Abstract
We characterized the expression and function of the endoplasmic reticulum protein GRP78 in glial tumors. GRP78 is highly expressed in glioblastomas but not in oligodendrogliomas, and its expression is inversely correlated with median patient survival. Overexpression of GRP78 in glioma cells decreases caspase 7 activation and renders the cells resistant to etoposide- and cisplatin- induced apoptosis, whereas silencing of GRP78 decreases cell growth and sensitizes glioma cells to etoposide, cisplatin, and γ-radiation. Thus, GRP78 contributes to the increased apoptosis resistance and growth of glioma cells and may provide a target for enhancing the therapeutic responsiveness of these tumors.
Keywords: apoptosis, caspase 7, glioblastoma, GRP78, survival
Glioblastomas (GBMs) are the most frequent primary CNS neoplasms, accounting for more than 50% of all brain tumors.1 Despite advances in diagnostic and surgical techniques, the prognosis of these patients remains poor and the median survival of 12 months has not significantly changed during the last several years.2 The latter is due primarily to the fact that GBMs are highly resistant to chemotherapy and radiotherapy.3
GRP78 is a member of the HSP70 family, which plays an important role in oncogenesis.4 GRP78 resides primarily in the endoplasmic reticulum (ER) and plays a role in the proper folding and assembly of functional proteins.5 The expression of GRP78 is up-regulated by glucose starvation and hypoxia, which are present in the tumor microenvironment.6 Indeed, GRP78 is highly expressed in various tumors and plays a role in their growth, metastasis, and immune regulation.7–10 GRP78 has been also implicated in tumor progression and maintenance.7 It plays an important role in cell survival and in promoting chemoresistance by interfering with various apoptotic signaling pathways.11–13 GRP78 expression and function in glial tumors, however, have not been characterized. Here, we demonstrate that the expression of GRP78 is elevated in GBM as compared to low-grade astrocytomas (LGAs) and oligodendrogliomas, and that GRP78 expression is inversely correlated with glioma patient survival. Moreover, GRP78 confers resistance to glioma cells from apoptosis induced by etoposide, cisplatin, and radiation.
Materials and Methods
Sample and Clinical Information
Snap frozen normal tissue (n = 21) and brain tumor (n = 169) specimens with clinical annotation were collected at the Hermelin Brain Tumor Center, Henry Ford Hospital (Detroit, MI, USA). All specimens were collected under an institutional review board–approved protocol and deidentified for patient confidentiality. Clinical annotation was provided for all samples.
Glioma Cell Lines
The glioma cell lines A172, U87, LNZ308, U251, LN-443, and LN-229 have been previously described.14
Microarray Data
Gene expression profiles of 163 normal and brain tumor specimens were captured using Affymetrix (Santa Clara, CA, USA) U133 Plus2 GeneChips according to the manufacturer’s protocol. Array data were processed according to the Affymetrix MAS5 (Microarray Suite 5) algorithm implemented in Affymetrix GCOS (GeneChip Operating Software). Text files exported from GCOS were uploaded into GeneSpring 7.2 for data management (Silicon Genetics, Redwood City, CA, USA). Expression profiling was performed by the Neuro-Oncology Branch at the National Cancer Institute.
Statistical Analysis of Tumor Samples
Differential gene expression between normal brain tissue and different tumor types was tested for significance with two-sample, unpaired t-tests under an assumption of unequal variance. Univariate Cox proportional-hazard regression was used to assess the relationship between gene expression and patient survival. Survival was measured in days from date of diagnosis to date of death (DOD) or date last seen (DLS). Censoring was done on samples with DLS instead of DOD. Normal specimens and samples without survival data were excluded from the survival analysis (n = 146). For the survival analysis, p < 0.05 was considered significant. Correlation (r) and Cox hazard regression coefficients were used to determine the nature of the relationship between expression and survival (i.e., r > 0 and a negative Cox coefficient indicates increased expression is associated with longer survival, and vice versa).
Real-Time PCR
Real-time PCR was performed as described previously.14 The following primers were used: GRP78 forward: CCAAGAGAGGGTTCTTGAATCTCG; GRP78 reverse: ATGGGCCAGCCTGGATATACAACA; S12 forward: TGCTGGAGGTGTAATGGACG; S12 reverse: CAAGCACACAAAGATGGGCT.
Reactions were run on an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA, USA). The cycling conditions were composed of 4 min polymerase activation at 95°C and 40 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min. Cycle threshold (Ct) values were obtained from the ABI 7000 software. S12 levels were also determined for each RNA sample as a control.
GRP78 Immunohistochemistry
Nine human GBM surgical specimens and six surgical specimens from epilepsy patients were assessed for GRP78 expression as described previously.15 Briefly, GRP78 (RB-10478; Labvision, Fremont, CA, USA) antibody was diluted to 1:750 in Sniper blocking reagent (Biocare Medical, Concord, CA, USA) and incubated on the sections at room temperature for 30 min. Detection was with 4Plus universal link and 4Plus horseradish peroxidase label followed by Betazoid diaminobenzidine for 4 min. Negative controls were incubated in rabbit IgG at the same concentration as the primary antibody.
Cell Transfection
Cells were transfected with the control vector (green fluorescent protein [GFP]) or with GFP-GRP78 expression vector by electroporation using the Nucleofector device (Amaxa Biosystems, Gaithersburg, MD, USA) as described.14 Small interfering (si)RNA duplexes targeting GRP78 and a control scrambled sequence were obtained from Dharmacon (Lafayette, CO, USA).
Measurement of Cell Apoptosis
Cell apoptosis was measured by flow cytometry after propidium iodide staining, as described previously.14
MTT Assay
MTT assay (Promega, Madison, WI, USA) was used to assess the number of viable cells. MTT assay was performed according to the manufacturer’s instructions. Briefly, cells transfected with control siRNA or GRP78 siRNA for 3 days were plated in a 96-well plate at the concentration of 25,000 cells/ml. Cells were then irradiated with 2 and 5 Gy and were incubated for 24 and 48 h thereafter. The relative absorbance was measured at 595 nm.
Western Blot Analysis
GFP-GRP78 expression was examined using Western blot analysis, as described.14
Statistical Analysis
The results are presented as the mean values ± SE. Data were analyzed using analysis of variance and a Student’s t-test. For real-time PCR data, Student’s t-test (with correction for data sets with unequal variances) was done using Prism 4 (GraphPad Software, Inc., San Diego, CA, USA).
Results
GRP78 Is Expressed in Gliomas and Is Inversely Correlated with Patient Survival
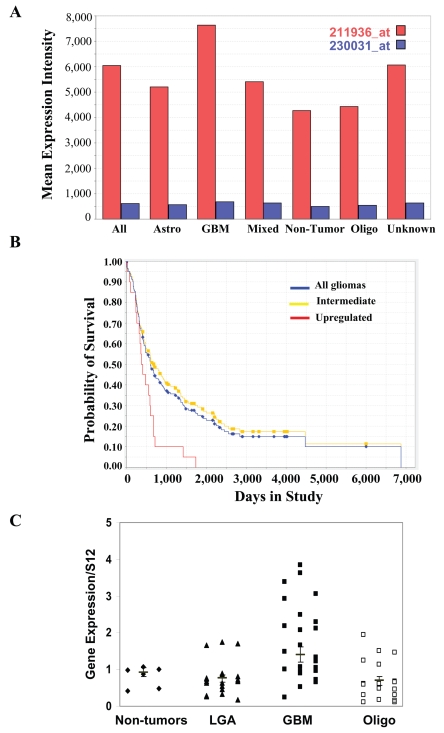
GRP78 has been shown to be highly expressed in various tumors7–10; however, its expression in glial tumors has not been reported. Using microarray analysis, we found that GRP78 expression was up-regulated in glioma specimens and that its expression correlated with tumor grade (Fig. 1A16). Thus, the expression of GRP78 was the highest in GBM as compared to specimens from control brains, LGAs, and oligodendrogliomas. Moreover, among glial tumor specimens, elevated GRP78 expression was significantly associated with poor survival (Fig. 1B16; p < 0.0012).
Fig. 1.
GRP78 expression is up-regulated in astrocytic tumors and is correlated with overall patient survival. (A) Microarray analysis was performed on tumor specimens from 190 patients, including 81 glioblastomas multiforme (GBM), 49 oligodendrogliomas, 28 astrocytomas, 11 mixed gliomas, and 21 nontumor brain tissues obtained from epilepsy patients. (B) The probability of GBM patient survival according to GRP78 expression level was determined for glioma patients. The yellow line indicates the survival of GBM patients with intermediate levels of GRP78 mRNA; the red line indicates the survival of GBM patients with high levels of GRP78 mRNA; and the blue line indicates the overall GBM patient survival rate. T-test analysis demonstrated that the p value between the intermediate and high levels was less than 0.001. (C) The expression of GRP78 in normal brain, GBM, low-grade astrocytoma (LGA), and oligodendroglioma (Oligo) specimens was analyzed by real-time PCR. Results are normalized relative to the levels of S12 mRNA and are presented relative to reference sample. Mean values are marked. GBM versus normal, p < 0.05; GBM versus Oligo, p < 0.001; GBM versus LGA, p < 0.001.
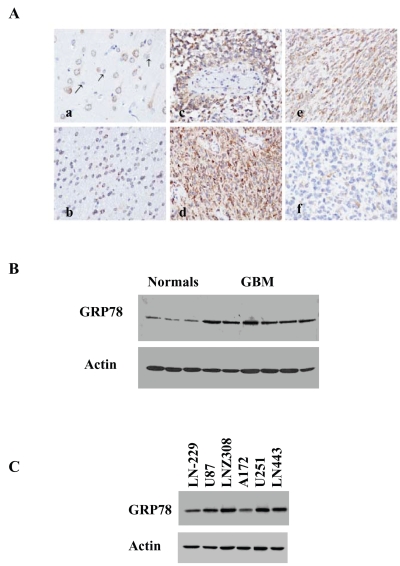
To validate the microarray results, we examined the expression of GRP78 in different types of glial tumors using tumor samples from LGA (grade II), anaplastic oligodendrogliomas (grade III), and GBMs (grade IV). The expression of GRP78 in these tumors was compared to that of normal brains using real-time PCR. As presented in Fig. 1C, the expression of GRP78 in normal brains was low. Similarly, most LGAs and oligodendrogliomas expressed low levels of GRP78. In contrast, higher levels of GRP78 were observed in GBMs. Immunohistochemical staining with the anti-GRP78 antibody showed cytoplasmic staining of cortical neurons; however, cortical astrocytes and microglia cells showed negative reactivity (Fig. 2A-a). Immunostaining of subcortical deep white matter revealed moderate GRP78 expression in oligodendrocytes (Fig. 2A-b). These were observed in all six normal brain sections examined (data not shown). Immuno-staining of GBM samples demonstrated that most of the tumors were strongly and diffusely positive for GRP78 (5/9; Fig. 2A-c,d). Some tumors showed moderate diffuse staining (3/9; Fig. 2A-e), and only one showed only weak and focal GRP78 positivity (Fig. 2A-f).
Fig. 2.
Expression of GRP78 in glioblastoma (GBM) specimens and glioma cell lines. (A) GRP78 expression in normal brain and GBM tissue sections: immunohistochemical staining of normal adult cortex with an anti-GRP78 antibody demonstrating cytoplasmic immunoreactivity of neurons and negative immunoreactivity of astrocytes (a); section of normal subcortical white matter showing positive GRP78 immunostaining of oligodendrocytes (b); anti-GRP78 immunohistochemical staining of human GBMs demonstrating a range of GRP78 immunoreactivity from strong diffuse positivity (c and d), to moderate diffuse positivity (e), to weak focal positivity (f). All original magnifications, ×400. The expression of GRP78 in normal brain and GBM specimens (B) and in glioma cell lines (C) was also examined using Western blot analysis.
The expression of the GRP78 protein was also examined by Western blot analysis. Similar to the mRNA results, specimens of normal brains expressed low levels of the GRP78 protein, whereas significantly higher levels of this protein were observed in GBM specimens (Fig. 2B). As presented in Fig. 2C, GRP78 was expressed in all the glioma cell lines examined.
Overexpression of GRP78 Protects Glioma Cells from Etoposide- and Cisplatin-Induced Apoptosis
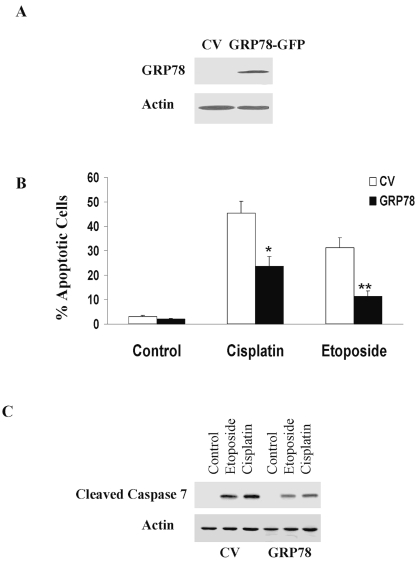
GRP78 has been reported to regulate cell apoptosis.11–13 To examine the role of GRP78 in glioma cell apoptosis, we overexpressed this protein in the A172 glioma cell lines that express relatively lower levels of GRP78 (Fig. 3A). As presented in Fig. 3B, overexpression of GRP78 significantly decreased the apoptosis of the A172 cells in response to etoposide (100 μM) and cisplatin (30 μM). Treatment of control vector A172 cells with etoposide or cisplatin for 48 h induced 31% and 43% of cell apoptosis, respectively (Fig. 3B). In contrast, overexpression of GRP78 decreased cell apoptosis to 22% in cisplatin-treated cells and to 11% in etoposide-treated cells (Fig. 3B). GRP78 has been reported to inhibit the activation of caspase 7 induced by etoposide.12 Similarly, we found that overexpression of GRP78 significantly decreased the activation of caspase 7 induced by both etoposide and cisplatin (Fig. 3C).
Fig. 3.
Overexpression of GRP78 decreases the sensitivity of glioma cells to etoposide and cisplatin. A172 cells were transfected with green fluorescent protein (GFP)-GRP78 or with GFP (control vector, CV). After 24 h, the cells were treated with etoposide (100 μM) or cisplatin (30 μM) for an additional 48 h. Overexpression of GRP78 was determined using Western blot analysis and an anti-GRP78 antibody (A). Cell apoptosis was determined using propidium iodide staining and fluorescence-activated cell sorting analysis (B), and the cleavage of caspase 7 was determined using an anti-caspase 7 antibody that recognizes the cleaved form (C). The results represent one of four experiments with similar results (A and C) or represent the means ± SE of three independent experiments (B). *p < 0.01, **p < 0.001.
GRP78 Silencing Enhances the Apoptotic Effect of Cisplatin, Etoposide, and γ-Radiation in Glioma Cells
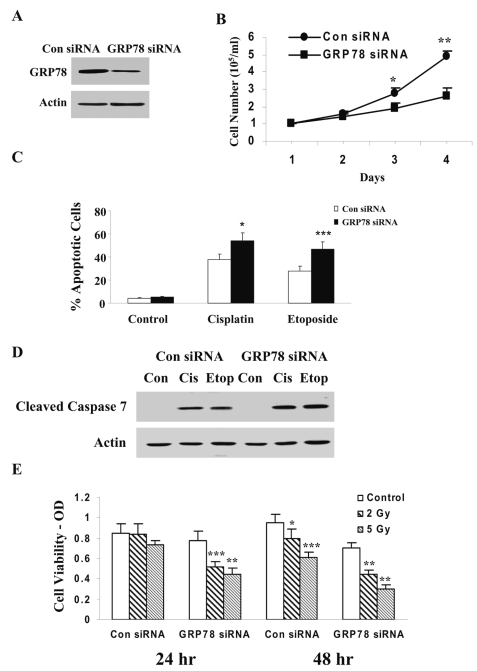
To further examine the effect of GRP78 in the regulation of glioma cell apoptosis, we silenced the expression of GRP78 using siRNA duplexes. For these experiments, we used LNZ308 cells, which express higher levels of GRP78. Fig. 4A shows decreased GRP78 expression in cells transfected with GRP78 siRNA duplex as compared with cells transfected with a control scrambled siRNA duplex. Silencing of GRP78 in the LNZ308 decreased the growth of the cells (Fig. 4B) but did not induce cell apoptosis (Fig. 4C). In contrast, silencing of GRP78 significantly increased the apoptotic response of the cells to etoposide and cisplatin (Fig. 4C). Thus, control siRNA-transfected cells exhibited 29.5% cell apoptosis in response to etoposide and 37.8% cell apoptosis in cisplatin-treated cells. Silencing of GRP78 increased the apoptosis of the cells in response to both etoposide and cisplatin. Similarly, silencing of GRP78 also induced an increase in the cleavage of caspase 7 in the etoposide- and cisplatin-treated cells (Fig. 4D).
Fig. 4.
Silencing of GRP78 increases glioma cell apoptosis in response to etoposide, cisplatin, and γ-radiation. LNZ308 cells were transfected with control or GRP78 small interfering (si)RNA duplexes. After 3 days, the expression of GRP78 was examined using Western blot analysis (A). Control and GRP78 siRNA-transfected cells were plated in plastic dishes, and cell number was monitored at 24-h intervals (B). Cells were treated with etoposide (100 μM) or cisplatin (30 μM) or irradiated with 2 and 5 Gy. Cell apoptosis was determined following 24 h using propidium iodide staining and fluorescence-activated cell sorting analysis (C), and cleaved caspase 7 was determined by Western blot analysis (D). Cell viability in the irradiated cells was determined using MTT assay (E). The results represent one of four experiments with similar results (A and D) or represent the means ± SE of three independent experiments (B, C, and E). *p < 0.01, **p < 0.001, ***p < 0.005. OD, optical density.
We also examined the role of GRP78 in the resistance of glioma cells to γ-radiation. LNZ308 cells transfected with control siRNA and GRP78 siRNA were irradiated with 2 and 5 Gy for 24 and 48 h and cell viability was determined using MTT assay. Irradiation of the cells induced only a small decrease in cell viability of the control siRNA transfected cells following 48 h. In contrast, silencing of GRP78 significantly enhanced the sensitivity of the cells to 2 Gy and 5 Gy radiation after 24 and 48 h (Fig. 4E).
Discussion
GRP78 is a molecular chaperone that resides in the ER and belongs to the HSP70 protein family.4 GRP78 is induced under certain stress conditions such as glucose starvation, hypoxia, and oxidative stress, which are characteristic in the tumor microenvironment.6 Indeed, the expression of GRP78 has been shown to be elevated in a variety of tumors, including prostate,17 lung,18 breast,10 colon,19 and gastric tumors8; however, its expression and function in gliomas has not yet been described. We found that GRP78 was highly expressed in GBMs as compared to normal brain specimens and that its expression in astrocytic tumors correlated with tumor grade. Thus, LGAs exhibited lower levels of GRP78 than GBMs. Interestingly, oligodendrogliomas also expressed low levels of GRP78, similar to those of normal brain specimens and LGAs. Anaplastic oligoden-drogliomas and GBMs have different clinical courses; anaplastic oligodendrogliomas show better response to chemotherapy, therefore portending a more favorable prognosis.20 Thus, the decreased expression of GRP78 in oligodendrogliomas, as compared to that of GBM, may partially explain their superior response to chemotherapy, and GRP78 may be a useful differential diagnostic marker of these neoplasms.
In addition to its increased expression in GBM, GRP78 expression was inversely correlated with patient survival. Thus, higher levels of GRP78 indicated lower median patient survival. Similar results have been previously described for prostate tumors, in which increased GRP78 expression was associated with poor overall survival.17 Similarly, the elevated expression of GRP78 correlated with poor prognosis in patients with gastric cancer8 as well as in patients with liver and colon cancer.9,19
We found that GRP78 regulated glioma cell growth and the responsiveness of glioma cells to etoposide, cisplatin, and γ-radiation. Overexpression of GRP78 decreased the sensitivity of glioma cells to etoposide and cisplatin, whereas silencing of GRP78 significantly increased the sensitivity of glioma cells to these stimuli as well as to γ-radiation. GRP78 has been associated with the regulation of cell apoptosis via different mechanisms. Reddy et al. reported that GRP78 associated with caspase 7 formed an antiapoptotic complex in the ER membrane, thereby inhibiting caspase 7 activation/ cleavage in etoposide-treated cells.12 In addition, GRP78 has been shown to associate with the BH3-only proapoptotic protein Bik in the ER and to inhibit the activation of Bax and the release of cytochrome C from the mitochondria.13 We found that in glioma cells, GRP78 overexpression decreased the cleavage of caspase 7 by etoposide and cisplatin and, conversely, that silencing of GRP78 increased the cleavage of caspase 7 by both drugs. The protective effect of GRP78 from cell apoptosis has been demonstrated to be an important factor in determining the response of various tumors to chemotherapy. Thus, GRP78 acts as a predictor of the responsiveness of breast cancer to Adriamycin10 and to hormone therapy.13
In summary, we found that GRP78 is overexpressed in GBM and that its expression is inversely correlated with patient survival. The expression of GRP78 is associated with the enhanced cell growth of glioma cells and with their sensitivity to apoptosis induced by chemotherapeutic drugs such as etoposide and cisplatin and to γ-radiation. Collectively, our results demonstrate that GRP78 is a potential prognostic marker and a therapeutic target in gliomas, particularly GBM.
Acknowledgments
This work was supported by CA-109196 (C.B.), CA-R21-96965 (T.M.), and CA-86997 (S.A.R.) from the National Cancer Institute and by the William and Karen Davidson Fund, Hermelin Brain Tumor Center.
References
- 1.Kleihues P, Louis DN, Scheithauer BW, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. [DOI] [PubMed] [Google Scholar]
- 2.Brandes AA. State-of-the-art treatment of high-grade brain tumors. Semin Oncol. 2003;30:4–9. doi: 10.1053/j.seminoncol.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Prados MD, Levin V. Biology and treatment of malignant glioma. Semin Oncol. 2000;27:1–10. [PubMed] [Google Scholar]
- 4.Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 5.Kleizen B, Braakman B. Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol. 2004;16:343–349. doi: 10.1016/j.ceb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 7.Fu Y, Lee AS. Glucose regulated proteins in cancer progression, drug resistance and immunotherapy. Cancer Biol Ther. 2006;5:741–744. doi: 10.4161/cbt.5.7.2970. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Jiang Y, Jia Z, et al. Association of elevated GRP78 expression with increased lymph node metastasis and poor prognosis in patients with gastric cancer. Clin Exp Metastasis. 2006;23:401–410. doi: 10.1007/s10585-006-9051-9. [DOI] [PubMed] [Google Scholar]
- 9.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–3499. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 10.Lee E, Nichols P, Spicer D, Groshen S, Yu MC, Lee AS. GRP78 as a novel predictor of responsiveness to chemotherapy in breast cancer. Cancer Res. 2006;66:7849–7853. doi: 10.1158/0008-5472.CAN-06-1660. [DOI] [PubMed] [Google Scholar]
- 11.Miyake H, Hara I, Arakawa S, Kamidono S. Stress protein GRP78 prevents apoptosis induced by calcium ionophore, ionomycin, but not by glycosylation inhibitor, tunicamycin, in human prostate cancer cells. J Cell Biochem. 2000;77:396–408. doi: 10.1002/(sici)1097-4644(20000601)77:3<396::aid-jcb5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Reddy RC, Mao C, Baumeister P, Austin RC, Kaufman RK, Lee AS. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: role of ATP binding site in suppression of caspase-7 activation, . J Biol Chem. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- 13.Fu Y, Li J, Lee AS. GRP78/BiP inhibits endoplasmic reticulum BIK and protects human breast cancer cells against estrogen starvation-induced apoptosis. Cancer Res. 2007;67:3734–3740. doi: 10.1158/0008-5472.CAN-06-4594. [DOI] [PubMed] [Google Scholar]
- 14.Rosenzweig T, Ziv-Av A, Xiang C, et al. Related to testes-specific, vespid, and pathogenesis protein-1 (RTVP-1) is overexpressed in gliomas and regulates the growth, survival, and invasion of glioma cells. Cancer Res. 2006;66:4139–4148. doi: 10.1158/0008-5472.CAN-05-2851. [DOI] [PubMed] [Google Scholar]
- 15.Schultz C, Lemke N, Ge S, Golembieski WA, Rempel SA. Secreted protein acidic and rich in cysteine promotes glioma invasion and delays tumor growth in vivo. Cancer Res. 2002;62:6270–6277. [PubMed] [Google Scholar]
- 16.National Cancer Institute. Rembrandt Database. Available at http://rembrandt.nci.nih.gov.
- 17.Pootrakul L, Datar RH, Shi SR, et al. Expression of stress response protein GRP78 is associated with the development of castration-resistant prostate cancer. Clin Cancer Res. 2006;12:5987–5993. doi: 10.1158/1078-0432.CCR-06-0133. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, He Z, Zhang J, et al. Overexpression of endoplasmic reticulum molecular chaperone GRP94 and GRP78 in human lung cancer tissues and its significance. Cancer Detect Prev. 2005;29:544–551. doi: 10.1016/j.cdp.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Xing X, Lai M, Wang Y, Xu E, Huang Q. Overexpression of glucose-regulated protein 78 in colon cancer. Clin Chim Acta. 2006;364:308–315. doi: 10.1016/j.cca.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 20.Miller CR, Perry A. Glioblastoma. Arch Pathol Lab Med. 2007;131:397–406. doi: 10.5858/2007-131-397-G. [DOI] [PubMed] [Google Scholar]