Abstract
The effects of combining histone deacetylase (HDAC) inhibitors and proteasome inhibitors were evaluated in both established glioblastoma multiforme (GBM) cell lines and short-term cultures derived from the Mayo Clinic xenograft GBM panel. Coexposure of LBH589 and bortezomib at minimally toxic doses of either drug alone resulted in a striking induction of apoptosis in established U251, U87, and D37 GBM cell lines, as well as in GBM8, GBM10, GBM12, GBM14, and GBM56 short-term cultured cell lines. Synergism of apoptosis induction was also observed in U251 cells when coexposing cells to other HDAC inhibitors, including LAQ824 and trichostatin A, with the proteasome inhibitor MG132, thus demonstrating a class effect. In U251 cells, bortezomib alone or in combination with LBH589 decreased Raf-1 levels and suppressed Akt and Erk activation. LBH589 or bortezomib alone increased expression of the cell cycle regulators p21 and p27. Additionally, the combination, but not the individual agents, markedly enhanced JNK activation. Synergistic induction of apoptosis after exposure to LBH589 and bortezomib was partially mediated by Bax translocation from the cytosol to the mitochondria resulting from Bax conformational changes. Bax translocation precedes cytochrome c release and apoptosis, and selective down-regulation of Bax using siRNA significantly mitigates the cytotoxicity of LBH589 and bortezomib. This combination regimen warrants further preclinical and possible clinical study for glioma patients.
Keywords: apoptosis, Bax, bortezomib (PS-341), glioma, LBH589
Glioblastoma multiforme (GBM) is an aggressive, invasive, and difficult to treat primary brain tumor. Standard therapy includes surgical resection, external beam radiation therapy, and chemotherapy, with no known curative therapy.1 Despite these treatment strategies, median survival has remained only approximately 1 year for decades. Thus, it is urgent to develop more effective therapies for this disease. A number of dysregulated signaling cascades have been described in gliomas, including the Ras/Raf/MEK/Erk mitogen-activated protein kinase pathway, the PI3K/Akt pathway, and the PLC-γ/PKC pathway. Dysregulation of these pathways is driven by mutation, amplification, or overexpression of multiple genes such as PTEN, EGFR, EGF, PDGFR-α, p53, and mTOR.2 These dysregulated pathways provide the basis for designing molecular- targeted therapies for the treatment of GBM.
The ubiquitin-proteasome pathway plays a critical role in the degradation of intracellular proteins involved in cell-cycle control, transcription factor activation, apoptosis, cell trafficking, and tumor growth.3 Recently, the ubiquitin-proteasome pathway has emerged as a new and exciting target in anticancer therapy. Bortezomib (PS341), an FDA-approved drug for the treatment of multiple myeloma, remains the first selective proteasome inhibitor to demonstrate significant preclinical activity in solid tumor models, including carcinomas of the breast, lung, colon, bladder, ovary, pancreas, prostate, and brain.4 Single agent activity with bortezomib in murine and human xenograft models is associated with enhanced apoptosis, specifically against transformed cells.5 The major biological effects of bortezomib include inhibition of NF-κB, CDK activity, angiogenesis, and cellular adhesion, as well as induction of PTEN, p53, p21, p27, ROS, and apoptosis.6,7 Several studies have described preclinical, in vitro activity of proteasome inhibitors in GBM cell lines, which is associated with G2/M cell cycle arrest, increased expression of p21 and p27, and induction of apoptosis.8,9 Initial data from a phase I trial in patients with recurrent malignant glioma demonstrated that bortezomib is well tolerated using a 3-week intravenous dosing schedule, but final efficacy data have not been presented.10
Posttranslational modification of histones by acetylation is a dynamic process that regulates chromatin structure and subsequent gene transcription. Acetylation is regulated by the opposing enzymes histone acetyltransferase and histone deacetylase (HDAC). In addition to histones, other proteins important in cancer biology are also regulated by acetylation, including p53 and hsp90.11 Mammalian HDACs are divided into three major classes: class I (HDACs 1, 2, 3, and 8), class II (HDACs 4, 5, 6, 7, 9a, 9b, and 10), class III (Sir2 family), and class IV (HDAC 11). A number of HDAC inhibitors have been developed that predominantly target the class I and class II HDACs, including the hydroxamates trichostatin A, SAHA, LBH589, and LAQ824. HDAC inhibitors induce cell-cycle arrest, growth inhibition, differentiation, and apoptosis in a wide variety of cancer cell lines.12,13 The precise molecular mechanism for the cytotoxicity of HDAC inhibitors is not well understood because they have multiple target effects. Indeed, treatment with HDAC inhibitors results in changes in approximately 2%–10% of expressed genes.14,15 HDAC inhibitor–induced cytotoxicity has been proposed to involve changes in expression of p21 or p27, VEGF, VEGFR, p53, HIFα, BAK, Bax, Mcl-1, TRAIL, FAS, and FASL, among many other proteins.12,16,17 Several HDAC inhibitors have demonstrated preclinical activity against glioma cell lines including SAHA, AN-9, AN-1, trichostatin A, and MS-275.18–21 SAHA is currently being tested in a phase II study in patients with recurrent GBM, and a preliminary interim analysis suggested promising activity (E. Galanis, Mayo Clinic, personal communication, September 20, 2006).
Multiple signaling cascades are dysregulated in gliomas; therefore, simultaneous targeting of multiple pathways is likely to produce a greater clinical benefit than targeting a single pathway. Both proteasome inhibitors and HDAC inhibitors have multiple target effects on pathways relevant to glioma biology. In combination, they have demonstrated synergistic cytotoxicity in a variety of cancer types including multiple myeloma, pancreas, colon, and myeloid leukemia.22–25 In this study, we tested the combination of proteasome inhibitors and HDAC inhibitors in GBM cell lines and found the combination to synergistically induce apoptosis via translocation of Bax from the cytosol to the mitochondria as well as growth inhibition, which is accompanied by changes in multiple signaling cascades.
Materials and Methods
Cells, Cell Culture, and Materials
U251 and U87 cell lines were purchased from American Type Culture Collection (Manassas, VA, USA). D37 cells were obtained from Dr. Jann Sarkaria (Mayo Clinic, Rochester, MN, USA). Short-term cultures of patient-derived glioblastoma multiforme (GBM), including GBM8 (G-8), GBM10 (G-10), GBM12 (G-12), GBM14 (G-14), and GBM56 (G-56) cells, have been previously described.26 All cells were grown following instructions provided by the suppliers. Subconfluent, logarithmically growing cells were placed in sterile plastic T-flasks, allowed to adhere, supplemented with the designated drugs, and incubated in a humidified incubator with 5% CO2 at 37°C for various time intervals as indicated. LBH589 and LAQ824 were provided by Dr. Peter Atadja (Novartis Pharmaceuticals, Basel, Switzerland). Bortezomib was provided by Millennium Pharmaceuticals (Cambridge, MA, USA). Trichostatin A and MG132 were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
Assessment of Apoptosis
Following drug treatment, apoptotic cells were detected by DAPI (4,6-diamidino-2-phenylindole dihydrochloride) staining, which allowed identification of apoptotic nuclear changes as described previously.27 Briefly, cells were washed with phosphate-buffered saline (PBS) and fixed with 1% glutaraldehyde at room temperature for 30 min. After washing with PBS, cells were resuspended in 20 μl of PBS and mixed with 5 μl of 10 μ/ml DAPI. Cell suspensions were mounted on slides and subjected to fluorescence microscopic examination. Apoptotic cells were identified by classical morphological features (i.e., nuclear condensation, cell shrinkage, and formation of apoptotic bodies). Five or more randomly selected fields, encompassing a total of ⩾500 cells/slide, were quantified for the percentage of apoptotic cells. To confirm the results of morphological analyses, annexin V/propidium iodide (PI; BD Pharmingen, San Diego, CA, USA) analysis was carried out per the manufacturer’s instructions.
Determination of Cell Proliferation MTS Assay
U251 and U87 cells were plated in a 96-well plate (1,500 cells/well) in 200 μl culture medium, incubated overnight, and then treated with LBH589 and/or bortezomib for 48 h. Cell proliferation was assessed by the CellTiter 96 AQueous One Solution (Promega, Madison, WI, USA) following the supplier’s protocol, which exploits the bioreduction of MTS to a colored tetrazolium salt by the cells.
Immunoblot Analysis
Following drug treatment, cell lysates were prepared and subjected to immunoblot analysis with 30 μg of cellular protein using standard sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) techniques. The sources of primary antibodies were caspase 9 (rabbit polyclonal), cleaved caspase 3 (rabbit polyclonal), Bid (rabbit polyclonal), PARP (mouse monoclonal), Bax (rabbit polyclonal), XIAP (rabbit polyclonal), phospho-MEK1/2 (rabbit polyclonal), phospho-Erk1/2 (Thr202/ Tyr204) (rabbit polyclonal), phospho-p38 (Thr180/ Tyr182) (rabbit polyclonal), phospho-JNK (mouse monoclonal), phospho-GSK3β (rabbit polyclonal), and phospho-p70S60K (rabbit polyclonal) from Cell Signaling Technology (Beverly, MA, USA); caspase 8 (mouse monoclonal) from AXXORA, LLC (San Diego, CA, USA); Bid (rabbit polyclonal), Bcl-xL (rabbit polyclonal), phospho-Akt, cyclin D1 (rabbit polyclonal), Raf-1 (mouse monoclonal), B-Raf (mouse monoclonal), JNK (mouse monoclonal), p53 (mouse monoclonal), p27 (mouse monoclonal), and cytochrome c (mouse monoclonal) from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mcl-1 (mouse monoclonal), p21 (mouse monoclonal), p57 (mouse monoclonal), and under-phospho-pRB were from BD Pharmingen. To ensure equivalent loading and transfer, blots were stripped and reprobed with antiactin antiserum. Immunoblots presented are representative of three independent experiments.
Assessment of Cytochrome c Release from Mitochondria
Following drug treatment, the release of cytochrome c from mitochondria was analyzed by a selective digitonin permeabilization method. For these assays, 4 × 106 cells per condition were resuspended in 50 μl of permeabilization buffer (containing 75 mM NaCl, 8 mM Na2PO4, 1 mM NaH2PO4 [pH 7.4], 250 mM sucrose, 1 mM EDTA, and 700 μg/ml digitonin). After incubation at room temperature for 1 min, cells were pelleted by centrifugation for 3 min at 13,000g, and the supernatants containing cytochrome c protein were analyzed using immunoblotting.
Examination of Bax Conformational Change
After drug treatment, cells were pelleted, washed twice in 1× PBS, and resuspended in lysis buffer containing 10 mmol/L HEPES (pH 7.4), 150 mmol/L NaCl, 1% CHAPS, and protease inhibitor cocktail (Roche, West Sussex, UK). Equal amounts of protein (1 mg in 0.5 ml lysis buffer) from whole cell lysates were immunoprecipitated using 3 μg conformation-specific anti-Bax 6A7 monoclonal antibody (Sigma-Aldrich, St. Louis, MO, USA) and protein G-agarose beads (50 μl) (GE Health-care, Piscataway, NJ, USA). The immunoprecipitated complexes were washed three times with lysis buffer and subjected to immunoblot analysis using Bax polyclonal antibody (Cell Signaling).
Preparation of Subcellular Fractions
Subcellular fractions were separated as described previously.28 Briefly, after drug treatment, cells were pelleted, washed twice with cold PBS, and incubated on ice in buffer containing 250 mM sucrose, 20 mM HEPES pH 7.4, 5 mM MgCl2, 10 mM KCl, 1 mM EDTA, and 1 mM EGTA supplemented with protease inhibitors and 0.05% digitonin for 30 min. Cytosolic (supernatant) and membrane (pellet) fractions were separated by centrifugation at 13,000g for 10 min.
Bax RNA Interference
Small interfering RNA (siRNA) specific for Bax was purchased from Ambion (Austin, TX, USA). Cells were transfected with siRNAs at a final concentration of 20 nM using siPORT NeoFX Transfection Agent (Ambion) following the manufacturer’s protocol.
Statistical Analysis
All data were expressed as mean ± SD from three individual experiments. Differences between groups were determined by using the student’s t-test for unpaired observations; p < 0.05 was considered significant. The effect of combining LBH589 and bortezomib was analyzed using Calcusyn software (Biosoft, Oxford, UK) as reported previously.29
Results
Induction of Enhanced Cell Death and Growth Inhibition in U251 and U87 Cells after Exposure to LBH589 and Bortezomib
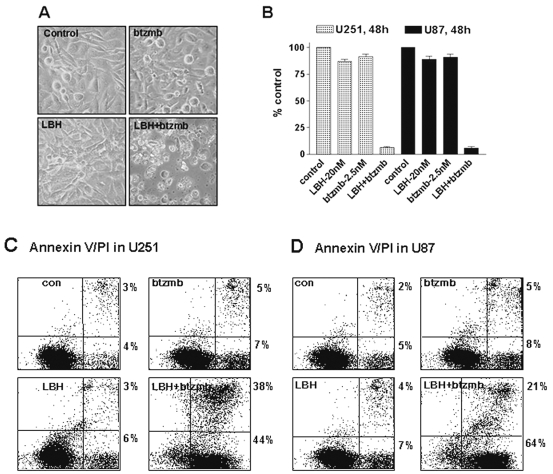
HDAC inhibitors and proteasome inhibitors target relevant pathways in glioma tumorigenesis and have been shown to have synergistic cytotoxicity in a variety of tumor types. However, nothing is known about this combination in glioma cells. Thus, in the present study, we set out to determine whether treatment with a combination of HDAC inhibitors and proteasome inhibitors would result in enhanced cytotoxicity in glioma cell lines. U251 glioma cells were treated with LBH589 or bortezomib alone, or in combination. LBH589 or bortezomib alone was minimally toxic, but the combination yielded a marked increase in apoptotic cell death as seen in Fig. 1A. In addition, significant inhibition of cell proliferation, as detected using an MTS assay, was only observed in U251 and U87 cells after exposure to LBH589 and bortezomib (Fig. 1B). Similar results were obtained using an annexin V/PI assay following the combined treatment of LBH589 and bortezomib in U251 or U87 cells (Fig. 1C, D), confirming that the morphological changes we observed were due to the induction of apoptosis.
Fig. 1.
Induction of enhanced cell death in U251 cells and growth inhibition in U251 and U87 cells after exposure to LBH589 (LBH) and bortezomib (btzmb). Morphology was determined using contrast microscopy (A), and MTS growth assays (B) were performed after U251 or U87 cells were treated at 20 nM LBH589 with or without 2.5 nM btzmb for 48 h. Values represent the means for three replicate determinations ± SD. Annexin V/propidium iodide (PI) staining (C, D) was employed to determine the extent of apoptosis after U251 or U87 cells were treated at 20 nM LBH589 with or without 2.5 nM btzmb for 48 h. Annexin V is plotted on the x-axis, and PI is plotted on the y-axis. Cells in the lower right quadrant (annexin V positive) represent early apoptotic cells; cells in the upper right quadrant (annexin V/PI positive) represent later apoptotic cells. Abbreviation: Con, control cells.
Synergistic Cytotoxicity of the Combination of LBH589 and Bortezomib in U251 Cells
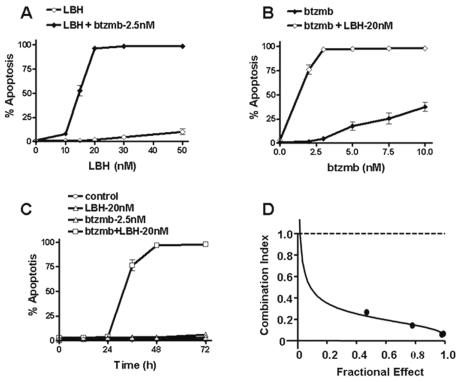
In order to gain further insight into the interaction between LBH589 and bortezomib in U251 cells, dose-response, time-course, and combination index studies were performed. Even though exposure of U251 cells to either 2.5 nM bortezomib or the indicated concentrations of LBH589 alone for 48 h did not induce significant apoptosis (less than 10%), the combination resulted in a marked increase in apoptosis, with the maximum cell death, about 98%, occurring at 20 nM LBH589 (Fig. 2A). Similarly, U251 cells were treated with 20 nM LBH589 either with or without graded concentrations of bortezomib. Again, neither LBH589 nor bortezomib alone induced significant apoptosis, but the combination yielded a marked increase in apoptosis, with the maximum amount of cell death, about 98%, observed at a bortezomib concentration of 2.5 nM (Fig. 2B). These data demonstrate a dose-dependent increase in the amount of apoptosis caused by the combination of LBH589 and bortezomib in U251 cells. Time-course studies were also performed in U251 cells by exposing cells to 20 nM LBH589 with or without 2.5 nM bortezomib for the indicated time intervals, and the results demonstrate that the induction of apoptosis is time dependent. Maximum apoptosis occurred at 48 h (Fig. 2C). To formally examine the synergistic interaction of the combination of LBH589 and bortezomib, U251 cells were treated with varying concentrations of LBH589 and bortezomib at a fixed ratio, and the combination index values for apoptosis induction were determined using the median effect method of Chou and Talalay.30 As shown in Fig. 2D, the combination index values were less than 1, indicating a synergistic interaction.
Fig. 2.
Interaction between bortezomib (btzmb) and LBH589 (LBH) in U251 is dose and time dependent and synergistic based on combination index studies. Apoptosis was determined by DAPI staining after U251 cells were treated at indicated concentrations of LBH with or without 2.5 nM btzmb (A) or at indicated concentrations of btzmb with or without 20 nM LBH (B) for 48 h, or at 20 nM LBH with or without 2.5 nM btzmb at indicated time points (C). U251 cells were treated with different concentrations of LBH with or without btzmb at a fixed ratio (8:1), and the combination index values of apoptosis induction were determined using the median effect method (D). Values represent the means for three replicate determinations ± SD.
Enhanced Cytotoxicity of the Combination of HDAC Inhibitors and Proteasome Inhibitors in Multiple Glioma Cell Lines
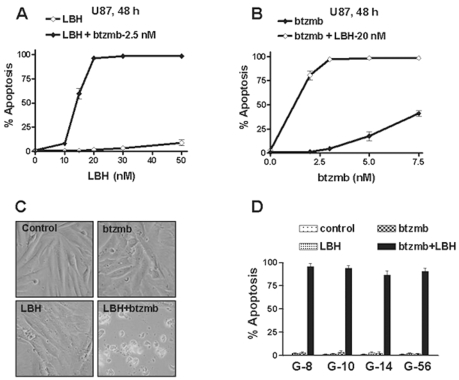
To confirm that the interaction between HDAC inhibitors and proteasome inhibitors seen in U251 cells was not cell-line specific, we further characterized the combination in other glioma cell lines. Dose-response studies performed in U87 glioma cells (Fig. 3A, B) indicated that enhanced cell killing occurred in a dose-dependent manner. We also observed enhanced cytotoxicity in D37 cells using LBH589 and bortezomib (Fig. 4A). In order to test the combination in a more clinically relevant model, we tested LBH589 and bortezomib against a panel of GBM cell lines derived from the Mayo GBM xenograft panel.31 In this model, patient tumor samples are implanted and maintained by serial passage in the flank of nude mice, and short-term explant cultures from four xenografts were utilized for our experiments, including G-56, G-8, G-10, and G-14. For each cell line, the effect of combining LBH589 and bortezomib on apoptosis induction was examined at multiple time points and dose levels. For each tumor line, monotherapy with either agent was ineffective, while combined therapy was highly effective at inducing apoptosis (Fig. 3C, D). These results suggest that LBH589 and bortezomib synergistically induce apoptosis against a broad panel of GBM cell lines.
Fig. 3.
Enhanced cell killing after exposure to histone deacetylase inhibitors with proteasome inhibitors in other tumor cell lines. Apoptosis was determined by DAPI staining after U87 cells were treated at indicated concentrations of LBH589 (LBH) with or without 2.5 nM bortezomib (btzmb; A), or at indicated concentrations of btzmb with or without 20 nM LBH (B). Cell morphology assay was examined using contrast microscopy after G-56 cells were exposed to LBH (50 nM) with or without btzmb (10 nM) for 72 h (C). Apoptosis was examined using DAPI staining after cotreatment with LBH and btzmb in G-8, G-10, G-14, and G-56 cells (D). Conditions are as follows: G-8 cells, 50 nM LBH, 10 nM btzmb, 72 h; G-10, 50 nM LBH, 5 nM btzmb, 72 h; G-14, 30 nM LBH, 5 nM btzmb, 48 h; G-56, 50 nM LBH, 10 nM btzmb, 72 h. Apoptosis was determined by DAPI staining after D37 cells were treated at indicated conditions for 72 h (C), or U251 cells were treated at indicated conditions (D). Values represent the means for three replicate determinations ± SD.
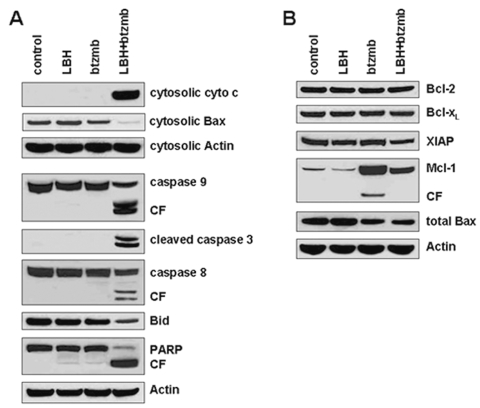
Fig. 4.
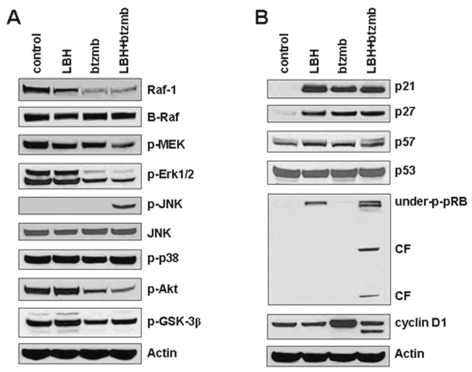
Effects of the LBH589 (LBH)/bortezomib (btzmb) combination on apoptosis pathways. After U251 cells were treated with control medium, 20 nM LBH alone, 2.5 nM btzmb alone, or the combination for 48 h, protein extracts were analyzed by Western blotting using indicated antigens, for cytochrome c (cyto c) and Bax from the cytosolic subcellular fraction, and for caspase 9, cleaved caspase 3, caspase 8, Bid, PARP, Bcl-2, Bcl-xL, XIAP, Mcl-1, and total Bax in whole-cell lysates. Abbreviation: CF, cleaved fragment.
Interaction between HDAC Inhibitors and Proteasome Inhibitors Is a Class Effect
To determine whether this synergistic interaction between LBH589 and bortezomib is a drug-specific interaction or a class interaction between HDAC inhibitors and proteasome inhibitors, we tested additional agents. In D37 cells, substituting the HDAC inhibitor LAQ824 for LBH589 similarly enhances cytotoxicity in combination with bortezomib (Fig. 3E). In U251 cells, the proteasome inhibitor MG-132 enhances the cytotoxicity of LBH, and the alternative combination of MG-132 and the HDAC inhibitor TSA results in synergistic cytotoxicity (Fig. 3F). Collectively, these results demonstrate that the interaction between LBH589 and bortezomib in GBM cell lines represents a class effect between HDAC inhibitors and proteasome inhibitors.
Effects of the Combination of LBH589 and Bortezomib on Apoptosis and Regulatory Signaling Cascades
To confirm that cytotoxicity from this combination is due to the induction of apoptotic cell death, we analyzed biological markers and regulatory proteins of apoptosis from extracts of U251 cells. Cytochrome c release in the combined treatment with LBH589 and bortezomib was detected in the cytosolic subcellular fraction using Western blot analysis, consistent with activation of the intrinsic pathway of apoptosis. Bax was markedly reduced in the cytosolic fraction with combined drug treatment, accompanied by only a small decrease in total Bax levels. Since Bax translocation to mitochondria is known to precede cytochrome c release,32 these results support a model in which Bax translocation enhances mitochondrial outer membrane permeability with subsequent release of cytochrome c to the cytosol, thus activating caspase 9 and the apoptotic cascade. Consistent with this model, combined therapy resulted in marked cleavage of procaspase 9, procaspase 3, Bid, and PARP, whereas the individual agents did not (Fig. 4A). LBH589 and bortezomib also may activate the extrinsic apoptotic pathway, because we also detected cleavage of caspase 8. The levels of other Bcl-2 family members and the anti-apoptotic protein XIAP were not changed except that bortezomib increased Mcl-1 levels and LBH589 abrogated this effect.
We next examined the effects of this combination on intracellular signaling cascades that regulate growth, cell-cycle progression, and apoptosis induction, in order to gain insight into the signaling changes that may participate in the synergistic interaction between HDAC and proteasome inhibitors in U251 cells (Fig. 5A, B). Bortezomib alone or in combination with LBH589 decreased Raf-1 protein levels with a subsequent decrease in phospho-Erk. Bortezomib also inhibited Akt signaling and enhanced expression of cyclin D1. Moreover, bortezomib treatment results in accumulation of Mcl-1, an effect abrogated by LBH589. LBH589 induces the appearance of the hypophosphorylated form of pRB, and cotreatment with bortezomib appears to enhance pRB hypophosphorylation, although interpretation of the results is complicated by cleavage of pRB. HDAC inhibitors have been previously described to induce the appearance of underphosphorylated pRB and the caspase-dependent cleavage of pRB.33 JNK is markedly activated by the combination but not by either agent alone. Both LBH589 and bortezomib were associated with elevated p21 and p27 levels, but there did not appear to be any further increase with combined treatment. Thus, LBH589 and bortezomib disrupt signaling in glioma cells via a number of pathways, both as single agents and in combination.
Fig. 5.
Effects of the LBH589 (LBH)/bortezomib (btzmb) combination on signaling pathways. After U251 cells were treated with control medium, 20 nM LBH alone, 2.5 nM btzmb alone, or the combination for 36 h, protein extracts in whole-cell lysates were analyzed by Western blotting against indicated antigens. Abbreviation: CF, cleaved fragment.
Bax Conformational Change and Mitochondrial Translocation Contribute to LBH589/Bortezomib-Mediated Apoptosis
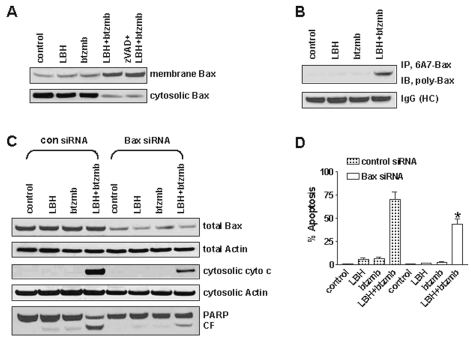
Bax conformational change, accompanied by translocation and integration into the mitochondrial membrane, is believed to be a crucial step for triggering apoptogenic factor release from mitochondria to the cytosol, including cytochrome c, apoptosis-inducing factor, and Smac/DIABLO.34,35 Bax conformational change and its redistribution to mitochondria are common and early features of B-CLL apoptosis in response to proteasome inhibitors,28 an effect that has also been proposed to mediate HDAC inhibitor–induced cytotoxicity.36 The data presented in Fig. 5 suggest that Bax may play an important role in the synergism between HDAC and proteasome inhibitors in glioma cells, since cytosolic Bax levels declined in response to the combination but total Bax levels remained constant, an effect that could be explained by translocation of Bax to the mitochondria. To further examine the role of Bax redistribution during drug treatment with LBH589, bortezomib, and the combination, subcellular fractionation was performed to evaluate Bax levels in both cytosolic and mitochondrial compartments.28 As shown in Fig. 6A, exposure of U251 cells to a combination of LBH589 and bortezomib resulted in a pronounced increase of membrane-associated Bax, which was accompanied by depletion of cytosolic Bax. The Bax translocation from the cytosol to the mitochondrial membrane mediated by LBH589/ bortezomib was not appreciably changed by the pancaspase inhibitor z-VAD-fmk, demonstrating that translocation occurs independently of caspase activation.
Fig. 6.
LBH589 (LBH) and bortezomib (btzmb) induce Bax conformational change and mitochondrial translocation. (A) After U251 cells were treated with vehicle control, 20 nM LBH, 2.5 nM btzmb, or the combination for 36 h, membrane and cytosolic proteins were extracted from each subcellular fraction and subjected to immunoblot analysis using polyclonal Bax antibody. (B) After U251 cells were treated as mentioned above, Bax immunoprecipitation from whole-cell lysates was performed using a conformation-specific Bax (6A7) monoclonal antibody, and the immunoprecipitated complexes were subjected to immunoblot analysis using a polyclonal Bax antibody. The membrane was reprobed with antimouse IgG as loading control (HC: heavy chain). (C) U251 cells were treated with vehicle control, 20 nM LBH, 2.5 nM btzmb, or the combination for 36 h after transfection with control or Bax siRNA for 48 h. Whole-cell lysates were analyzed by immunoblotting for indicated antigens. (D) U251 cells were treated with vehicle control, 20 nM LBH, 2.5 nM btzmb, and the combination for 36 h; apoptosis was examined by DAPI staining. Values represent the means for three replicate determinations ± SD.
Translocation of Bax to mitochondria requires a conformational change that exposes sequences required for oligomerization, membrane insertion, and pore formation.37 Bax molecules that have undergone this conformational change can be detected using a monoclonal antibody to Bax (6A7) that only recognizes Bax after the conformational change. To determine if LBH589 and bortezomib induce Bax conformational changes, conformation-specific Bax was immunoprecipitated from whole-cell lysates using the 6A7 antibody and subjected to immunoblot analysis using a polyclonal anti-Bax antibody. As shown in Fig. 6B, coexposure with LBH589/bortezomib resulted in a marked increase in Bax that had undergone a conformational change.
To determine the functional role of Bax in LBH589/ bortezomib-mediated apoptosis, total Bax levels were reduced using transient transfection of Bax siRNA. As shown in Fig. 6C, cytochrome c release and the appearance of cleaved PARP were reduced in cells containing lower levels of Bax, suggesting Bax reduction may protect cells from LBH589/bortezomib-induced apoptosis. Using a morphological assay, only 40%–50% of the U251 cells expressing low levels of Bax became apoptotic after LBH589/bortezomib treatment, compared to 65%–75% of the cells expressing high Bax levels (Fig. 6D, p < 0.05). Together, these findings indicate that Bax conformational change and mitochondrial translocation play an important role in LBH589/bortezomib-induced apoptosis in glioma cells.
Discussion
The present study examines the effects of combining a proteasome inhibitor with an HDAC inhibitor in GBM cell lines. Both proteasome inhibitors and HDAC inhibitors disrupt cellular signaling in multiple pathways, including pathways important in GBM tumorigenesis, growth, and invasion. Preclinical data have suggested that each class of agents may have utility in the treatment of GBM,8,9,18–21 and clinical testing of HDAC and proteasome inhibitors as single agents in GBM is ongoing. Here, we report that combined HDAC and proteasome inhibition synergistically suppresses growth and induces apoptosis in established GBM cell lines as well as in short-term cultures from patient-derived GBM xenografts, which is a model system that retains the growth and invasive characteristics of GBM.31 Apoptosis induced by the combination is associated with depletion of Bax from the cytosol, Bax conformational change, translocation of Bax to the mitochondria, and subsequent activation of the intrinsic pathway via cytochrome c release from the mitochondria. LBH589 and bortezomib disrupt multiple signaling pathways, both as single agents and in combination. Importantly, LBH589 and bortezomib induce the expression of the cell-cycle inhibitory proteins p21 and p27. LBH589 activates pRB, while bortezomib inhibits MAPK and Akt signaling, and the combination synergistically activates the JNK pathway.
The data presented in this study clearly demonstrate synergistic cytotoxicity when combining LBH589 and bortezomib in GBM cell lines. A number of studies have examined the effects of combining proteasome inhibitors and HDAC inhibitors in a broad range of tumor histologies. Various mechanisms have been proposed. Inhibition of the proteasome results in the formation of aggresomes, which are aggregates of ubiquitin- conjugated proteins. Aggresome formation is thought to be cytoprotective. In multiple myeloma and pancreatic cancer cells, bortezomib treatment induced the formation of aggresomes, but this was blocked by concurrent HDAC inhibitor treatment, resulting in enhanced cytotoxicity.24,25,38 In non-small-cell lung carcinoma and gastrointestinal adenocarcinoma cells, generation of reactive oxygen species by combined HDAC inhibitor and proteasome inhibitor treatment is thought to mediate synergistic cytotoxicity.23,39 In human myeloid leukemia cells, the HDAC inhibitor depsipeptide induces Bax translocation to the mitochondria with subsequent release on cytochrome c, which was markedly enhanced by the addition of bortezomib.36 In CML cells, combining proteasome and HDAC inhibitors induced apoptosis via the intrinsic pathway, which was associated with activation of JNK and required protein synthesis.22
Our results demonstrate that multiple signaling pathways are disrupted by HDAC and proteasome inhibitors in glioma cell lines. One important component necessary for synergistic cytotoxicity is activation of Bax via conformational change and translocation to the mitochondria. The link between Bax translocation and cytochrome c release with subsequent induction of apoptosis is well established. Our results support this model in that suppression of Bax expression by RNA interference decreases both cytochrome c release and apoptosis induced by the combination. However, the cytoprotection offered by siRNA-mediated Bax knockdown is incomplete, which may be due to several causes. First, Bax knockdown in our experiments is incomplete and the residual Bax may be sufficient to induce apoptosis in the presence of HDAC and proteasome inhibitors. Second, the efficiency of Bax knockdown at the cellular level may be variable such that the knockdown in some cells is sufficient to offer cytoprotection, whereas in other cells knockdown may be inefficient and not offer protection. Finally, both HDAC and proteasome inhibitors affect multiple signaling pathaways and alternative proapoptotic signals are likely activated by the combination. Supporting the final cause is our observation that both the intrinsic and extrinsic apoptotic pathways are activated by HDAC and proteasome inhibitors, however, the appearance of cleaved caspase 8 can also occur as a result of feedback through the intrinsic pathway. Thus, while our data clearly support a role for the mitochondrial translocation of Bax in mediating synergistic cytotoxicity between HDAC and proteasome inhibitors, we cannot rule out the participation of other factors.
Both HDAC inhibitors and proteasome inhibitors have been shown to induce Bax translocation as single agents in multiple cell types.28,36,40 Although these studies define Bax translocation as an important component of the cytotoxicity, little is known about the precise signals that regulate Bax translocation in response to HDAC and proteasome inhibitors. Regulation of Bax translocation is complex and depends on changes in phosphorylation, temperature, pH, and interaction with multiple binding partners such as the BH3-only proteins, humanin, Ku70, 14-3-3, and Bif-1.37 In our present study, several of the signaling pathways affected by HDAC and proteasome inhibitors, such as disruption of Akt signaling and hyperactivation of JNK signaling, have been implicated in Bax regulation. Direct phosphorylation of Bax by Akt on Ser184 inhibits translocation to the mitochondria in neutrophils.41 Alternatively, phosphorylation of Bax by JNK stimulates mitochondrial translocation.42 Effects on Bax-interacting proteins may also contribute to the effects induced by HDAC and proteasome inhibitors. Ku70 was initially identified as a component of the apparatus responsible for repair of DNA strand breaks, but it has also been shown to have an important role in sequestering Bax in the cytoplasm. Sequestration of Bax by Ku70 depends on the acetylation of several key residues within the c-terminal linker domain that is adjacent to the Bax interaction domain.43 Treating cells with an HDAC inhibitor is therefore expected to result in accumulation of acetylated Ku70, which may be unable to sequester Bax in the cytoplasm to suppress apoptosis. Thus, it is likely that multiple cellular changes induced by LBH589 and bortezomib in glioma cell lines contribute to the activation of Bax and subsequent induction of apoptosis.
Extended survival for patients with GBM is rare despite aggressive, multimodality therapy. Thus, novel treatment approaches are urgently needed. Trials testing HDAC and proteasome inhibitors as single agents in patients with recurrent glioma are ongoing. In this study, we demonstrate potent synergistic cytotoxicity between HDAC inhibitors and proteasome inhibitors in multiple GBM cell lines. The combination disrupts signaling in multiple signaling cascades that play key roles in cell growth, survival, and apoptosis. Induction of apoptosis by this combination depends on the induced conformational change of the pro-apoptotic protein Bax, which translocates to the mitochondria. High Bax expression is correlated with high-grade gliomas44 and may represent a valuable target in the treatment of GBM. The data presented in this study provide a strong rationale to pursue further preclinical and clinical development of therapeutic combinations of HDAC and proteasome inhibitors for the treatment of GBM.
References
- 1.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359:1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 2.Soni D, King JA, Kaye AH, Hovens CM. Genetics of glioblastoma multiforme: mitogenic signaling and cell cycle pathways converge. J Clin Neurosci. 2005;12:1–5. doi: 10.1016/j.jocn.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Adams J, Palombella VJ, Sausville EA, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 4.Spano JP, Bay JO, Blay JY, Rixe O. Proteasome inhibition: a new approach for the treatment of malignancies. Bull Cancer. 2005;92:E61–66. 945–952. [PubMed] [Google Scholar]
- 5.Williams S, Pettaway C, Song R, Papandreou C, Logothetis C, McConkey DJ. Differential effects of the proteasome inhibitor bortezomib on apoptosis and angiogenesis in human prostate tumor xenografts. Mol Cancer Ther. 2003;2:835–843. [PubMed] [Google Scholar]
- 6.Almond JB, Cohen GM. The proteasome: a novel target for cancer chemotherapy. Leukemia. 2002;16:433–443. doi: 10.1038/sj.leu.2402417. [DOI] [PubMed] [Google Scholar]
- 7.Fujita T, Doihara H, Washio K, et al. Proteasome inhibitor bortezomib increases PTEN expression and enhances trastuzumab-induced growth inhibition in trastuzumab-resistant cells. Anticancer Drugs. 2006;17:455–462. doi: 10.1097/01.cad.0000198910.90819.06. [DOI] [PubMed] [Google Scholar]
- 8.Yin D, Zhou H, Kumagai T, et al. Proteasome inhibitor PS-341 causes cell growth arrest and apoptosis in human glioblastoma multiforme (GBM) Oncogene. 2005;24:344–354. doi: 10.1038/sj.onc.1208225. [DOI] [PubMed] [Google Scholar]
- 9.Laurent N, de Bouard S, Guillamo JS, et al. Effects of the proteasome inhibitor ritonavir on glioma growth in vitro and in vivo. Mol Cancer Ther. 2004;3:129–136. [PubMed] [Google Scholar]
- 10.Phuphanich S, Supko J, Carson KA, et al. Phase I trial of bortezomib in adults with recurrent malignant glioma [abstract] J Clin Oncol. 2006;24:1567. doi: 10.1007/s11060-010-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glozak MA, Sengupta N, Zhang X, Seto E. Acetylation and deacetylation of non-histone proteins. Gene. 2005;363:15–23. doi: 10.1016/j.gene.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Liu T, Kuljaca S, Tee A, Marshall GM. Histone deacetylase inhibitors: multifunctional anticancer agents. Cancer Treat Rev. 2006;32:157–165. doi: 10.1016/j.ctrv.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 14.Van Lint C, Emiliani S, Verdin E. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr. 1996;5:245–253. [PMC free article] [PubMed] [Google Scholar]
- 15.Glaser KB, Staver MJ, Waring JF, Stender J, Ulrich RG, Davidsen SK. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol Cancer Ther. 2003;2:151–163. [PubMed] [Google Scholar]
- 16.Kelly WK, Marks PA. Drug insight: histone deacetylase inhibitors—development of the new targeted anticancer agent suberoylanilide hydroxamic acid. Nat Clin Pract Oncol. 2005;2:150–157. doi: 10.1038/ncponc0106. [DOI] [PubMed] [Google Scholar]
- 17.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 18.Entin-Meer M, Rephaeli A, Yang X, Nudelman A, VandenBerg SR, Haas-Kogan DA. Butyric acid prodrugs are histone deacetylase inhibitors that show antineoplastic activity and radiosensitizing capacity in the treatment of malignant gliomas. Mol Cancer Ther. 2005;4:1952–1961. doi: 10.1158/1535-7163.MCT-05-0087. [DOI] [PubMed] [Google Scholar]
- 19.Wetzel M, Premkumar DR, Arnold B, Pollack IF. Effect of trichostatin A, a histone deacetylase inhibitor, on glioma proliferation in vitro by inducing cell cycle arrest and apoptosis. J Neurosurg. 2005;103:549–556. doi: 10.3171/ped.2005.103.6.0549. [DOI] [PubMed] [Google Scholar]
- 20.Eyupoglu IY, Hahnen E, Trankle C, et al. Experimental therapy of malignant gliomas using the inhibitor of histone deacetylase MS-275. Mol Cancer Ther. 2006;5:1248–1255. doi: 10.1158/1535-7163.MCT-05-0533. [DOI] [PubMed] [Google Scholar]
- 21.Eyupoglu IY, Hahnen E, Buslei R, et al. Suberoylanilide hydroxamic acid (SAHA) has potent anti-glioma properties in vitro, ex vivo and in vivo. J Neurochem. 2005;93:992–999. doi: 10.1111/j.1471-4159.2005.03098.x. [DOI] [PubMed] [Google Scholar]
- 22.Drexler HC, Euler M. Synergistic apoptosis induction by proteasome and histone deacetylase inhibitors is dependent on protein synthesis. Apoptosis. 2005;10:743–758. doi: 10.1007/s10495-005-2942-4. [DOI] [PubMed] [Google Scholar]
- 23.Adachi M, Zhang Y, Zhao X, et al. Synergistic effect of histone deacetylase inhibitors FK228 and m-carboxycinnamic acid bis-hydroxamide with proteasome inhibitors PSI and PS-341 against gastrointestinal adenocarcinoma cells. Clin Cancer Res. 2004;10:3853–3862. doi: 10.1158/1078-0432.CCR-03-0806. [DOI] [PubMed] [Google Scholar]
- 24.Catley L, Weisberg E, Kiziltepe T, et al. Aggresome induction by proteasome inhibitor bortezomib and α-tubulin hyperacetylation by tubulin deacetylase (TDAC) inhibitor LBH589 are synergistic in myeloma cells. Blood. 2006;108:3441–9. doi: 10.1182/blood-2006-04-016055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nawrocki ST, Carew JS, Pino MS, et al. Aggresome disruption: a novel strategy to enhance bortezomib-induced apoptosis in pancreatic cancer cells. Cancer Res. 2006;66:3773–3781. doi: 10.1158/0008-5472.CAN-05-2961. [DOI] [PubMed] [Google Scholar]
- 26.Giannini C, Sarkaria JN, Saito A, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro-Oncology. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu C, Bruzek LM, Meng XW, et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43–9006. Oncogene. 2005;24:6861–6869. doi: 10.1038/sj.onc.1208841. [DOI] [PubMed] [Google Scholar]
- 28.Dewson G, Snowden RT, Almond JB, Dyer MJ, Cohen GM. Conformational change and mitochondrial translocation of Bax accompany proteasome inhibitor-induced apoptosis of chronic lymphocytic leukemic cells. Oncogene. 2003;22:2643–2654. doi: 10.1038/sj.onc.1206326. [DOI] [PubMed] [Google Scholar]
- 29.Adjei AA, Davis JN, Bruzek LM, Erlichman C, Kaufmann SH. Synergy of the protein farnesyltransferase inhibitor SCH66336 and cisplatin in human cancer cell lines. Clin Cancer Res. 2001;7:1438–1445. [PubMed] [Google Scholar]
- 30.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 31.Giannini C, Sarkaria JN, Saito A, et al. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro-Oncology. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Giorgi F, Lartigue L, Bauer MK, et al. The permeability transition pore signals apoptosis by directing Bax translocation and multimerization. FASEB J. 2002;16:607–609. doi: 10.1096/fj.01-0269fje. [DOI] [PubMed] [Google Scholar]
- 33.Rosato RR, Almenara JA, Dai Y, Grant S. Simultaneous activation of the intrinsic and extrinsic pathways by histone deacetylase (HDAC) inhibitors and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) synergistically induces mitochondrial damage and apoptosis in human leukemia cells. Mol Cancer Ther. 2003;2:1273–1284. [PubMed] [Google Scholar]
- 34.Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC. Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci U S A. 1998;95:4997–5002. doi: 10.1073/pnas.95.9.4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosse T, Olivier R, Monney L, et al. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature. 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 36.Sutheesophon K, Kobayashi Y, Takatoku MA, et al. Histone deacetylase inhibitor depsipeptide (FK228) induces apoptosis in leukemic cells by facilitating mitochondrial translocation of Bax, which is enhanced by the proteasome inhibitor bortezomib. Acta Haematol. 2006;115:78–90. doi: 10.1159/000089471. [DOI] [PubMed] [Google Scholar]
- 37.Er E, Oliver L, Cartron PF, Juin P, Manon S, Vallette FM. Mitochondria as the target of the pro-apoptotic protein Bax. Biochim Biophys Acta. 2006;1757:1301–1311. doi: 10.1016/j.bbabio.2006.05.032. [DOI] [PubMed] [Google Scholar]
- 38.Hideshima T, Bradner JE, Wong J, et al. Small-molecule inhibition of proteasome and aggresome function induces synergistic antitumor activity in multiple myeloma. Proc Natl Acad Sci U S A. 2005;102:8567–8572. doi: 10.1073/pnas.0503221102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Denlinger CE, Rundall BK, Keller MD, Jones DR. Proteasome inhibition sensitizes non-small-cell lung cancer to gemcitabine-induced apoptosis. Ann Thorac Surg. 2004;78:1207–1214. doi: 10.1016/j.athoracsur.2004.04.029. [DOI] [PubMed] [Google Scholar]
- 40.Wang S, Yan-Neale Y, Cai R, Alimov I, Cohen D. Activation of mitochondrial pathway is crucial for tumor selective induction of apoptosis by LAQ824. Cell Cycle. 2006;5:1662–1668. doi: 10.4161/cc.5.15.3099. [DOI] [PubMed] [Google Scholar]
- 41.Gardai SJ, Hildeman DA, Frankel SK, et al. Phosphorylation of Bax Ser184 by Akt regulates its activity and apoptosis in neutrophils. J Biol Chem. 2004;279:21085–21095. doi: 10.1074/jbc.M400063200. [DOI] [PubMed] [Google Scholar]
- 42.Kim B-J, Ryu S-W, Song B-J. JNK- and p38 kinase–mediated phosphorylation of Bax leads to its activation and mitochondrial translocation and to apoptosis of human hepatoma hepG2 cells. J Biol Chem. 2006;281:21256–21265. doi: 10.1074/jbc.M510644200. [DOI] [PubMed] [Google Scholar]
- 43.Cohen HY, Lavu S, Bitterman KJ, et al. Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell. 2004;13:627–638. doi: 10.1016/s1097-2765(04)00094-2. [DOI] [PubMed] [Google Scholar]
- 44.Martin S, Toquet C, Oliver L, et al. Expression of bcl-2, bax and bcl-xl in human gliomas: a re-appraisal. J Neurooncol. 2001;52:129–139. doi: 10.1023/a:1010689121904. [DOI] [PubMed] [Google Scholar]