Abstract
Human malignant glioma cell lines and adult brain tumors overexpress high levels of interleukin-13 receptor α2 chain (IL-13Rα2). Because the IL-13Rα2 chain is an important target for cancer therapy and prognosis for patients with brainstem glioma (BSG) remains dismal, we investigated the expression of this receptor in specimens of diffusely infiltrative pediatric BSG relative to normal brain tissue. Twenty-eight BSG specimens and 15 normal brain specimens were investigated for IL-13Rα2 protein expression by immunohistochemical analysis (IHC) using two different antibodies in two different laboratories. Highly sensitive Q-dot–based IHC and in situ hybridization (ISH) assays were also developed to identify IL-13Rα2 protein and RNA in these specimens. The results were evaluated independently in two laboratories in a blinded fashion. By Q-dot IHC or a standard IHC assay, 17 of 28 (61%) tumor specimens showed modest to strong staining for IL-13Rα2, while 15 normal brain tissue samples showed weak expression for IL-13Rα2 protein. Significant interrater agreement between the two laboratories was seen in the assessment of IL-13Rα2 intensity. High-level IL-13Rα2 RNA expression was detected in tumor samples by Q-dot ISH, but only weak RNA expression was observed in normal brain. Significant agreement between ISH and IHC assays was observed (simple kappa [κ] estimate = 0.358, weighted κ = 0.89, p = 0.001). IL-13Rα2 protein and mRNA are expressed to significantly higher levels in BSG than in normal brain tissue. Both IHC and ISH represent robust methods to detect expression of the IL-13Rα2 receptor in BSG that could represent an important new drug target for treatment of this disease.
Keywords: brainstem glioma, IL-13 receptor α2, immunohistochemical analysis (IHC), in situ hybridization (ISH), pediatric tumors
Malignant astrocytoma/glioblastoma multiforme (GBM) is the third leading cause of cancer-related death among children and adolescents in the United States.1 In contrast to adult brain tumors, which occur typically in the cerebral hemispheres, pediatric tumors are localized predominantly in the posterior fossa and brainstem.2 Brainstem glioma (BSG) accounts for ~20% of all childhood brain tumors. Despite numerous attempts to improve treatment of this disease, more than 90% of children with BSG develop diffusely infiltrative tumors and succumb to disease within 2 years of diagnosis.3,4 The intimate relation of BSG to vital control centers renders extensive surgical resection of the tumor impossible, and conventional chemotherapy and radio-therapy are ineffective in the treatment of this tumor. Therefore, new treatments of BSG are urgently needed.
Several proteins expressed by brain tumor cells that may serve as targets of new treatments have been identified. These include cell surface expression of fibroblast growth factor receptor-1b,2 epidermal growth factor receptor,5,6 and tranferrin receptor.7 We have demonstrated abundant expression of receptors for interleukin 4 (IL-4) and IL-13 on adult and pediatric brain tumors and meningiomas.8–12 In contrast, normal human brain expresses barely detectable levels of IL-4R or IL-13R.8,10 The differential expression of certain receptors between normal and malignant brain tissue may identify important biologic processes in cancer development. Further, proteins that are expressed selectively on cancer cells could be used as targets of therapeutic agents that deliver toxic agents specifically to malignant cells.
The expression and structure of IL-4 and IL-13 receptors on glioma and other human tumors have been studied extensively. IL-4 receptor complex exists in two different types. Type I IL-4 receptors are composed of IL-4Rα (also known as IL-4Rβ) and IL-2R gamma common (γc) subunits, whereas type II receptors have IL-4Rα and IL-13Rα1 subunits.13,14 IL-13R receptors also exist in at least two different types. Type I IL-13R is composed of IL-13Rα1 (also known as IL-13Rα′), IL-13Rα2 (also known as IL-13Rα), and IL-4Rα chains, whereas type II IL-13R consists of IL-4Rα and IL-13Rα1 chains.15,16 The role of IL-2 receptor common γc chain in the formation of IL-13R complex is not clear. It has been shown that the introduction of IL-2R γc can decrease IL-13 and IL-4 binding and interferes in the functioning of both receptors in cells that normally do not express this chain.15,17–19 These and other studies have shown that IL-4Rα and IL-13α1 chains are shared between IL-4R and IL-13R complexes. Furthermore, both chains are required for signal transduction through type II IL-4R and both type I and II IL-13R.15 IL-4 and IL-13 mediate signal transduction through JAK/STAT pathways. They phosphorylate and activate different JAK kinases, but both cytokines phosphorylate and activate the same STAT6 protein.20,21 In contrast to IL-4Rα and IL-13Rα1, the IL-13Rα2 chain does not seem to signal through the STAT6 pathway; it inhibits signaling through the STAT6 pathway by both IL-13R and IL-4R.13,22–24 Most recently, we have reported that IL-13 could signal through IL-13Rα2 in a STAT6- independent, AP-1–dependent manner to induce activation of the TGFβ1 promoter resulting in inflammation and fibrosis in animals.25
To target IL-13R, we have developed an IL-13 cytotoxin that consists of IL-13 and a mutated form of Pseudomonas exotoxin (PE). Recombinant IL-13PE cytotoxin is found to be highly cytotoxic to IL-13R–positive renal cell carcinoma (RCC) cells and other IL-13Rα2–positive cancer cells derived from malignant glioma, AIDS-associated Kaposi’s sarcoma (AIDS-KS), squamous cell carcinoma of the head and neck (SCCHN), ovarian carcinoma, and prostate carcinoma.26–31 In various animal models of human cancer (e.g., glioblastoma, AIDS-KS, SCCHN, and ovarian carcinoma), IL-13PE has shown remarkable antitumor effects in vivo.28,31–35 On the basis of these and other preclinical studies, several phase I/II clinical trials were initiated at various medical centers to determine safety and tolerability of IL-13PE in patients with malignant brain tumors.36–47 All these studies have demonstrated that intratumoral and peritumoral infusion of IL13-PE is well tolerated and seems to show clinical benefit to patients.48 Based on these results, a phase III clinical study was initiated in which two to three catheters were placed peritumorally and IL-13PE at a concentration of 0.5 μg/ml was infused.38 This multicenter trial was completed for patient accrual in December 2005. The results for safety and overall survival when compared with standard Gliadel treatment are being carefully evaluated. The preliminary findings suggest that there was no difference in survival in patients treated with these two therapies. The data analysis is underway to examine the group of patients with clinical benefit and correlation with IL-13Rα2 expression. In addition, limitations for drug delivery to infiltrating tumors are being evaluated.
Since IL-13R targeting by IL-13PE may provide clinical benefit to patients with IL-13R–positive tumors, here we have examined whether BSGs express IL-13R. We have demonstrated previously that primary explants of human malignant gliomas and pediatric brain tumors overexpress IL-13R in more than 72% of specimens.49,50 The information on IL-13R expression in BSGs will be useful to further determine whether these are potential targets for IL-13PE. Therefore, both IL-13 receptor α2 mRNA and protein expression were examined in 28 cases of pediatric BSG and in 15 normal brain specimens. Our data show that IL-13R is expressed selectively to high levels in BSG and can be detected reliably using either immunohistochemical analysis (IHC) or in situ hybridization (ISH).
Materials and Methods
Tissue Specimens
Tumor and normal brain samples were obtained after securing approval from the U.S. Food and Drug Administration (FDA) Research Involving Human Subjects Committee (RIHSC) and the St. Jude Children’s Research Hospital (SJCRH) Institutional Review Board. Formalin- fixed tumor sections were collected from 28 children (≤ 17 years old) with a radiological or histological (biopsy performed at time of diagnosis or postmortem) diagnosis of intrinsic BSG recruited under the Pediatric Brain Tumor Consortium (PBTC) study PBTC-N06. The histologic grading and pathology review of these samples has been described previously. Sixteen patients were female, and 12 were male. The mean age at diagnosis was 6.9 years (range, 2–14 years).6
Fifteen normal brain specimens including nine pediatric and six adult brain samples were obtained from the National Cancer Institute–supported Co-operative Human Tissue Network (CHTN) and commercially available human tissue arrays (Cybrdi, Frederick, MD, USA; Biomax, Gaithersburg, MD, USA).
IHC Analysis
IL-13Rα2 levels in BSG and normal tissue sections were determined using two different antibodies at two different institutions. Polyclonal chicken antibody against IL-13Rα2 protein (Genway, San Diego, CA, USA) was used for IHC assay at SJCRH, and goat polyclonal antibody against IL-13Rα2 (R&D, Minneapolis, MN, USA) was used at the Tumor Vaccines and Biotechnology Branch (TVBB), Center for Biologics Evaluation and Research (CBER), FDA (Bethesda, MD, USA). At both institutions, 5-μ paraffin sections were deparaffinized, treated with 100%, 75%, and 50% 200-proof ethyl alcohol prepared in RNase-free water, and microwaved for 15 min in antigen retrieval reagent to unmask the antigen. Auto-fluorescence in tissue sections was minimized by sodium borohydride treatment, and the sections were blocked for 2 h in block buffer consisting of 5% rabbit serum and 1% biotin-free bovine serum albumin in 1× phosphate-buffered saline (PBS). At TVBB, tissue sections were then incubated in primary antibody at a concentration of 0.5 μg/ml for 16 h at 4° C, washed, and incubated with biotinylated rabbit antigoat antibody. These sections were then reacted with Q-dot 655 streptavidin (0.5 μg/ml) for 45 min, washed, and incubated further with biotinylated antistreptavidin antibody (1 μg/ml) for 45 min to amplify the fluorescent signals of the immunostaining. In the final step of the assay, the samples were incubated with Q-dot 655 streptavidin (0.5 μg/ml) for 45 min at room temperature. After washing with PBS three times, the sections were mounted with 90% glycerol and viewed in a Nikon fluorescence microscope using Q-dot 655 filters (Chroma, Rockingham, VT, USA). For control, the samples were immunostained with isotype control goat immunoglobulin G (IgG) and processed through all steps of the protocol. At SJCRH 3,3′-diaminobenzidine/peroxidase assay was used to localize antibody binding in IHC as described previously.51
The tumor sections were evaluated and graded for IL-13Rα2 immunostaining twice at different time points independently at SJCRH and at TVBB (B.H.J. and F.V.) in a blinded fashion. Normal brain sections were evaluated at TVBB. The percentage of IL-13Rα2–positive fields in tissue sections was counted by viewing the tumor or tissue section under the same magnification (×200). Intensity of immunostaining was also recorded on a semiquantitative scale (<1+, 1+, 2+, and 3+). After staining and positive field assessment, codes were deidentified, and results were compared between the two institutions.
ISH Analysis
ISH assay was performed in BSG and normal brain specimens for the expression of IL-13Rα2 RNA using Q-dot 525–labeled probe hybridization technique (Joshi et al., unpublished results). For ISH, we used the same tumor specimens previously used for IHC assay after stripping off the antigen–primary antibody–secondary antibody complex by incubating with Restore Western Stripping buffer (Pierce, Rockford, IL, USA) for 2 h at room temperature. The specimens were washed with 1× PBS and incubated in 25 mM glycine buffer (pH 2.5) for 30 min to inactivate residual Q-dot–based fluorescence on the specimens. The samples were washed with 1× PBS and permeabilized by incubating with 5 μg/ml proteinase K (Sigma-Aldrich, St. Louis, MO, USA) for 15 min at room temperature. The DNA in the sections was destroyed by incubating with 5 U/ml of DNase for 6 h at room temperature. The sections were washed three times and hybridized with an in vitro–transcribed biotinylated antisense riboprobe for IL-13Rα2; RNA was dissolved in 2× hybridization buffer (4× saline sodium citrate [SSC], 0.2 M sodium phosphate [pH 6.50], 2× Denhardt’s solution, and 0.1mg/ml sodium azide) and 20% dextran sulfate in deionized formamide. An in vitro transcribed biotinylated sense riboprobe for IL-13Rα2 was used as a negative control. The slides were heated to 65° C for 5 min, hybridized for 16 h at 42° C, and subsequently washed with 0.2× SSC three times and 1× PBS two times. The sections were then incubated with 0.5 μg/ml streptividin–Q-dot 525 for 45 min, washed three times with PBS, and reacted with biotinylated anti-streptavidin antibody and with 0.5 μg/ml streptividin–Q-dot 525 for 45 min for amplification of the hybridized signals. The slides were washed three times with PBS, dried, mounted with 90% glycerol, and viewed under a Nikon fluorescence microscope using Q-dot 525 filters. The fluorescence microscopic images were digitized and analyzed using IP lab software (Scanalytics, Alexandria, VA, USA). The tissue sections were evaluated and graded for IL-13Rα2 hybridized fluorescence intensity twice at different time points by two authors (B.H.J. and F.V.) in a blinded fashion.
Statistical Analysis
The results of the IHC and ISH analyses were analyzed at the Operations and Biostatistics Center of PBTC and also at the Biostatistics Branch, Office of Biostatistics and Epidemiology, CBER, FDA. To investigate agreement between the SJCRH and the FDA labs and between ISH and IHC assays, simple kappa (κ) and weighted κ statistics were estimated, and 95% confidence intervals and p values are provided. Fisher’s exact test was used to investigate the association between two dichotomous factors. The Cochran-Armitage trend test was used to detect trend differences between the levels of a dichotomous variable and an ordinal variable, the Cochran-Mantel-Haenszel test for correlations between two ordinal categorical variables, and Spearman’s rank correlation to investigate rank correlations between two continuous variables.
Results
Expression of IL-13Ra2 Chain by IHC Analysis
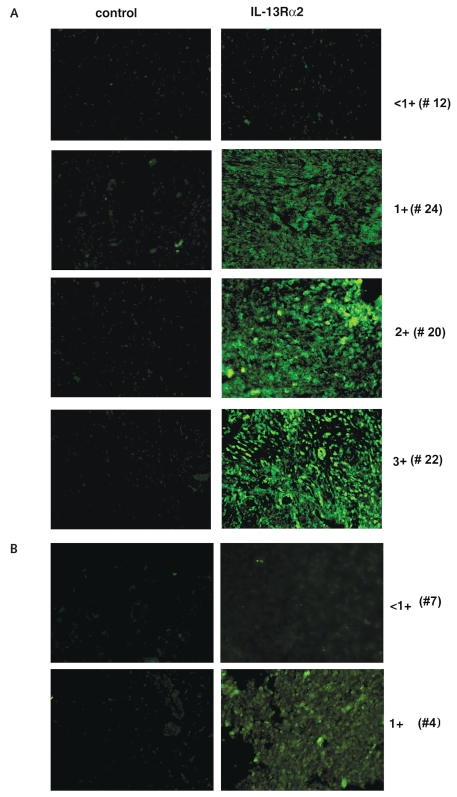
At FDA/CBER, 28 BSG tumor specimens were analyzed for IL-13Rα2 expression by highly sensitive Q-dot–based IHC analysis (Table 1). This IHC technique is highly sensitive for the detection of low fluorescence signals because of the amplification of signal through biotin-streptavidin complex on the tissue section. Overall, 17 of 28 (61%) BSG specimens expressed IL-13Rα2 with varying degrees of immunostaining that ranged from weak to strong staining (1+, 2+, and 3+). Representative BSG-positive specimens are shown in Fig. 1A. Five samples showed 1+, 3 samples 2+, 9 samples 3+, and 11 samples <1+ staining intensity. IHC staining was observed at both the cell surface and intracellularly (Fig. 1A; results not shown). Tissue sections stained with isotype IgG showed no or weak staining. In contrast to tumors, eight pediatric and six adult normal brain tissue samples demonstrated weak staining for IL-13Rα2 chain (<1+). However, sample of brain tissue taken from a pediatric patient with epilepsy showed 1+ fluorescent intensity (Table 2, Fig. 1B).
Table 1.
IL-13Rα2 expression in brainstem glioma
| IL-13Rα2 Expressiona |
% Fields with Positive Fluorescenceb |
||||||
|---|---|---|---|---|---|---|---|
| Tumor Number | Sex | Age | Tumor Grade | Protein (IHC) | RNA (ISH) | Protein (IHC) | RNA (ISH) |
| 1 | F | 3 | 4 | <1+ | <1+ | 0 | 0 |
| 2 | M | 7 | 3 | 3+ | 2+ | 89 | 74 |
| 3 | M | 7 | 2 | <1+ | <1+ | 0 | 0 |
| 4 | M | 7 | 4 | 1+ | 2+ | 25 | 32 |
| 5 | M | 14 | 4 | <1+ | <1+ | 0 | 0 |
| 6 | M | 7 | 4 | <1+ | 1+ | 33 | 0 |
| 7 | F | 3 | 4 | <1+ | <1+ | 0 | 0 |
| 8 | F | 2 | 2 | <1+ | <1+ | 11 | 8 |
| 9 | F | 7 | 3 | 2+ | 3+ | 82 | 78 |
| 10 | M | 6 | 4 | 3+ | 4+ | 88 | 93 |
| 11 | F | 8 | 3 | 1+ | 2+ | 40 | 34 |
| 12 | M | 7 | 3 | <1+ | <1+ | 8 | 6 |
| 13 | F | 16 | 2 | 3+ | 2++ | 96 | 92 |
| 14 | F | 4 | 3 | <1+ | <1+ | 5 | 3 |
| 15 | F | 6 | 3 | <1+ | <1+ | 3 | 2 |
| 16 | M | 10 | 4 | 3+ | 3+ | 90 | 86 |
| 17 | F | 8 | 2 | <1+ | 1+ | 8 | 5 |
| 18 | F | 7 | 3 | <1+ | <1+ | 22 | 13 |
| 19 | F | 4 | 2 | 3+ | 4+ | 73 | 96 |
| 20 | M | 17 | 2 | 2+ | 4+ | 88 | 79 |
| 21 | M | 4 | 2 | 3+ | 3+ | 84 | 76 |
| 22 | F | 3 | NA | 3+ | 4+ | 95 | 90 |
| 23 | F | 5 | 1 | 3+ | 4+ | 70 | 95 |
| 24 | F | 7 | 1 | 1+ | 1+ | 73 | 68 |
| 25 | NA | NA | NA | 3+ | 2+ | 81 | 78 |
| 26 | NA | NA | NA | 1+ | 1+ | 55 | 36 |
| 27 | NA | NA | NA | 1+ | 1+ | 54 | 28 |
| 28 | NA | NA | NA | 2+ | 3+ | 80 | 69 |
Abbreviations: IHC, immunohistochemical analysis; ISH, in situ hybridization; F, female; M, male; NA, not available.
Positivity of the sample was ascertained by fluorescence intensity of brainstem glioma specimen immunostained with anti-IL-13Rα2 antibody and Q-dot as described in “Materials and Methods”: <1+, weakly positive; 1+, modestly positive; 2+, moderately positive; 3+, strongly positive.
A field was defined as a field viewed under ×200 magnification. The percentage of positive fields were counted in a blinded manner by viewing the entire tumor section under the same magnification.
Fig. 1.
Immunohistochemical analysis for IL-13Rα2 chain in high-grade diffusely infiltrative pediatric brainstem glioma (BSG) specimens. (A) A representative staining of <1+ (weakly positive), 1+ (modestly positive), 2+ (moderately positive), and 3+ (strongly positive) BSG specimens. BSG tissue sections were immunostained with IL-13Rα2 antibody, and immunofluorescence was developed with Q-dot 655 as described in “Materials and Methods.” For control, tissue sections from the same specimen were treated with the same concentration of respective immunoglobulin G protein. The specimen number is shown in parentheses. (B) Normal brain specimens were treated similarly with the same concentration of anti-IL-13Rα2 antibody, and immunofluorescence was observed in a Nikon fluorescence microscope.
Table 2.
IL-13Rα2 expression in normal brain specimens
| IL-13Rα2 Expressiona |
% Fields with Positive Fluorescenceb |
||||||
|---|---|---|---|---|---|---|---|
| Specimen Number | Sex | Age | Diagnosis | Protein (IHC) | mRNA (ISH) | Protein (IHC) | mRNA (ISH) |
| 1 | F | 2 | Normal autopsy | <1+ | <1+ | 0 | 0 |
| 2 | M | 7 | Epilepsy | <1+ | <1+ | 4 | 3 |
| 3 | M | 7 | Normal autopsy | <1+ | <1+ | 0 | 0 |
| 4 | M | 12 | Epilepsy | 1+ | 1+ | 8 | 6 |
| 5 | M | 14 | Normal autopsy | <1+ | <1+ | 0 | 0 |
| 6 | M | 22 | Normal autopsy | <1+ | <1+ | 5 | 3 |
| 7 | F | 8 | Normal autopsy | <1+ | <1+ | 0 | 0 |
| 8 | M | 69 | Normal autopsy | <1+ | <1+ | 4 | 7 |
| 9 | F | 2 | Normal autopsy | <1+ | <1+ | 4 | 5 |
| 10 | M | 54 | Epilepsy | <1+ | <1+ | 0 | 0 |
| 11 | F | 8 | Normal autopsy | <1+ | <1+ | 3 | 4 |
| 12 | M | 28 | Normal autopsy | <1+ | <1+ | 5 | 5 |
| 13 | F | 36 | Epilepsy | <1+ | <1+ | 4 | 2 |
| 14 | F | 20 | Normal autopsy | <1+ | <1+ | 3 | 4 |
| 15 | M | 16 | Normal autopsy | <1+ | <1+ | 4 | 4 |
Abbreviations: IHC, immunohistochemical analysis; ISH, in situ hybridization; F, female; M, male.
Positivity of the sample was ascertained by fluorescence intensity of normal brain specimen immunostained with anti-IL-13Rα2 antibody and Q-dot as described in “Materials and Methods”: <1+, weakly positive; 1+, modestly positive.
A field was defined as a field viewed under ×200 magnification. The percentage of positive fields were counted in a blinded manner by viewing the entire tumor section under the same magnification.
IL-13Rα2 expression by IHC performed at SJCRH also identified IL-13Rα2 expression in 17 of 28 tumors. The mean percentage of IL-13Rα2 tumor cell expression in the 17 immunopositive BSG sections was 80% (range, 60%–100%). Significant agreement was seen in the IHC score recorded by the two different methods and the two different research sites (simple κ = 0.284; weighted κ = 0.424; exact p = 0.025). Interestingly, the percentage of IL-13R–positive tumor samples recorded in the two different sites for each tumor also significantly rank-correlated with each other (Spearman’s rank correlation coefficient, rS = 0.55; p = 0.0023). Normal brain sections showed few positive cells (Table 2).
ISH Analysis
ISH analysis of the same 28 BSG specimens and 15 normal brain specimens employed for IHC was performed after stripping sections of primary and secondary antibodies. Nineteen (68%) showed >1+ staining, while nine BSG samples showed <1+ staining. Among >1+ intensity samples, five showed 1+, five showed 2+, four showed 3+, and five showed 4+ staining intensity. Fig. 2A shows examples of ISH staining of four randomly selected BSG specimens displaying <1+ to 3+ ISH intensity. In contrast, no staining was seen in sections in which an in vitro–transcribed biotinylated sense control riboprobe to IL-13Rα2 was used. Consistent with IHC, only one normal brain sample showed 1+ ISH staining intensity, while 14 of 15 tested normal brain specimens showed <1+ staining intensity (Table 2, Fig. 2B).
Fig. 2.
In situ hybridization analysis for IL-13Rα2 mRNA in high-grade diffusely infiltrative pediatric brainstem glioma (BSG) specimens. (A) BSG tissue specimens were hybridized with an in vitro–transcribed biotinylated antisense riboprobe for IL-13Rα2, and immunofluorescence was developed with Q-dot 525 conjugate as described in “Materials and Methods.” For control, the same specimen was treated with the same concentration of a sense biotinylated riboprobe. Staining intensity is scaled as follows: <1+, weakly positive; 1+, modestly positive; 2+, moderately positive; 3+, strongly positive. The specimen numbers are shown in parentheses. (B) Normal brain specimens were treated similarly with the same concentration of control or biotinylated IL-13Rα2 probe, and immunofluorescence was observed in a Nikon fluorescence microscope.
We also analyzed the results to determine whether any relationship existed between IL-13Rα2 expression and clinical variables. As shown in Table 1, the immunofluorescence intensity of immunostaining for IL-13Rα2 expression had no correlation with the clinical variables (e.g., age, sex, or different grades of tumor), even though a majority of specimens overexpressed this receptor chain at protein and mRNA levels. Although this conclusion was made at both SJCRH and FDA/CBER laboratories, it is important to note that the sample size is rather small. It is difficult to conclude whether IL-13Rα2 expression correlates with tumor types. Additional samples need to be analyzed for a definite conclusion.
As shown in Table 3, 61%–68% of BSG specimens had at least >1+ IL-13Rα2 intensity measured by both IHC and ISH analyses. Agreement between ISH and IHC assays for IL-13Rα2 intensities was significantly correlated (p < 0.0001); simple κ estimate = 0.358, and weighted κ = 0.89 (p = 0.001). Further, analysis of these data suggested that percent positive fields for IL-13Rα2 intensities obtained from IHC and ISH techniques was also highly rank-correlated (rS = 0.88, p < 0.0001). A similar relationship between ISH and IHC was observed in normal brain specimens, suggesting that ISH and IHC intensities of IL-13Rα2 expression correlated significantly (p < 0.001).
Table 3.
Correlation of IL-13Rα2 protein and RNA expression in BSG specimens
| No. of Samples with IL-13Rα2–Positive Staininga |
||||
|---|---|---|---|---|
| Diagnosis | No. of Samples | <1+ | >1+ | % Positive Specimens |
| BSG specimens | ||||
| IHC | 28 | 11 | 17 | 61.0* |
| ISH | 28 | 9 | 19 | 68.0** |
| Normal specimens | ||||
| IHC | 15 | 14 | 1 | 7.0 |
| ISH | 15 | 14 | 1 | 7.0 |
Abbreviations: BSG, brainstem glioma; IHC, immunohistochemical analysis; ISH, in situ hybridization.
Positivity of the sample was ascertained by fluorescence intensity of BSG specimen immunostained with anti-IL-13Rα2 antibody or hybridized with biotinylated probe for IL-13Rα2 and Q-dot as described in “Materials and Methods”: <1+, weakly positive; 1+, modestly positive; 2+, moderately positive; 3+, strongly positive.
p < 0.0004;
p < 0.0001; Fisher’s exact test, both compared to normal IHC or ISH.
Discussion
We demonstrate that diffusely infiltrative pediatric BSG expresses readily detectable levels of IL-13Rα2 RNA and protein. In contrast, this receptor was not detected in the majority of normal pediatric and adult brain samples. Importantly, RNA and protein IL-13Rα2 expression levels were significantly correlated in our studies, and the results of two different IHC studies conducted in two separate research facilities were also in close agreement. Our results also suggest heterogeneity in IL-13Rα2 expression, particularly in high-grade BSG tumors. More than 50% of grade 4 tumors had low levels of IL-13α2 expression. Since the sample size is small, additional samples are needed to confirm this conclusion. Together, these data indicate that IL-13Rα2 is expressed to relatively high levels in pediatric BSG and can be readily and reliably detected using either IHC or ISH.
These data are important because IL-13Rα2 is being developed as a potential drug target for pediatric glioma, and reliable detection of this protein will be crucial for the conduct of appropriate clinical trials. We have previously demonstrated that the IL-13Rα2 chain is over-expressed in adult GBM primary culture cells and cell lines.49,50,52 We have also reported that IL-13Rα2 is over-expressed in 83% of pediatric brain tumor specimens of non-BSG.50 These pediatric brain tumors express type I IL-13R, which is composed of the IL-13Rα1, IL-13Rα2, and IL-4Rα chains. Thus, the current study extends these previous observations to show that IL-13Rα2 is also expressed in BSG.
It is important to note that we were able to successfully perform ISH assays on the same tissue sections employed for IHC analysis following stripping of antigen- antibody immune complexes. This technology may be used to study protein and RNA expression and perhaps multiple gene products in BSG when often small biopsy specimens are available.
The significance of IL-13Rα2 expression in BSG or any other tumor tissue is not clear. IL-13Rα2 has been shown to be a major IL-13 binding component of the IL-13R complex.16 After binding to IL-13, the IL-13Rα2 chain internalizes inside the cell for biological activity.53 Whether IL-13Rα2 signals in BSG cells is not known. We have also considered the role of IL-13Rα2 in tumorigenicity and metastasis. In that regard, the IL-13Rα2 gene may undergo mutations or rearrangements causing transformation of normal cells. Although our initial studies have found no mutations in the IL-13Rα2 gene in cell cultures derived from adult glioma samples, it is still possible that this gene undergoes unknown changes.54 Mutation analysis of IL-13Rα2 in BSG has not been performed.
Previous studies have demonstrated that the IL-13Rα2 chain can sensitize human glioma cells to a chimeric fusion protein comprising IL-13 and a mutated form of Pseudomonas exotoxin (termed IL-13–PE38QQR). This cytotoxin is highly cytotoxic to IL-13R–positive malignancies, including brain tumors in vitro and in vivo.29,32,55–57 Based on these observations, it is predicted that IL-13 cytotoxin will also be active in pediatric patients with BSG. However, because of heterogeneity in receptor expression, it is noted that IL-13Rα2 expression could be a critical factor in BSG patients for evaluating response to IL-13 immunotoxin-based therapy. Also, as not all pediatric BSG cells are positive for the IL-13Rα2 chain, it is possible that IL-13 cytotoxin will not eliminate all malignant cells. Therefore, it is noteworthy and we have reported that plasmid-mediated gene transfer of IL-13Rα2 sensitizes tumors to IL-13 cytotoxin in vitro and in vivo.34,58 IL-13 cytotoxin eliminated the majority of malignant cells in in vivo animal models after IL-13Rα2 plasmid injection. Tumor regression was attributed not only to cytotoxic effect of IL-13 cytotoxin but also to infiltration by innate immune cells. Therefore, it is possible that in the clinical setting, IL-13 cytotoxin will be effective in eliminating most tumor cells, even if they do not uniformly express the IL-13Rα2 chain.58,59 Thus, direct intratumoral administration of IL-13 cytotoxin may provide a useful strategy for patients in whom surgical resection cannot be achieved. Because IL-13 cytotoxin has been shown to be well tolerated when administered directly to adult glioma or to brain tissue adjoining the tumor resection cavity37,48,49,56,60 and in the brainstem in monkeys,61 this agent may have utility in the treatment of pediatric patients with BSG. Therefore, additional preclinical and clinical studies need to be performed to determine the utility of IL-13R targeting for BSG therapy.
Acknowledgment
This work was supported in part by NIH grant U01 CA81457 for the Pediatric Brain Tumor Consortium (PBTC) and the American Lebanese Syrian Associated Charities. We thank Drs. Brent McCright and Robert Aksamit, both from CBER, for their help in reading the manuscript and providing critical comments. We also thank Dr. James Boyett of PBTC, SJCRH for his help in statistical analysis. The views presented in this article do not necessarily reflect those of the FDA. These studies were conducted as part of collaboration between the FDA and NeoPharm, Inc., under a Cooperative Research and Development Agreement (CRADA).
References
- 1.Levin VA, Leibel SA, Gutin PH. Neoplasms of the central nervous system. In: DeVita VT Jr, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott; 1989. pp. 1557–1611. [Google Scholar]
- 2.Cohen KJ, Broniscer A, Glod J. Pediatric glial tumors. Curr Treat Options Oncol. 200;2:529–536. doi: 10.1007/s11864-001-0074-9. [DOI] [PubMed] [Google Scholar]
- 3.Jennings MT, Sposto R, Boyett JM, et al. Preradiation chemotherapy in primary high-risk brainstem tumors: phase II study CCG-9941 of the Children’s Cancer Group. J Clin Oncol. 2002;20:3431–3437. doi: 10.1200/JCO.2002.04.109. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan AM, Albright AL, Zimmerman RA, et al. Brainstem gliomas in children: a Children’s Cancer Group review of 119 cases. Pediatr Neurosurg. 1996;24:185–192. doi: 10.1159/000121036. [DOI] [PubMed] [Google Scholar]
- 5.Smith MA, Freidlin B, Ries LA, Simon R. Trends in reported incidence of primary malignant brain tumors in children in the United States. J Natl Cancer Inst. 1998;90:1269–1277. doi: 10.1093/jnci/90.17.1269. [DOI] [PubMed] [Google Scholar]
- 6.Gilbertson RJ, Hill DA, Hernan R, et al. ERBB1 is amplified and overexpressed in high-grade diffusely infiltrative pediatric brain stem glioma. Clin Cancer Res. 2003;9:3620–3624. [PubMed] [Google Scholar]
- 7.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3:1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 8.Puri RK, Hoon DS, Leland P, et al. Preclinical development of a recombinant toxin containing circularly permuted interleukin 4 and truncated Pseudomonas exotoxin for therapy of malignant astrocytoma. Cancer Res. 1996;56:5631–5637. [PubMed] [Google Scholar]
- 9.Puri R, Leland P, Kreitman R, Pastan I. Human neurological cancer cells express interleukin-4 (IL-4) receptors which are targets for the toxic effects of IL4–Pseudomonas exotoxin chimeric protein. Int J Cancer. 1994;58:574–581. doi: 10.1002/ijc.2910580421. [DOI] [PubMed] [Google Scholar]
- 10.Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin Cancer Res. 1995;1:1253–1258. [PubMed] [Google Scholar]
- 11.Debinski W, Miner R, Leland P, Obiri NI, Puri RK. Receptor for interleukin (IL) 13 does not interact with IL4 but receptor for IL4 interacts with IL13 on human glioma cells. J Biol Chem. 1996;271:22428–22433. doi: 10.1074/jbc.271.37.22428. [DOI] [PubMed] [Google Scholar]
- 12.Batra JK, Fitzgerald DJ, Chaudhary VK, Pastan I. Single-chain immunotoxins directed at the human transferrin receptor containing Pseudomonas exotoxin A or diphtheria toxin: anti-TFR(Fv)-PE40 and DT388–anti-TFR(Fv) Mol Cell Biol. 1991;11:2200–2205. doi: 10.1128/mcb.11.4.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murata T, Obiri NI, Debinski W, Puri RK. Structure of IL-13 receptor: analysis of subunit composition in cancer and immune cells. Biochem Biophys Res Commun. 1997;238:90–94. doi: 10.1006/bbrc.1997.7248. [DOI] [PubMed] [Google Scholar]
- 14.Joshi BH, Hogaboam C, Dover P, Husain SR, Puri RK. Role of interleukin-13 in cancer, pulmonary fibrosis, and other T(H)2-type diseases. Vitam Horm. 2006;74:479–504. doi: 10.1016/S0083-6729(06)74019-5. [DOI] [PubMed] [Google Scholar]
- 15.Murata T, Obiri NI, Puri RK. Structure of and signal transduction through interleukin-4 and interleukin-13 receptors (review) Int J Mol Med. 1998;1:551–557. doi: 10.3892/ijmm.1.3.551. [DOI] [PubMed] [Google Scholar]
- 16.Obiri NI, Leland P, Murata T, Debinski W, Puri RK. The IL-13 receptor structure differs on various cell types and may share more than one component with IL-4 receptor. J Immunol. 1997;158:756–764. [PubMed] [Google Scholar]
- 17.Obiri NI, Murata T, Debinski W, Puri RK. Modulation of interleukin (IL)-13 binding and signaling by the gamma c chain of the IL-2 receptor. J Biol Chem. 1997;272:20251–20258. doi: 10.1074/jbc.272.32.20251. [DOI] [PubMed] [Google Scholar]
- 18.Obiri NI, Debinski W, Leonard WJ, Puri RK. Receptor for interleukin 13. Interaction with interleukin 4 by a mechanism that does not involve the common gamma chain shared by receptors for interleukins 2, 4, 7, 9, and 15. J Biol Chem. 1995;270:8797–8804. doi: 10.1074/jbc.270.15.8797. [DOI] [PubMed] [Google Scholar]
- 19.Kuznetsov VA, Puri RK. Kinetic analysis of high affinity forms of interleukin (IL)-13 receptors: suppression of IL-13 binding by IL-2 receptor gamma chain. Biophys J. 1999;77:154–172. doi: 10.1016/S0006-3495(99)76879-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murata T, Husain SR, Mohri H, Puri RK. Two different IL-13 receptor chains are expressed in normal human skin fibroblasts, and IL-4 and IL-13 mediate signal transduction through a common pathway. Int Immunol. 1998;10:1103–1110. doi: 10.1093/intimm/10.8.1103. [DOI] [PubMed] [Google Scholar]
- 21.Murata T, Noguchi PD, Puri RK. IL-13 induces phosphorylation and activation of JAK2 Janus kinase in human colon carcinoma cell lines: similarities between IL-4 and IL-13 signaling. J Immunol. 1996;156:2972–2978. [PubMed] [Google Scholar]
- 22.Wood N, Whitters MJ, Jacobson BA, et al. E nhanced interleukin (IL)-13 responses in mice lacking IL-13 receptor alpha 2. J Exp Med. 2003;197:703–709. doi: 10.1084/jem.20020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahaman SO, Sharma P, Harbor PC, Aman MJ, Vogelbaum MA, Haque SJ. IL-13R(alpha)2, a decoy receptor for IL-13 acts as an inhibitor of IL-4–dependent signal transduction in glioblastoma cells. Cancer Res. 2002;62:1103–1109. [PubMed] [Google Scholar]
- 24.Rahaman SO, Vogelbaum MA, Haque SJ. Aberrant Stat3 signaling by interleukin-4 in malignant glioma cells: involvement of IL-13Ralpha2. Cancer Res. 2005;65:2956–2963. doi: 10.1158/0008-5472.CAN-04-3592. [DOI] [PubMed] [Google Scholar]
- 25.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha(2) receptor is involved in induction of TGF-beta(1) production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 26.Debinski W, Obiri NI, Pastan I, Puri RK. A novel chimeric protein composed of interleukin 13 and Pseudomonas exotoxin is highly cytotoxic to human carcinoma cells expressing receptors for interleukin 13 and interleukin 4. J Biol Chem. 1995;270:16775–16780. doi: 10.1074/jbc.270.28.16775. [DOI] [PubMed] [Google Scholar]
- 27.Maini A, Hillman G, Haas GP, et al. Interleukin-13 receptors on human prostate carcinoma cell lines represent a novel target for a chimeric protein composed of IL-13 and a mutated form of Pseudomonas exotoxin. J Urol. 1997;158:948–953. doi: 10.1097/00005392-199709000-00077. [DOI] [PubMed] [Google Scholar]
- 28.Husain SR, Puri RK. Interleukin-13 fusion cytotoxin as a potent targeted agent for AIDS-Kaposi’s sarcoma xenograft. Blood. 2000;95:3506–3513. [PubMed] [Google Scholar]
- 29.Joshi BH, Husain SR, Puri RK. Preclinical studies with IL-13PE38QQR for therapy of malignant glioma. Drug News Perspect. 2000;13:599–605. doi: 10.1358/dnp.2000.13.10.858450. [DOI] [PubMed] [Google Scholar]
- 30.Kawakami K, Kawakami M, Joshi BH, Puri RK. Interleukin-13 receptor-targeted cancer therapy in an immunodeficient animal model of human head and neck cancer. Cancer Res. 2001;61:6194–6200. [PubMed] [Google Scholar]
- 31.Kioi M, Husain SR, Croteau D, Kunwar S, Puri RK. Convection-enhanced delivery of interleukin-13 receptor-directed cytotoxin for malignant glioma therapy. Technol Cancer Res Treat. 2006;5:239–250. doi: 10.1177/153303460600500307. [DOI] [PubMed] [Google Scholar]
- 32.Husain SR, Joshi BH, Puri RK. Interleukin-13 receptor as a unique target for anti-glioblastoma therapy. Int J Cancer. 2001;92:168–175. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1182>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 33.Husain SR, Gill P, Kreitman RJ, Pastan I, Puri RK. Interleukin-4 receptor expression on AIDS-associated Kaposi’s sarcoma cells and their targeting by a chimeric protein comprised of circularly permuted interleukin-4 and Pseudomonas exotoxin. Mol Med. 1997;3:327–338. [PMC free article] [PubMed] [Google Scholar]
- 34.Kawakami K, Joshi BH, Puri RK. Sensitization of cancer cells to interleukin 13-pseudomonas exotoxin-induced cell death by gene transfer of interleukin 13 receptor alpha chain. Hum Gene Ther. 2000;11:1829–1835. doi: 10.1089/10430340050129459. [DOI] [PubMed] [Google Scholar]
- 35.Kawakami K, Husain SR, Bright RK, Puri RK. Gene transfer of interleukin 13 receptor alpha2 chain dramatically enhances the antitumor effect of IL-13 receptor-targeted cytotoxin in human prostate cancer xenografts. Cancer Gene Ther. 2001;8:861–868. doi: 10.1038/sj.cgt.7700373. [DOI] [PubMed] [Google Scholar]
- 36.Croteau D, Kunwar S, Ram Z, et al. A cytokine tumor targeting agent, IL-13PE38QQR (cintredekin besudotox), administered by intraparenchymal convection-enhanced delivery (CED) for the treatment of recurrent malignant glioma (MG) brain tumors. Presentation at Cytokines and inflammation; San Diego, CA. 2006. [Google Scholar]
- 37.Kunwar S, Pai LH, Pastan I. Cytotoxicity and antitumor effects of growth factor-toxin fusion proteins on human glioblastoma multiforme cells. J Neurosurg. 1993;79:569–576. doi: 10.3171/jns.1993.79.4.0569. [DOI] [PubMed] [Google Scholar]
- 38.Kunwar S, Prados M, Chang SM, et al. Convection-enhanced delivery of IL-13PE38QQR: results of a multicenter phase I study in recurrent malignant glioma. Presentation at the annual meeting of the American Association of Neurological Surgeons; New Orleans, LA. 2005. [Google Scholar]
- 39.Kunwar S, Ram Z, Sampson JH, et al. Peritumoral convection-enhanced delivery (CED) of IL-13PE38QQR (IL13PE): results of multicenter phase 1 studies in recurrent high grade glioma (HGG) Neuro-Oncology. 2005;7:114. [Google Scholar]
- 40.Lang F, Kunwar S, Strauss L, et al. A clinical study of convection-enhanced delivery of IL-13PE38QQR cytotoxin pre- and post-resection of recurrent GBM. Presentation at the meeting of the American Society of Neuro-Oncologists; Chicago. April 2002. [Google Scholar]
- 41.Prados M, Kunwar S, Lang F, et al. Final results of phase I/II studies of IL-13PE38QQR administered intratumorally (IT) and/or peritumorally (PT) via convection-enhanced delivery (CED) in patients undergoing tumor resection for recurrent malignant glioma. J Clin Oncol. 2005;23:115S. [Google Scholar]
- 42.Prados M, Lang F, Strauss L, et al. Intratumoral and intracerebral microinfusion of Il13–PE38QQR cytotoxin: phase I/II study of pre- and post-resection infusions in recurrent resectable malignant glioma. Presentation at the 8th annual meeting of the American Society of Clinical Oncology; Orlando, FL. May 18–21, 2002; abstract 2087. [Google Scholar]
- 43.Prados M, Lang F, Strauss L, et al. Pre and post-resection interstitial infusions of IL13-PE38QQR cytotoxin: phase I study in recurrent respectable malignant glioma. Presentation at the First Quadrennial Meeting of the World Federation of Neuro-Oncology; Washington, DC. November 15–November 17, 2001. [Google Scholar]
- 44.Sampson JH, Friedman AH, Reardon DA, et al. Convection-enhanced delivery of IL-13PE38QQR in malignant glioma: effect of catheter placement on drug distribution. J Neurosurg. 2004;100:A772. [Google Scholar]
- 45.Weingart J, Grossman SA, Bohan E, Fisher JD, Strauss L, Puri RK. Phase I/II study of interstitial infusion of IL13–PE38QQR cytotoxin in recurrent malignant glioma. Presentation at the First Quadrennial Meeting of the World Federation of Neuro-Oncology; Washington, DC. November 15–17, 2001. [Google Scholar]
- 46.Weingart J, Strauss L, Grossman SA, et al. Phase I/II study: intra-tumoral infusion of IL13-PE38QQR cytotoxin for recurrent supratentorial malignant glioma. Neuro-Oncology. 2002;4:379. [Google Scholar]
- 47.Weingart J, Tatter SSR, Mikkelsen T, et al. Intratumoral convection-enhanced delivery of IL-13PE38QQR cytototxin for recurrent malignant glioma without planned resection: a phase I/II study. Presentation at the 8th annual meeting of the Society for Neuro-Oncology; Keystone, CO. 2003. [Google Scholar]
- 48.Kunwar S, Prados MD, Chang SM, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25:837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 49.Joshi BH, Plautz GE, Puri RK. Interleukin-13 receptor alpha chain: a novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res. 2000;60:1168–1172. [PubMed] [Google Scholar]
- 50.Kawakami M, Kawakami K, Takahashi S, Abe M, Puri RK. Analysis of interleukin-13 receptor alpha2 expression in human pediatric brain tumors. Cancer. 2004;101:1036–1042. doi: 10.1002/cncr.20470. [DOI] [PubMed] [Google Scholar]
- 51.Gilbertson RJ, Perry RH, Kelly PJ, Pearson AD, Lunec J. Prognostic significance of HER2 and HER4 coexpression in childhood medulloblastoma. Cancer Res. 1997;57:3272–3280. [PubMed] [Google Scholar]
- 52.Debinski W, Hulet SW, Connor JR, Gillespie GY, Puri R. Overexpression of a receptor for Interleukin 13 in human glioblastoma multiforme detected in situ. Presentation at the 2nd annual meeting of the Society for Neuro-Oncology; Charlottesville, VA. 1997. [Google Scholar]
- 53.Murata T, Taguchi J, Puri RK, Mohri H. Sharing of receptor subunits and signal transduction pathway between the IL-4 and IL-13 receptor system. Int J Hematol. 1999;69:13–20. [PubMed] [Google Scholar]
- 54.Kawakami M, Leland P, Kawakami K, Puri RK. Mutation and functional analysis of IL-13 receptors in human malignant glioma cells. Oncol Res. 2000;12:459–467. doi: 10.3727/096504001108747468. [DOI] [PubMed] [Google Scholar]
- 55.Joshi B, Husain SR, Leland P, Puri R. Interleukin-13 receptors and development of IL-13 Pseudomonas exotoxin for human cancer therapy. In: Kawakami K, Aggarwal BB, Puri R, editors. Cytotoxins and Immunotoxins for Cancer Therapy: Clinical Applications. Boca Raton, FL: CRC Press; 2005. pp. 45–69. [Google Scholar]
- 56.Husain SR, Puri RK. Interleukin-13 receptor-directed cytotoxin for malignant glioma therapy: from bench to bedside. J Neurooncol. 2003;65:37–48. doi: 10.1023/a:1026242432647. [DOI] [PubMed] [Google Scholar]
- 57.Husain SR, Obiri NI, Gill P, et al. Receptor for interleukin 13 on AIDS-associated Kaposi’s sarcoma cells serves as a new target for a potent Pseudomonas exotoxin-based chimeric toxin protein. Clin Cancer Res. 1997;3:151–156. [PubMed] [Google Scholar]
- 59.Kawakami K, Kawakami M, Puri RK. IL-13 receptor-targeted cytotoxin cancer therapy leads to complete eradication of tumors with the aid of phagocytic cells in nude mice model of human cancer. J Immunol. 2002;169:7119–7126. doi: 10.4049/jimmunol.169.12.7119. [DOI] [PubMed] [Google Scholar]
- 60.Kunwar S, Prados M, Lang F, et al. Intratumoral and peritumoral convection-enhanced delivery of IL-13PE38QQR, a recombinant tumor-targeted cytotoxin in a recurrent malignant glioma phase I trial. J Neurosurg. 2003;98:697. [Google Scholar]
- 61.Murad GJ, Walbridge S, Morrison PF, et al. Real-time, image-guided, convection-enhanced delivery of interleukin 13 bound to Pseudomonas exotoxin. Clin Cancer Res. 2006;12:3145–3151. doi: 10.1158/1078-0432.CCR-05-2583. [DOI] [PubMed] [Google Scholar]