM significantly decreased the IA inactivation time constant (τinact) from 9.8 ± 3.0 ms to 4.9 ± 1.2 ms. The changes in IA τinact were unlikely to be due to a surface charge effect. The IA amplitude was not affected by TEA at any concentration or membrane potential. The isosmotic replacement of NaCl with choline chloride had no effect in IA kinetics demonstrating that the TEA effects were not due to a reduction of extracellular NaCl. We conclude that TEA modulates, in a concentration dependent manner, τinact but not IA amplitude in hamster SCN neurons.
Section: Neurophysiology, Neuropharmacology and other forms of Intercellular Communication
Keywords: potassium current, suprachiasmatic nucleus, circadian rhythm, KV4 channel
1. Introduction
Neurons in the hypothalamic suprachiasmatic nucleus (SCN) contain a molecular clock that drives circadian rhythms. The circadian clock determines many facets of SCN neuronal activity including the frequency of action potential firing and also the strength of afferent excitatory and inhibitory synapses (Gompf and Allen, 2004; Itri et al., 2005; Lundkvist et al., 2002; Pennartz et al., 2001). The mechanisms underlying the circadian regulation of cellular electrical activity remain largely unknown. Several ionic currents including a persistent Na+ current, delayed rectifier K+ channels (IDR), large conductance Ca2+-activated K+ channels (BK), transient K+ current (A-type, IA), and voltage-dependent L- and T-type Ca2+ channels regulate action potential firing or show significant day-night differences in activity or both (Itri et al., 2004; Jackson et al., 2004; Kim et al., 2005; Kononenko et al., 2004; Kuhlman and McMahon, 2004; Meredith et al., 2006; Pennartz et al., 2002; Pitts et al., 2006). Identification of the contribution that each ion channel makes to the firing of SCN neurons requires selective chemical tools with defined mechanisms of action.
IA is a rapidly activating, rapidly inactivating K+ current that contributes to setting the timing between action potentials and the postsynaptic responses to synaptic inputs (Connor and Stevens, 1971). IA is observed in the majority of SCN neurons and may play a role in setting the action potential firing frequency in these cells (Bouskila and Dudek, 1995; Huang, 1993; Huang et al., 1993). IA is carried by ion channels composed of α subunits of the Kv4 family (Shal, (Jerng et al., 2004)). In neurons, the observed IA activation and inactivation kinetics require the presence of Kv Channel Interacting Proteins (KChIP). KChIP are EF-hand Ca2+-binding proteins that associate with the cytoplasmic tail of the Kv α subunits and alter the expression of the α subunits and IA inactivation kinetics (An et al., 2000; Rhodes et al., 2004).
Activation of IA increases the interspike interval and slows the action potential firing frequency by reducing the rate of membrane depolarization (Rudy et al., 1999). IA is largely inactivated in the range of resting membrane potentials (−40 mV to −55 mV) recorded from SCN neurons during the daytime (Kuhlman et al., 2003; Schaap et al., 1999; Teshima et al., 2003). IA rapidly recovers from inactivation during the afterhyperpolarization that follows the upstroke an action potential. The membrane hyperpolarization moves the Kv4 channels to closed states from which IA can be activated during a subsequent subthreshold depolarization (Bardoni and Belluzzi, 1993; Campbell et al., 1993). Therefore, a careful characterization of IA properties is required to understand the physiological properties of SCN neurons and for the development of accurate computational models of neuronal function and circuitry in the SCN.
In addition to IA, SCN neurons have both fast and slow delayed rectifier K+ currents (IDR) and depolarization of an SCN neuron will activate both IA and IDR (Bouskila and Dudek, 1995; Itri et al., 2005; Kuhlman and McMahon, 2004). Two experimental strategies are usually used individually or together to isolate IA from the IDR. The first takes advantage of the fact that IA and IDR have different rates of inactivation at depolarizing voltages. Holding the membrane potential at relatively depolarized levels inactivates IA and a subsequent membrane depolarization only activates IDR. Alternately, tetraethylammonium (TEA) is used to separate IA from the IDR because IA is much less sensitive to TEA block than IDR (Andreasen and Hablitz, 1992). To produce a significant reduction in the IDR requires a TEA concentration of 20 mM to 60 mM (Bardoni and Belluzzi, 1993; Zhou and Hablitz, 1996). Together, the low TEA sensitivity of IA and the voltage-dependence of IDR activation and IA inactivation, allow accurate recording of IA. However, some authors have found that TEA alters IA amplitude but not the kinetics (Bardoni and Belluzzi, 1993; Sanchez et al., 1998). While studying the kinetics of IA in hamster SCN neurons we observed that the TEA concentrations required to block IDR significantly increased IA inactivation time constant.
2. Results
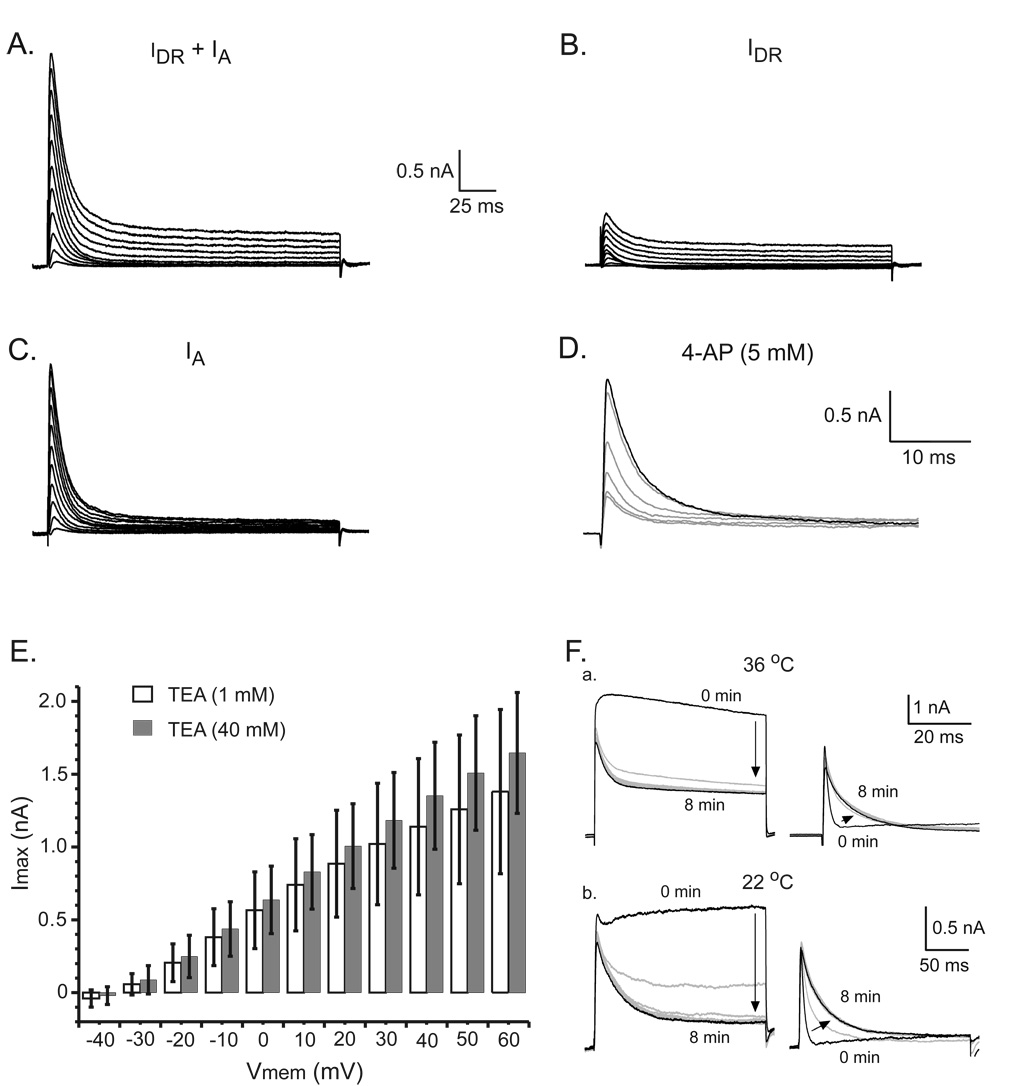
The first experiments were designed to determine IA inactivation time constant (τinact) in hamster SCN neurons. In the presence of TEA (40 mM), SCN neurons were voltage-clamped at −100 mV then sequentially stepped in 10 mV increments to membrane potentials ranging from −30 mV and +60 mV (Fig. 1A). The IDR recorded under these conditions was only a small fraction of the total IDR due to TEA inhibition (Fig. 1B). The membrane holding potential was then set at −40 mV to inactivate IA and a second set of depolarizing pulses applied (range −30 mV to +60 mV). IA was isolated by digitally subtracting the currents recorded at the −40 mV holding potential from those recorded using the −100 mV holding potential (Fig. 1C). The identity of IA was confirmed by an 8 min bath application of 4-aminopyridine (4-AP, 5 mM), which significantly reduced the IA amplitude 83 ± 10% (Fig. 1D). The mean IA amplitude recorded at +60 mV was 964 ± 231 pA during control recordings and 162 ± 64 pA (n = 4, p < 0.04) after 4-AP application. IA amplitude was not affected by TEA (1 mM and 40 mM) when the experiments were performed at 36°C (Fig. 1E). The mean maximal IA amplitudes, recorded in 1 mM and 40 mM TEA with a voltage step to +60 mV were not significantly different (p > 0.21); 1.4 ± 0.6 nA (n = 14) and 1.6 ± 0.4 nA (n = 10), respectively. Recently it was demonstrated that IA activation kinetics are modified in preparations maintained at room temperature (Campbell et al., 1993). Therefore, experiments were performed at 22 °C to determine whether the TEA effects on IA were temperature dependent. However, neither 1 mM nor 40 mM TEA had an effect on IA amplitude when the experiments were performed at 22°C (n = 3).
Figure 1.
Effects of TEA and 4-AP on IA of hamster SCN neurons. The bath solution contained TTX (0.5 µM), picrotoxin (50 µM), TEA (40 mM) and a low extracellular Ca2+ concentration (0.2 mM) to reduce Ca2+ currents. A. Currents were activated by sequence of voltage steps to potentials between −30 mV and +60 mV from a holding potential of −100 mV. This depolarization protocol activated both IDR and IA. B. Holding the membrane potential at −40 mV and applying a sequence of voltage steps to depolarized values between −30 mV to +60 mV activated the IDR. C. Digital subtraction of the currents recorded in A and B. D. Example of the inhibition of IA by 4-AP (5 mM). The currents shown were recorded each minute during an 8 min 4-AP application. E. A high concentration of TEA (40 mM) did not alter IA amplitude. Each bar represents the mean IA amplitude at each step potential (mean ± standard deviation, n = 10). F. a. TEA (40 mM) added to the recording chamber (0 min) produced a progressive decrease in the IDR activated from a holding potential of −80 mV by a voltage step to +60 mV. Note that the remaining current shows a rapid activation followed by inactivation. IA recorded during the same experiment. IA τinact increased from 3.3 ms to 28 ms during the 8 min TEA application (36 °C). b. A similar experiment performed at 22°C. TEA (40 mM) increased IA τinact from 1.3 ms to 10.4 ms.
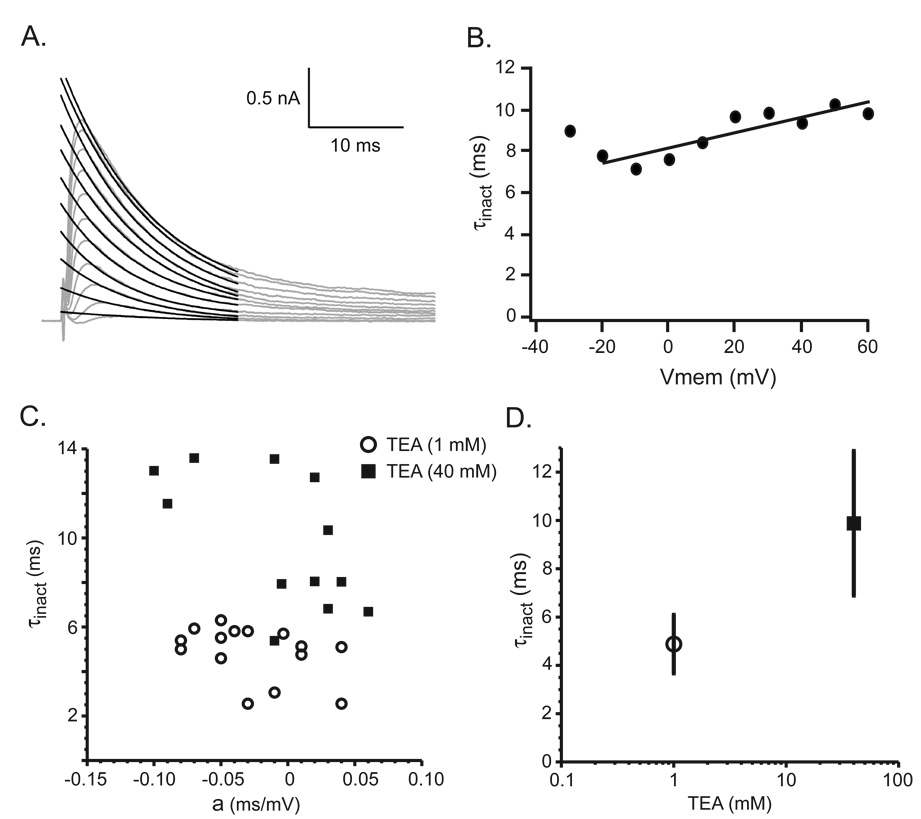
IA inactivation progressively slowed during the 8 min TEA (40 mM) perfusion at both 36 °C and 22 °C (Fig. 1F). To further examine the effect of TEA on IA kinetics, a sequence of voltage steps were used to activate IA in each SCN neuron. A τinact was calculated for IA activated by each voltage step, the τinact plotted versus membrane potential (Vmemb), and the data fit with a line, τinact = a*Vmemb + b (Fig. 2A, B). The τinact for each SCN neuron was estimated from the linear fit at Vmem = 0 (parameter b). The τinact, estimated in the presence of 40 mM TEA, ranged from 5.4 ms to 13.6 ms with mean of 9.8 ± 3.0 ms (n = 12). Reducing the TEA concentration from 40 mM to 1 mM decreased the mean estimated τinact to 4.9 ± 1.2 ms with a range of 2.6 ms to 6.3 ms (n = 15). The τinact recorded in 40 mM TEA was significantly longer than that recorded in 1 mM TEA (p < 0.0001) and the individual τinact fell into two distinct populations (Fig. 2C, D). The slope of the linear fit (parameter a) in 1 mM and 40 mM TEA were similar (−0.26 ± 0.01 vs. −0.07 ± 0.02 ms/10 mV, n = 15 and n = 12, respectively, p < 0.30). The shallowness of the τinact voltage in the presence of both low and high TEA concentrations suggests that the TEA induced lengthening of τinact was not due to a surface charge effect.
Figure 2.
Sensitivity of IA τinact to TEA (40 mM). A, B. Example of the calculated IA τinact. IA (gray lines) and the black lines the fit to the equation (see Experimental Procedures). B. The value of the IA τinact was directly related to the value of the membrane potential (Vmem). C. The effect of high (40 mM) and low (1 mM) TEA concentrations on parameters of IA τinact. Distribution of the values for the parameter (a) and IA τinact calculated from the linear fits to the data from each neuron. The parameter (a) represents the voltage dependence of IA τinact while parameter (b) represents the IA τinact at 0 mV. D. The mean ± standard deviation of IA τinact in the presence of either 40 mM or 1 mM TEA shows the strong effect of the TEA on IA inactivation kinetics.
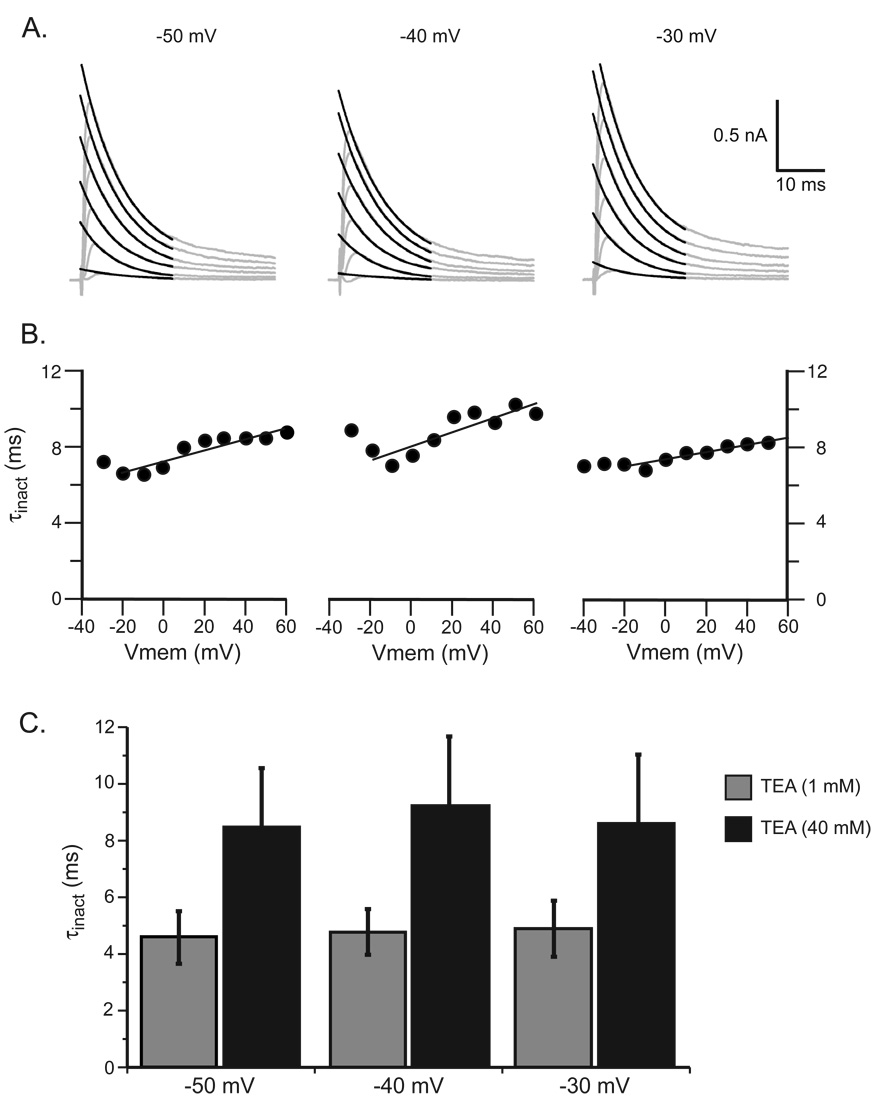
In Helix aspersa neurons, TEA in the extracellular solution shifts the voltage-dependence of IA activation by altering the membrane potential near the voltage sensing domains of the Kv4 channels (Denton and Leiter, 2002). Experiments were performed using an initial holding potential of −100 mV followed by three holding potentials, −50 mV, −40 mV, and −30 mV to determine whether the holding potential used to inactivate IA altered the TEA effect on τinact. The τinact in the presence of 1 mM TEA at the three different holding potentials were not significantly different: 4.6 ± 0.3 at −50 mV, 4.8 ± 0.3 at −40 mV and 4.9 ± 0.4 at −30 mV (n = 8, p > 0.22, Fig. 3). In the presence of 40 mM TEA, the τinact were 8.5 ± 0.7 at −50 mV, 8.8 ± 0.7 at −40 mV and 7.9 ± 0.6 at −30 mV (n = 8, p > 0.29). These data demonstrate that the holding potential used to inactivate IA did not alter the lengthening τinact by 1 mM or 40 mM TEA. It further demonstrated that the subtraction procedure used to isolate IA produced similar results at each holding voltage.
Figure 3.
Determination of IA τinact at holding potentials of −50 mV, −40 mV and −30 mV. A. The gray lines were IA and the black lines the fit to the equation B. The calculated IA τinact was not affected by the holding potential. C. The mean ± standard deviation of IA τinact recorded at three holding potentials in the presence of high (40 mM) and low (1 mM) TEA concentration.
During the experiments described above, NaCl (39 mM) was replaced isosmotically by TEA to increase the TEA concentration from 1 mM to 40 mM. Therefore, additional experiments were performed to insure that the effect of TEA on IA τinact was not due to the reduction of extracellular NaCl. IA τinact was similar in normal ACSF (1 mM TEA) and when NaCl was isosmotically replaced with choline chloride (39 mM, 8 min perfusion): 2.7 ± 0.9 ms vs. 4.0 ± 1.5 ms (n = 8, p > 0.06).
3. Discussion
Identification of the mechanisms that drive SCN neurons to fire action potentials with a higher frequency during the day than during the night is an important topic in circadian neurobiology. IA plays an active role in neuronal action potential repolarization and may contribute to setting the circadian firing frequency (Bouskila and Dudek, 1995; Huang, 1993; Huang et al., 1993). IA may also modulate the response of SCN neurons to retinohypothalamic tract excitatory input. Thus, the accurate determination of IA kinetics is very important for the development of realistic models of SCN neurons that faithfully reproduce the spiking activity. SCN neurons express many types of K+ channels that may also determine the membrane potential and firing frequency (Itri et al., 2004; Kuhlman and McMahon, 2004; Pitts et al., 2006; Teshima et al., 2003). It is an experimental challenge to isolate and characterize these diverse K+ currents. A frequently used strategy to isolate IA is to block IDR with TEA. Unfortunately, in hamster SCN neurons application of TEA at concentrations sufficient to inhibit the IDR altered IA τinact that was shown in our experiments.
TEA has variable effects on IA amplitude and kinetics depending on the concentration and type of the cell. In, TEA had no effect on IA guinea pig laterodorsal tegmental neurons (40 mM), sensormotor cortical neurons, or cerebellar granule cells (20 mM) (Bardoni and Belluzzi, 1993; Sanchez et al., 1998; Zhou and Hablitz, 1996). In contrast, 60 mM TEA reduces IA amplitude by more than 40% in layer 1 neurons and layer II/III pyramidal cells of the neocortex. In these neurons the τinact was found to be approximately 15 ms (Zhou and Hablitz, 1996). In rat dorsal nucleus neurons, a TEA concentration of 10 mM reduced the peak IA by 23% but did not alter the τinact value of 12.3 ms (Fu et al., 1996). In hamster SCN neurons, we found that while IA amplitude was insensitive to TEA, IA τinact was significantly increased from 4.9 ms in 1 mM of TEA to 9.8 ms in 40 mM TEA. The last value is within the range of IA τinact (10 – 20 ms) observed in other neurons (Bardoni and Belluzzi, 1993; Fu et al., 1996; Zhou and Hablitz, 1996).
TEA is a quaternary cation that may alter ion channel kinetics by multiple mechanisms. One possibility is a surface charge effect where the electrical potential across the membrane is reduced by cations neutralizing negatively charged membrane components. For example, TEA in the extracellular solution shifts the voltage-dependence of IA activation, in Helix aspersa neurons, by altering the membrane potential near the voltage sensing domains of the Kv4 channels (Denton and Leiter, 2002). It is unlikely that the TEA alteration of IA τinact was due to a surface charge effect since there was a very shallow voltage dependence of τinact of hamster SCN neurons in either high or low TEA concentrations. We therefore conclude that TEA is not a useful blocker for the evaluation of IA kinetics in hamster suprachiasmatic nucleus neurons because it alters IA τinact.
4. Experimental Procedure
Golden hamsters (Mesocricetus auratus) were housed under a 14:10 hr light–dark cycle for at least a week. The hamsters were deeply anesthetized with halothane at least 1 hour before the beginning of the dark period, decapitated and the brain removed (Gillette, 1986). The Oregon Health & Science University Institutional Animal Care and Use Committee approved in advance all procedures involving animals. Thin slices containing the SCN (250 µm) were cut with a vibrating blade microtome (Leica, VT1000S, Nussloch, Germany) and incubated in a storage chamber with a physiological solution at 33°C saturated with 95% O2 - 5% CO2.
After 2–3 hr, SCN slices were placed in a small recording chamber and perfused with a solution containing in mM: NaCl 139, KCl 3, NaH2PO4 1.2, MgCl2 3.3, CaCl2 0.2, NaHCO3 20, glucose 9 saturated with 95% O2 - 5% CO2 at 36°C. Recording electrodes were pulled using a PP-83 electrode puller (Narishige, Japan) and had resistances of 8–10 MΩ when filled with a solution composed of (in mM): K-gluconate 125, KCl 10, EGTA 5, HEPES 10, MgATP 2 and Tris GTP 0.2. Individual SCN neurons were visualized using infrared illumination and differential interference contrast optics on a Leica DFLMS microscope (Nussloch, Germany). Currents were recorded in the whole-cell patch-clamp configuration using an Axopatch-1D amplifier (Axon Instruments, USA). Series resistances were compensated a minimum of 70%. The evoked currents were low-pass filtered at 2 KHz and acquired at a sampling rate of 20 KHz with an ITC16 interface (Instrutech, USA) and Pulse+PulseFit software (HEKA, Lambrecht, Germany). The recorded currents were analyzed with Excel (Microsoft, USA) and plotted with Igor Pro (WaveMetrics, USA). The reported voltages were not corrected for a liquid junction potential of approximately −13 mV.
The experimental strategy used to study IA in neurons takes advantage of the observation that IA is inactivated at depolarized membrane potentials. IA was isolated by subtracting the currents activated from a holding potential of −100 mV from those activated by depolarizing pulses from a holding potential of −40 mV. IA inactivation time constant (τinact) was determined by fitting the subtracted currents with the equation:
where τ is the inactivation time constant, io is the baseline current and K is the maximal IA amplitude. The data are presented as the mean ± standard deviation.
Voltage-dependent Na+ channels were blocked with tetrodotoxin (TTX, 0.5 µM) and spontaneous GABAA receptor-mediated currents were inhibited with picrotoxin (50 µM). Voltage-dependent Ca2+ currents were insignificant at the external Ca2+ concentration of 0.2 mM. The minimal concentration of TEA used was 1 mM, which inhibited the Ca2+-activated K+ currents and the fast IDR (Wong and Adler, 1986).
Acknowledgements
We would like to thank Dr. Mykhaylo Moldavan and Dr. David W. Robinson for critically reading the manuscript. The experimental work was supported by grant NS36607 (CNA) from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature References
- An WF, et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- Andreasen M, Hablitz JJ. Kinetic properties of a transient outward current in rat neocortical neurons. J Neurophysiol. 1992;68:1133–1142. doi: 10.1152/jn.1992.68.4.1133. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Belluzzi O. Kinetic study and numerical reconstruction of A-type current in granule cells of rat cerebellar slices. J Neurophysiol. 1993;69:2222–2231. doi: 10.1152/jn.1993.69.6.2222. [DOI] [PubMed] [Google Scholar]
- Bouskila Y, Dudek FE. A rapidly activating type of outward rectifier K+ current and A-current in rat suprachiasmatic nucleus neurones. J Physiol (Lond) 1995;488:339–350. doi: 10.1113/jphysiol.1995.sp020970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DL, et al. The calcium-independent transient outward potassium current in isolated ferret right ventricular myocytes. I. Basic characterization and kinetic analysis. J Gen Physiol. 1993;101:571–601. doi: 10.1085/jgp.101.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JA, Stevens CF. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol (Lond) 1971;213:21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton JS, Leiter JC. Anomalous effects of external TEA on permeation and gating of the A-type potassium current in H. aspersa neuronal somata. J Membr Biol. 2002;190:17–28. doi: 10.1007/s00232-002-1021-9. [DOI] [PubMed] [Google Scholar]
- Fu XW, et al. Potassium currents and membrane excitability of neurons in the rat's dorsal nucleus of the lateral lemniscus. J Neurophysiol. 1996;76:1121–1132. doi: 10.1152/jn.1996.76.2.1121. [DOI] [PubMed] [Google Scholar]
- Gillette MU. The suprachiasmatic nuclei: circadian phase-shifts induced at the time of hypothalamic slice preparation are preserved in vitro. Brain Res. 1986;379:176–181. doi: 10.1016/0006-8993(86)90273-8. [DOI] [PubMed] [Google Scholar]
- Gompf HS, Allen CN. GABAergic synapses of the suprachiasmatic nucleus exhibit a diurnal rhythm of short-term synaptic plasticity. European Journal of Neuroscience. 2004;19:2791–2798. doi: 10.1111/j.1460-9568.2004.03382.x. [DOI] [PubMed] [Google Scholar]
- Huang R-C. Sodium and calcium currents in acutely dissociated neurons from rat suprachiasmatic nucleus. J Neurophysiol. 1993;70:1692–1703. doi: 10.1152/jn.1993.70.4.1692. [DOI] [PubMed] [Google Scholar]
- Huang R-C, et al. Zinc modulation of a transient potassium current and histochemical localization of the metal in neurons of the suprachiasmatic nucleus. Proc Natl Acad Sci USA. 1993;90:11806–11810. doi: 10.1073/pnas.90.24.11806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri J, et al. Circadian rhythm in inhibitory synaptic transmission in the mouse suprachiasmatic nucleus. J Neurophysiol. 2004;92:311–319. doi: 10.1152/jn.01078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itri JN, et al. Fast delayed rectifier potassium current is required for circadian neural activity. Nat Neurosci. 2005;8:650–656. doi: 10.1038/nn1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, et al. Mechanism of spontaneous firing in dorsomedial suprachiasmatic nucleus neurons. J Neurosci. 2004;24:7985–7998. doi: 10.1523/JNEUROSCI.2146-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng HH, et al. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004;27:343–369. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Kim DY, et al. Voltage-gated calcium channels play crucial roles in the glutamate-induced phase shifts of the rat suprachiasmatic circadian clock. Eur J Neurosci. 2005;21:1215–1222. doi: 10.1111/j.1460-9568.2005.03950.x. [DOI] [PubMed] [Google Scholar]
- Kononenko NI, et al. Riluzole-sensitive slowly inactivating sodium current in rat suprachiasmatic nucleus neurons. J Neurophysiol. 2004;91:710–718. doi: 10.1152/jn.00770.2003. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, McMahon DG. Rhythmic regulation of membrane potential and potassium current persists in SCN neurons in the absence of environmental input. Eur J Neurosci. 2004;20:1113–1117. doi: 10.1111/j.1460-9568.2004.03555.x. [DOI] [PubMed] [Google Scholar]
- Kuhlman SJ, et al. Phase resetting light pulses induce Per1 and persistent spike activity in a subpopulation of biological clock neurons. J Neurosci. 2003;23:1441–1450. doi: 10.1523/JNEUROSCI.23-04-01441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist GB, et al. The suprachiasmatic nucleus exhibits diurnal variations in spontaneous excitatory postsynaptic activity. J Biol Rhythms. 2002;17:40–51. doi: 10.1177/074873002129002320. [DOI] [PubMed] [Google Scholar]
- Meredith AL, et al. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennartz CMA, et al. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature. 2002;416:286–290. doi: 10.1038/nature728. [DOI] [PubMed] [Google Scholar]
- Pennartz CMA, et al. Enhanced NMDA receptor activity in retinal inputs to the rat suprachiasmatic nucleus during the subjective night. Journal of Physiology. 2001;532:181–194. doi: 10.1111/j.1469-7793.2001.0181g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts GR, et al. Daily rhythmicity of large-conductance Ca2+ -activated K+ currents in suprachiasmatic nucleus neurons. Brain Res. 2006;1071:54–62. doi: 10.1016/j.brainres.2005.11.078. [DOI] [PubMed] [Google Scholar]
- Rhodes KJ, et al. KChIPs and Kv4 alpha subunits as integral components of A-type potassium channels in mammalian brain. J Neurosci. 2004;24:7903–7915. doi: 10.1523/JNEUROSCI.0776-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, et al. Contributions of Kv3 channels to neuronal excitability. Ann N Y Acad Sci. 1999;868:304–343. doi: 10.1111/j.1749-6632.1999.tb11295.x. [DOI] [PubMed] [Google Scholar]
- Sanchez RM. Voltage-clamp analysis and computer simulation of a novel cesium-resistant A-current in guinea pig laterodorsal tegmental neurons. J Neurophysiol. 1998;79:3111–3126. doi: 10.1152/jn.1998.79.6.3111. [DOI] [PubMed] [Google Scholar]
- Schaap J, et al. Neurons of the rat suprachiasmatic nucleus show a circadian rhythm in membrane properties that is lost during prolonged whole-cell recording. Brain Res. 1999;815:154–166. doi: 10.1016/s0006-8993(98)01025-7. [DOI] [PubMed] [Google Scholar]
- Teshima K, et al. Characterization of an apamin-sensitive potassium current in suprachiasmatic nucleus neurons. Neuroscience. 2003;120:65–73. doi: 10.1016/s0306-4522(03)00270-7. [DOI] [PubMed] [Google Scholar]
- Wong BS, Adler M. Tetraethylammonium blockade of calcium-activated potassium channels in clonal anterior pituitary cells. Pflugers Arch. 1986;407:279–284. doi: 10.1007/BF00585303. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Hablitz JJ. Layer I neurons of the rat neocortex. II. Voltage-dependent outward currents. J Neurophysiol. 1996;76:668–682. doi: 10.1152/jn.1996.76.2.668. [DOI] [PubMed] [Google Scholar]