Abstract
The purpose of this study is to estimate the maximum-tolerated dose (MTD) and describe toxicities and preliminary clinical effects of tipifarnib, a farnesyltransferase (FTase) inhibitor, administered concurrently with radiation therapy in children with newly diagnosed intrinsic diffuse brainstem glioma (BSG). Children ≥3 and ≤21 years of age with newly diagnosed nondisseminated intrinsic diffuse BSG were treated with concurrent tipifarnib and radiation, followed by adjuvant tipifarnib. Escalating doses of tipifarnib were administered orally twice daily, continuously, for the entire duration of radiation, followed by a 2-week break. Postradiation tipifarnib, 200 mg/m2/dose, was administered twice daily for 21 consecutive days, in 28-day cycles. Seventeen patients, median age 5.9 years (range, 3.6–13.8), received external beam radiation therapy administered concurrently with tipifarnib at dose levels ranging from 100 to 150 mg/m2/dose, followed by adjuvant tipifarnib for up to 24 months in the absence of tumor progression or unacceptable toxicity. Dose-limiting toxicities were grade 3 skin rash in one patient at the 125 mg/m2 dose level and two patients at the 150 mg/m2 dose level, and grade 3 pneumonia with a normal absolute neutrophil count (ANC) in one patient at the 150 mg/m2 dose level.One patient had isolated grade 4 neutropenia at the 150 mg/m2 dose level. The MTD of tipifarnib administered was estimated as 125 mg/m2/dose b.i.d. When administered concurrently with radiation, the dose-limiting toxicities of tipifarnib are rash, infection with normal ANC, and neutropenia. The MTD of tipifarnib with concurrent radiation is 125 mg/m2/dose b.i.d. One-year survival and progression-free survival estimates are 36.4% (SE 16.7%) and 9.4% (SE 6.3%), respectively.
Keywords: brainstem glioma, farnesyltransferase inhibitors, pediatric
Children with diffuse brainstem glioma (BSG) have a notoriously poor prognosis, with a median survival of less than 1 year.1 Despite concerted efforts to improve survival for BSG patients, outcome documented in national clinical studies has been essentially unchanged since patients with low-grade BSG were restricted from enrollment more than a decade ago. Long-term survival remains less than 10% despite attempts to increase radiation doses, alter radiation fractionation schemes, and add agents such as chemotherapy and radiosensitizers.2
As new approaches are explored for the treatment of BSG, targeted agents have emerged in the forefront. However, the dearth of BSG tissue available for molecular analyses and the heterogeneity of molecular aberrations in gliomas more generally have hindered the successful incorporation of targeted agents into BSG therapy. Recently, studies have defined specific signal transduction pathways activated in pediatric BSG and have lent support to the use of signaling inhibitors for these patients.3 Epidermal growth factor receptor (EGFR) signaling plays an important role in the development of childhood BSG, leading to the logical choice of the enzyme farnesyltransferase (FTase) as a target for therapeutic inhibition.
Signals that emanate from growth factor receptors such as EGFR activate Ras, a small guanosine triphosphatase (GTPase) that requires FTase-mediated post-translational modifications for its activity. FTase inhibitors (FTIs) impede Ras functions, including promotion of oncogenesis and radiation resistance. FTIs not only directly block the function of Ras but can also interrupt the effects of tyrosine kinase receptors that signal through Ras.4,5 Thus, although gliomas rarely contain mutated, oncogenic forms of Ras, common genetic aberrations such as EGFR overexpression may be susceptible to therapeutic targeting by FTIs.6–8
The precise mode of FTI action remains unclear, as the mutational status of Ras does not consistently correlate with response of cells to FTI treatment.5,8–10 Furthermore, an enlarging body of evidence suggests that FTI activity is mediated in part through inhibition of farnesylation of other Ras family members, such as RhoB.4,11–15 These ambiguities notwithstanding, treatment of gliomas in vitro with FTIs results in decreased proliferation and induction of apoptosis.6–9 Furthermore, glioma cells overexpressing EGFR exhibit enhanced sensitivity to such FTI treatment.8 Key to the rationale of the current study are promising results indicating that treatment with FTIs sensitizes human cancer cells to irradiation, specifically if they harbor Ras mutations or exhibit elevated Ras activity due to constitutive activation of upstream signals.16 These data support the hypothesis that FTIs will exhibit selective efficacy against BSGs compared to normal brain tissue and will augment tumor responses to radiation.
Tipifarnib (R115777, Zarnestra; Johnson and Johnson Pharmaceutical Research and Development, Raritan, NJ, USA) is a potent and selective, orally available, nonpeptidomimetic FTI that undergoes both phase I (oxidative) and phase II (conjugative) metabolism in the liver.17,18 A phase I study of tipifarnib was performed by the Pediatric Brain Tumor Consortium (PBTC) to describe the dose-limiting toxicities (DLTs) and to estimate the maximum-tolerated dose (MTD) of concurrent oral administration of tipifarnib and radiation therapy to pediatric patients with nondisseminated, diffuse, intrinsic BSG. This trial has formed the basis for an ongoing PBTC phase II study of tipifarnib administered concurrently with and following radiation therapy in children with BSG.
Materials and Methods
Study Aims
The primary objective of the study was to estimate the MTD of tipifarnib administered concurrently with radiation therapy to pediatric patients with nondisseminated, diffuse, intrinsic BSG. A secondary aim was to describe toxicities associated with tipifarnib treatment in combination with and following radiation therapy. A further secondary objective, results of which will be reported separately, was to characterize radiographic changes in BSG treated with radiation and tipifarnib using MRI, MR spectroscopy, perfusion and diffusion imaging, and PET scans.
Patient Eligibility
Children 3 years or older and 21 years or younger with newly diagnosed nondisseminated intrinsic diffuse BSG were eligible for this study. Other eligibility criteria included (1) a Karnofsky performance score (>16 years of age) or Lansky performance score (≤16 years of age) ≥50; (2) adequate bone marrow function (absolute neutrophil count [ANC] ≥1,000/mm3, hemoglobin ≥8 gm/dL, and a platelet count of 100,000/mm3); and (3) adequate renal function (serum creatinine normal for age or glomerular filtration rate >70 ml/min/1.73 m2) and adequate hepatic function (serum bilirubin ≤ 1.5; serum glutamate-pyruvate transaminase [alanine aminotransferase] and serum glutamic-oxaloacetic transaminase [aspartate aminotransferase] < 2.5 times upper limit of normal). Patients who had received prior radiation, chemotherapy, or experimental anticancer agents, with the exception of steroids, and patients with known allergy to topical or systemic imidazoles were excluded. Patients receiving enzyme-inducing anticonvulsants were also excluded because these agents have been shown to markedly increase the clearance of tipifarnib.19
The institutional review boards (IRBs) of each PBTC institution approved the protocol before initial patient enrollment, and continuing approval was maintained throughout the study. Patients, parents, or legal guardians gave written informed consent, and assent was obtained, as appropriate, in accordance with local IRB policies prior to enrollment.
Studies before and during Treatment
A detailed history was obtained and physical and neurological exams were performed prior to treatment, at weekly intervals during the first 8 weeks of treatment and monthly thereafter. Pretreatment laboratory evaluations included complete blood counts with differential, blood electrolytes including calcium, magnesium and phosphorous, creatinine, blood urea nitrogen, urinalysis, and liver function tests. These were repeated at 1- to 4-week intervals throughout the treatment. Pretreatment pregnancy tests were required for girls of childbearing potential.
Neuroimaging included pretreatment brain MRI with gradient echo sequences, MR spectroscopy, and perfusion/diffusion MR. These studies were repeated at 8-week intervals. At institutions with PET facilities, patients underwent 18F-fluorodeoxyglucose PET imaging pretreatment and again 4 months later. We were cognizant of the potential for postradiation effects such as increased edema to be incorrectly categorized as progressive disease. However, such erroneous assessment is unlikely in this study because none of the patients who remained on study for at least 8 weeks displayed progressive disease at their 8-week (2 weeks postradiation) disease evaluation.
Radiation and Drug Administration
Tipifarnib was given orally twice a day, beginning 1–2 days prior to the start of radiation therapy. In this study we aimed to capitalize on the reported radiosensitizing function of tipifarnib, and therefore administered the drug continuously throughout the course of radiotherapy. However, due to concerns about toxicities potentially associated with prolonged, continuous dosing of tipifarnib, a 2-week rest period followed radiation therapy. Actual drug administration was monitored using diaries in which patients and their families recorded the date, time, and dose of tipifarnib for each treatment day.
At approximately week 9, patients resumed taking tipifarnib at 200 mg/m2/dose administered orally twice daily, the previously established pediatric MTD in the absence of radiation therapy, or at one dose level lower than the initially assigned dose for patients who experience a DLT during the DLT observation period. Thereafter, tipifarnib was administered twice a day on a 28-day schedule consisting of 3 weeks on the drug followed by a 1-week rest period. The first 8 weeks of treatment during radiation constituted the first two courses, and each subsequent 28-day period was defined as a course.
Patients received local irradiation using conventional or conformal, volume-based delivery techniques. The gross tumor volume (GTV) was defined as the abnormal signal on MRI (usually T2 weighted, but a combination of T1 with contrast and T2 was acceptable). The clinical target volume (CTV) for subclinical disease was defined as a 1.5-cm anatomic margin beyond the GTV, targeting at least the entire anatomic section of the brainstem within the CTV. This margin was constrained by the anatomic limits of brain structures and skull. The planning target volume (PTV) was an institution-specific margin to allow for daily patient setup uncertainties (typically 3–5 mm).
Doses were delivered uniformly to the target volumes and prescribed to the isocenter or to an isodose surface that encompassed the PTV (e.g., 100% isodose surface for three-dimensional techniques. Conventional fractionation was used, with radiation administered once daily, 5 days per week, to a total dose of 5,580 cGy.
Dose Escalation, DLT, and MTD
A traditional phase I dose escalation design was used to estimate the MTD. Cohorts of three to six patients were treated at each dose level beginning at 150 mg/m2/dose b.i.d. Dose escalation was determined by toxicity observed during the DLT observation period that began with the first dose of tipifarnib and concluded at the end of the 2-week break that followed the course of radiation therapy (approx. 8 weeks from the initiation of therapy). The MTD was defined as the dose at which at most one of six patients experienced a DLT and the next higher dose level proved too toxic. Patients who seemed likely to benefit clinically and who did not experience unacceptable toxicities were permitted to remain on treatment for up to 2 years (26 courses).
Toxicities were graded according to the NCI Common Terminology Criteria for Adverse Events (CTCAE version 3.0) scale. DLT was defined as grade 4 neutropenia; grade 3 or 4 thrombocytopenia; any grade 3 or 4 nonhematologic toxicity except skin toxicity; grade 3 or 4 skin toxicity that persisted longer than 7 days despite withholding tipifarnib and treatment with topical agents and oral prednisone; grade 2 skin rash that progressed to grade 3 or greater, despite treatment; symptomatic intratumoral hemorrhage or progressive asymptomatic hemorrhage; any toxicity that required interruption of radiation therapy for longer than 5 consecutive days or 10 days total; or failure to recover sufficiently from toxicities to be eligible for retreatment with tipifarnib within 2 weeks of the last dose of drug.
Survival was defined as the interval from initiation of treatment to death or date of last contact for surviving patients. Progression-free survival was defined as the interval from initiation of treatment to the earliest of disease progression (tumor increase of 25% over baseline tumor measurement; appearance of new lesion(s); or progressive/worsening neurological status) or death for patients who failed and to the last date of follow-up for patients without failure.
Results
A total of 17 children were enrolled in the study from July 2004 to January 2006. Patient characteristics are listed in Table 1. Three patients were not assessable for toxicity: one patient withdrew before initiation of protocol treatment; one patient received only 3 days of treatment and discontinued therapy due to disease progression; and one patient received only 9 days of therapy and discontinued treatment due to adverse events unrelated to the study.
Table 1.
Patient characteristics for eligible patients (n = 17)
| Characteristics | No. of Patients |
|---|---|
| Sex | |
| Males | 6 |
| Females | 11 |
| Ethnicity | |
| Hispanic or Latino | 3 |
| Non-Hispanic | 14 |
| Age (years) | |
| Median (range) | 5.9 (3.6–13.8) |
| Karnofsky Lansky score | |
| Median (range) | 70 (50–100) |
Table 2 lists adverse events that occurred during the DLT observation period, were at least grade 3 in severity, and were considered possibly, probably, or likely related to tipifarnib.
Table 2.
Grade 3–4 toxicities during dose-limiting toxicity observation period attributed to tipifarnib
| Dose (mg/m2/dose)
|
|||
|---|---|---|---|
| 125 (n = 6)
|
150 (n = 5)
|
||
| Toxicity | Grade 3 | Grade 3 | Grade 4 |
| Anorexia | 1 | ||
| Febrile neutropenia | 1 | 1 | |
| Hemoglobin | 1 | ||
| Hypoxia and infection with normal ANC | 1a | ||
| Leukocytes | 1 | 1 | |
| Lymphopenia | 2 | 1 | |
| Absolute neutrophil count | 1a | ||
| Rash/desquamation | 1a | 2a | |
Abbreviation: ANC, absolute neutrophil count.
If a given patient had the same toxicity more than once at various grades, only the highest grade is shown.
Dose-limiting toxicities (DLTs).
The first two patients at the starting dose, 150 mg/m2/dose administered twice daily, experienced DLTs consisting of grade 4 neutropenia and grade 3 rash, respectively (Table 2). The dose was therefore de- escalated to 100 mg/m2 b.i.d. and the protocol amended to include two additional dose levels (dose level −1, 75 mg/m2 b.i.d.; and dose level 0.5, 125 mg/m2 b.i.d.). The amended protocol added a section delineating specific instructions for treatment of skin toxicities. With these explicit guidelines, the traditional phase I dose-finding design was reinitiated at 100 mg/m2 b.i.d., allowing for dose reescalation to 150 mg/m2 b.i.d. and higher, if supported by the data. None of the three patients treated at 100 mg/m2 b.i.d. experienced a DLT, and the dose was escalated to 125 mg/m2 b.i.d.
At the 125 mg/m2 b.i.d. dose level, one dose-limiting grade 3 rash was observed among six evaluable patients and the dose was subsequently reescalated to 150 mg/m2/dose b.i.d. Two of three patients treated at the 150 mg/m2 b.i.d. dose level experienced DLTs, one patient with grade 3 skin rash and one patient with grade 3 infection without neutropenia consisting of pneumonitis of unknown etiology. This latter patient, who had a prior history of reactive airway disease, developed a chronic, persistent cough and intermittent hypoxia 2 weeks after completion of radiation therapy and prior to receiving any postradiation tipifarnib. Infectious work-up, including bronchoscopy, was negative. Administration of tipifarnib was discontinued, and she did not resume tipifarnib treatment in the postradiation phase of the study. The patient’s cough and chest CT findings subsequently resolved. Thus, including patients treated prior to the amendment, four of five patients treated at 150 mg/m2/dose administered twice daily experienced a DLT. The MTD of tipifarnib administered concurrently with radiation was declared as 125 mg/m2/dose b.i.d.
In the postradiation phase of the study (200 mg/m2/dose administered b.i.d.), 63 courses of tipifarnib were administered to 12 patients. Table 3 delineates the adverse events, equal to or greater than grade 3, that occurred after the DLT observation period (in the post-radiation portion of the study) and were considered possibly, probably, or likely related to tipifarnib. One patient was removed from treatment during the postradiation therapy phase for an adverse event possibly related to tipifarnib. Tipifarnib was discontinued in this patient during the seventh month of therapy, during course 8 of therapy, when a routine MRI showed interval development of punctate foci consistent with microhemorrhages in the white matter of the temporal and occipital lobes and the right periatrial region. The patient was asymptomatic, but a scan 1 month later showed interval development of increased T1- and T2-weighted signal in the globi pallidi as well as increased T1 signal in the sub-stantia nigra. The patient subsequently developed a constellation of behavioral changes consistent with KluverBucy syndrome. The radiographic changes and the patient’s symptoms slowly improved thereafter.
Table 3.
Grade 3–4 toxicities during postradiation period attributed to tipifarnib (n = 12; total number of treatment courses = 63)
| Toxicity | Grade 3 |
|---|---|
| Febrile neutropenia | 1 |
| Leukocytes | 1 |
| Neurology: KluverBucy syndrome | 1 |
| Absolute neutrophil count | 1 |
| Hypokalemia | 2 |
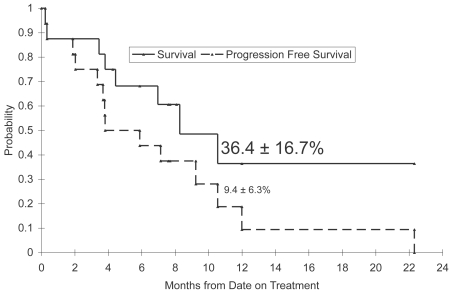
Secondary objectives of this study included assessing outcome as measured by survival and progression-free survival. Of the 17 enrolled patients, one patient withdrew prior to initiating protocol therapy and is excluded from the assessment of outcome. Kaplan-Meier estimates based on the 16 remaining patients show 1-year survival and progression-free survival rates of 36.4% (SE 16.7%) and 9.4% (SE 6.3%), respectively (Fig. 1).
Fig. 1.
Survival and progression-free survival distributions. Small triangles on the survival distribution indicate censored observations.
Discussion
The introduction of FTIs as potential antineoplastic agents propelled the movement toward rationally designed and targeted drugs. The promise of FTIs has rested in their inhibition of posttranslational modification of Ras, a process required for all Ras activities, including promotion of oncogenesis and radiation resistance.20–23 Ras constitutes one of the most promising therapeutic targets since 30% of human cancers contain oncogenic mutations of one of the three known human ras genes. However, FTIs have shown clinical efficacy against numerous tumors that lack Ras mutations. Culprits to explain such Ras-independent antineoplastic efficacy of FTIs include the 300 or so cellular farnesylated proteins as well as aberrant pathways that signal through Ras.24
These mechanistic enigmas notwithstanding, treatment of gliomas in vitro with FTIs results in decreased proliferation and induction of apoptosis.6–9 Furthermore, glioma cells overexpressing EGFR exhibit enhanced sensitivity to such FTI treatment.8 Proliferative signals from receptor tyrosine kinases expressed by gliomas utilize the Ras mitogenic pathway, providing a rational therapeutic target in gliomas.25 Promising activity of tipifarnib against gliomas has been extended to in vivo studies. In mouse models, treatment of human glioma xenografts with FTIs inhibits glioma cell proliferation in vivo, induces regression of established subcutaneous tumors, and prolongs survival of mice bearing intracranial tumors.26
Despite promising in vitro and in vivo activity of FTIs, such inhibitors of cell signaling are most likely to impact the treatment of human cancers when combined with standard forms of antineoplastic therapy such as chemotherapy and radiation. This is particularly true in BSGs, in which radiotherapy generally produces only transient tumor regression and neither pre- nor post-radiation chemotherapy has improved patient survival. Thus, there is a strong rationale for identifying agents that potentiate the efficacy of radiation. An enlarging body of evidence indicates that FTIs function as radiosensitizers in vitro.16,27,28 In a recently published study, tipifarnib enhanced the radiation sensitivity of radioresistant human glioma cell lines but did not affect the radiation response of more sensitive glioma cell lines.15
Given that tipifarnib reverses radiation resistance of human glioma cells in vitro, the aim of this study is to ultimately evaluate the efficacy of tipifarnib administered concurrently with and following radiation in pediatric patients with nondisseminated, diffuse, intrinsic BSGs. This phase I study has established the MTD of tipifarnib administered with radiation as 125 mg/m2/dose. Two of three patients treated at the 150 mg/m2 dose level experienced DLTs: one patient with grade 3 rash and one patient with grade 3 infection without neutropenia.
The North American Brain Tumor Consortium (NABTC) has conducted phase I and II studies of tipifarnib for patients with recurrent malignant gliomas. DLTs consist primarily of rash for patients taking enzyme-inducing epileptic drugs (EIAEDs) and myelosuppression in patients not taking EIAEDs.19,29 Hematologic toxicities were reported as mild to moderate, and all grade 3 toxicities were nonhematologic and consisted of rash, headache, and fatigue. The phase II NABTC study reported modest yet promising evidence of activity, with progression-free survival of 9 weeks for non-EIAED glioblastoma multiforme (GBM) patients and 6 weeks for EIAED GBM patients, a difference that reached statistical significance.29
Although the primary endpoint of the PBTC phase I study was to evaluate toxicities of tipifarnib in combination with radiation, 16 patients were assessable for response, revealing 1-year survival and progression-free survival estimates of 36.4% (SE 16.7%) and 9.4% (SE 6.3%), respectively.
Cooperative groups within the pediatric neurooncology community have discussed the best approach to evaluating novel therapies for BSGs and have established a more uniform approach to assessing the efficacy of such treatments. Outcome for children with BSGs has been essentially unchanged for over a decade, and therefore historical control data are relatively homogeneous among several trials, all revealing 1-year overall survival of 30% ± 3% and 1-year event-free survival of 12% ± 2%. This allows for identification of a “winner” within relatively small screening trials, followed by larger single arm trials to confirm clinical promise. Although the ultimate goal is to conduct a subsequent phase III trial, we have yet to reach this milestone for pediatric BSGs.
Based on results of this phase I trial, the PBTC is now accruing to the phase II component of PBTC-014 using 125 mg/m2/dose b.i.d. of tipifarnib, administered concurrently with radiation therapy followed by 200 mg/m2/dose b.i.d. and adjuvant tipifarnib administered for 21 continuous days out of 28 days. The primary goal of this continuing trial is to evaluate the overall feasibility and efficacy of this regimen for pediatric patients with nondisseminated diffuse intrinsic BSG who are not receiving EIAEDs. The results of this ongoing efficacy trial will be updated after completion of patient accrual and follow-up.
Acknowledgments
This work was supported in part by NIH grant U01 CA81457 for the Pediatric Brain Tumor Consortium (PBTC), American Lebanese Syrian Associated Charities, and The Nancy and Stephen Grand Philanthropic Fund (D.A.H.-K.), NCRR M01 RR00188.
References
- 1.Freeman CR, Farmer JP. Pediatric brain stem gliomas: a review. Int J Radiat Oncol Biol Phys. 1998;40:265–271. doi: 10.1016/s0360-3016(97)00572-5. [DOI] [PubMed] [Google Scholar]
- 2.Donaldson SS, Laningham F, Fisher PG. Advances toward an understanding of brainstem gliomas. J Clin Oncol. 2006;24:1266–1272. doi: 10.1200/JCO.2005.04.6599. [DOI] [PubMed] [Google Scholar]
- 3.Gilbertson RJ, Hill DA, Hernan R, et al. ERBB1 is amplified and over-expressed in high-grade diffusely infiltrative pediatric brain stem glioma. Clin Cancer Res. 2003;9:3620–3624. [PubMed] [Google Scholar]
- 4.Prendergast GC. Farnesyltransferase inhibitors: antineoplastic mechanism and clinical prospects. Curr Opin Cell Biol. 2000;12:166–173. doi: 10.1016/s0955-0674(99)00072-1. [DOI] [PubMed] [Google Scholar]
- 5.Sepp-Lorenzino L, Ma Z, Rands E, et al. A peptidomimetic inhibitor of farnesyl: protein transferase blocks the anchorage-dependent and -independent growth of human tumor cell lines. Cancer Res. 1995;55:5302–5309. [PubMed] [Google Scholar]
- 6.Bredel M, Pollack IF, Freund JM, Hamilton AD, Sebti SM. Inhibition of Ras and related G-proteins as a therapeutic strategy for blocking malignant glioma growth. Neurosurgery. 1998;43:124–131. doi: 10.1097/00006123-199807000-00081. [DOI] [PubMed] [Google Scholar]
- 7.Pollack IF, Bredel M, Erff M. Application of signal transduction inhibition as a therapeutic strategy for central nervous system tumors. Pediatr Neurosurg. 1998;29:228–244. doi: 10.1159/000028729. [DOI] [PubMed] [Google Scholar]
- 8.Feldkamp MM, Lau N, Roncari L, Guha A. Isotype-specific Ras.GTP-levels predict the efficacy of farnesyl transferase inhibitors against human astrocytomas regardless of Ras mutational status. Cancer Res. 2001;61:4425–4431. [PubMed] [Google Scholar]
- 9.Feldkamp MM, Lau N, Guha A. Growth inhibition of astrocytoma cells by farnesyl transferase inhibitors is mediated by a combination of anti-proliferative, pro-apoptotic and anti-angiogenic effects. Oncogene. 1999;18:7514–7526. doi: 10.1038/sj.onc.1203105. [DOI] [PubMed] [Google Scholar]
- 10.Nagasu T, Yoshimatsu K, Rowell C, Lewis MD, Garcia AM. Inhibition of human tumor xenograft growth by treatment with the farnesyl transferase inhibitor B956. Cancer Res. 1995;55:5310–5314. [PubMed] [Google Scholar]
- 11.Lebowitz PF, Du W, Prendergast GC. Prenylation of RhoB is required for its cell transforming function but not its ability to activate serum response element-dependent transcription. J Biol Chem. 1997;272:16093–16095. doi: 10.1074/jbc.272.26.16093. [DOI] [PubMed] [Google Scholar]
- 12.Du W, Lebowitz PF, Prendergast GC. Cell growth inhibition by farnesyltransferase inhibitors is mediated by gain of geranylgeranylated RhoB. Mol Cell Biol. 1999;19:1831–1840. doi: 10.1128/mcb.19.3.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu A, Du W, Liu JP, Jessell TM, Prendergast GC. RhoB alteration is necessary for apoptotic and antineoplastic responses to farnesyltransferase inhibitors. Mol Cell Biol. 2000;20:6105–6113. doi: 10.1128/mcb.20.16.6105-6113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebowitz PF, Prendergast GC. Non-Ras targets of farnesyltransferase inhibitors: focus on Rho. Oncogene. 1998;17:1439–1445. doi: 10.1038/sj.onc.1202175. [DOI] [PubMed] [Google Scholar]
- 15.Delmas C, Heliez C, Cohen-Jonathan E, et al. Farnesyltransferase inhibitor, R115777, reverses the resistance of human glioma cell lines to ionizing radiation. Int J Cancer. 2002;100:43–48. doi: 10.1002/ijc.10439. [DOI] [PubMed] [Google Scholar]
- 16.Jones HA, Hahn SM, Bernhard E, McKenna WG. Ras inhibitors and radiation therapy. Semin Radiat Oncol. 2001;11:328–337. doi: 10.1053/srao.2001.26020. [DOI] [PubMed] [Google Scholar]
- 17.Garner RC, Goris I, Laenen AA, et al. Evaluation of accelerator mass spectrometry in a human mass balance and pharmacokinetic study-experience with 14C-labeled (R)-6-[amino(4-chlorophenyl) (1-methyl-1H-imidazol-5-yl)methyl]-4-(3-chlorophenyl)-1-methyl-2(1H)-quinolinone (R115777), a farnesyl transferase inhibitor. Drug Metab Dispos. 2002;30:823–830. doi: 10.1124/dmd.30.7.823. [DOI] [PubMed] [Google Scholar]
- 18.Zujewski J, Horak ID, Bol CJ, et al. Phase I and pharmacokinetic study of farnesyl protein transferase inhibitor R115777 in advanced cancer. J Clin Oncol. 2000;18:927–941. doi: 10.1200/JCO.2000.18.4.927. [DOI] [PubMed] [Google Scholar]
- 19.Cloughesy TF, Kuhn J, Robins HI, et al. Phase I trial of tipifarnib in patients with recurrent malignant glioma taking enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study. J Clin Oncol. 2005;23:6647–6656. doi: 10.1200/JCO.2005.10.068. [DOI] [PubMed] [Google Scholar]
- 20.Rowinsky EK, Windle JJ, Von Hoff DD. Ras protein farnesyltransferase: a strategic target for anticancer therapeutic development. J Clin Oncol. 1999;17:3631–3652. doi: 10.1200/JCO.1999.17.11.3631. [DOI] [PubMed] [Google Scholar]
- 21.Gibbs JB, Pompliano DL, Mosser SD, et al. Selective inhibition of farnesyl-protein transferase blocks ras processing in vivo. J Biol Chem. 1993;268:7617–7620. [PubMed] [Google Scholar]
- 22.Kato K, Cox AD, Hisaka MM, Graham SM, Buss JE, Der CJ. Isoprenoid addition to Ras protein is the critical modification for its membrane association and transforming activity. Proc Natl Acad Sci U S A. 1992;89:6403–6407. doi: 10.1073/pnas.89.14.6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reiss Y, Goldstein JL, Seabra MC, Casey PJ, Brown MS. Inhibition of purified p21ras farnesyl:protein transferase by Cys-AAX tetrapeptides. Cell. 1990;62:81–88. doi: 10.1016/0092-8674(90)90242-7. [DOI] [PubMed] [Google Scholar]
- 24.Rowinsky EK. Lately, it occurs to me what a long, strange trip it’s been for the farnesyltransferase inhibitors. J Clin Oncol. 2006;24:2981–2984. doi: 10.1200/JCO.2006.05.9808. [DOI] [PubMed] [Google Scholar]
- 25.Guha A, Feldkamp MM, Lau N, Boss G, Pawson A. Proliferation of human malignant astrocytomas is dependent on Ras activation. Oncogene. 1997;15:2755–2765. doi: 10.1038/sj.onc.1201455. [DOI] [PubMed] [Google Scholar]
- 26.Pollack IF, Bredel M, Erff M, Hamilton AD, Sebti SM. Inhibition of Ras and related guanosine triphosphate-dependent proteins as a therapeutic strategy for blocking malignant glioma growth: II—preclinical studies in a nude mouse model. Neurosurgery. 1999;45:1208–1214. doi: 10.1097/00006123-199911000-00039. [DOI] [PubMed] [Google Scholar]
- 27.Bernhard EJ, Kao G, Cox AD, et al. The farnesyltransferase inhibitor FTI-277 radiosensitizes H-ras- transformed rat embryo fibroblasts. Cancer Res. 1996;56:1727–1730. [PubMed] [Google Scholar]
- 28.Bernhard EJ, McKenna WG, Hamilton AD, et al. Inhibiting Ras prenylation increases the radiosensitivity of human tumor cell lines with activating mutations of ras oncogenes. Cancer Res. 1998;58:1754–1761. [PubMed] [Google Scholar]
- 29.Cloughesy TF, Wen PY, Robins HI, et al. Phase II trial of tipifarnib in patients with recurrent malignant glioma either receiving or not receiving enzyme-inducing antiepileptic drugs: a North American Brain Tumor Consortium Study. J Clin Oncol. 2006;24:3651–3656. doi: 10.1200/JCO.2006.06.2323. [DOI] [PubMed] [Google Scholar]