Abstract
Although radionecrosis has been exhaustively described in depth in the neurooncological literature, its diagnosis is still a challenging issue because its radiological pattern is frequently indistinguishable from that of tumor recurrence. This review discusses the causes of radionecrosis and the potential effect of adjuvant chemotherapy concomitant with radiotherapy on its rate and onset. The potential pitfalls in clinical studies attempting to make a differential diagnosis between radionecrosis and disease progression are also discussed.
Keywords: chemotherapy, glioma, pseudoprogression, radionecrosis, radiotherapy
Glioblastoma multiforme (GBM), the most common primitive malignant type of CNS tumor in adults, is characterized by intrinsic aggressiveness and carries a dismal prognosis. Despite the efforts made in recent years, the median overall survival of patients with GBM has never exceeded 14 months. An important step forward in the treatment of patients with this disease was made in the randomized phase III trial conducted by the European Organization for Research and Treatment of Cancer (EORTC) and the National Cancer Institute of Canada (NCIC), in which both progression-free survival (PFS) and overall survival in the concomitant and adjuvant temozolomide (TMZ) arm were longer than that in the radiotherapyalone arm.1 Yet, even with this new multimodality treatment, GBM recurs after a median time of 7 months following diagnosis, requiring a second-line treatment.
Moreover, decisions regarding disease management are complicated by the fact that any change in the patient’s radiological image suggesting disease progression may be due to radiation-induced injury. In 1990, Macdonald et al.2 defined the criteria for evaluating response to treatment and disease progression. They suggested that variations in the tumor-enhancing area, neurological function, and steroid dosage should be considered in the assessment of tumor response. Although these criteria have since been the cornerstone in response evaluation in many phase II clinical trials, recently some doubts have been expressed regarding the evaluation of disease progression in patients treated with TMZ concomitant with radiotherapy.
Radiation Injury
In patients under treatment for brain tumors, worsening of the preexisting neurological focal deficits, suggesting tumor progression or recurrence, can be accompanied by a neuroradiological image of edema and contrast-enhancing lesion within the tumor bed. However, this radiological pattern is not necessarily associated with any clinical deterioration. These alterations, described in 1979 by Hoffman et al.3 in a group of patients treated with radiotherapy and carmustine (BCNU) at an interval of 8 weeks, were investigated with serial CT or MRI scans. Within 18 weeks following radiotherapy, 49% of patients had a deterioration that strongly suggested tumor progression. In 28% of the cases, however, spontaneous improvement occurred without a change in therapy. Following this pattern, which is indistinguishable from that of tumor recurrence, improvement usually occurs within a few weeks or months, with a thorough neuroradiological follow-up showing that these signs regress within 4–8 weeks. The timing of these clinical features, known as “early delayed reactions” following radiotherapy,4 seems to correspond to the turnover time of myelin. Hoffman et al.3 therefore postulated that they might be caused by demyelination. However, several years ago, Pratt et al.5 reported the presence of necrosis with vascular endothelial proliferation in tissue removed from a patient whose condition suddenly deteriorated following radiotherapy and chemotherapy. Overall, these findings may underscore the role of oligo-dendrocytes as putative targets of radiotherapy in early delayed reactions, leading to a final pattern of necrosis. This is contradicted, however, by the evidence that other demyelinating conditions, such as multiple sclerosis, do not often lead to necrosis.6
Complications that follow radiotherapy by a few months to many years, classified as “late delayed reactions,”4 are considered a major hazard of CNS exposure to irradiation. According to the vascular pathogenic hypothesis, radionecrosis, one such reaction and a challenging complication of radiotherapy in neurooncology, would be triggered by ischemia secondary to blood vessel damage,7 and, according to DeAngelis et al.,8 it may depend on increased capillary permeability induced by radiotherapy, leading to fluid transudation into the interstitial space and consequent brain edema. Furthermore, if capillary permeability is altered, damage from chemotherapy may occur earlier and be more severe; radiotherapy may enhance the efficacy of chemotherapy by maximizing drug uptake, either at the cell membrane, through a disruption of the blood–brain barrier, or through an alteration in cell metabolism.
As occurs in GBM patients with methylated O6- methylguanine DNA methyltransferase (MGMT), necrosis can be the consequence of highly effective combined radiochemotherapy. The mechanisms underlying this effect have been described by Chakravarti et al.,9 who showed that TMZ enhances radiation response in MGMT-methylated glioblastoma cells by enhancing double-strand DNA damage, a critical factor underlying radiation-induced cell death. However, these types of necrotic lesions should not be considered strictly radionecrosis as they are included in the effects of radiochemotherapy against glioma cells, implying a potential difference in the treatment outcome. Effective treatment, such as TMZ concomitant with radiotherapy, can lead to the disruption of the blood–brain barrier, allowing the passage of chemotherapeutic agents and thus enhancing their activity. However, 1 month after completion of concomitant treatment, the blood–brain barrier may be still altered, allowing the passage of gadolinium and thus evidencing a lesion that often appears larger than it was before radiotherapy (the field of radiotherapy is larger than the primary tumor) and simulating disease progression; only after several months can the area containing gadolinium enhancement be progressively reduced.
Incidence
Little is known about the incidence of radionecrosis after radiotherapy for gliomas. Moreover, data on the actuarial risk of radiation necrosis in the general glioma population are scarce, mainly because of the difficulty in differentiating necrosis from tumor recurrence radiologically and the low reoperation and autopsy rates in these patients.
In a cohort of 426 glioma patients, Ruben et al.10 found that radiation necrosis was documented in 4.9% of patients who had been treated with radiotherapy prior to the recent advent of concurrent and adjuvant TMZ chemotherapy. The mean time interval from the end of radiotherapy until onset of necrosis was 11.6 months. The authors also found that adjuvant chemotherapy produced a greater than fourfold increase in the risk of cerebral necrosis. Of 232 patients treated with radiotherapy alone, only three (1.3%) had evidence of radionecrosis, whereas among 194 patients given radiotherapy and chemotherapy as part of their treatment, 18 (9.3%) had radionecrosis. Adjuvant chemotherapy was therefore added to the series of known risk factors for the development of radiation necrosis, which includes total radiation dose, fraction size, and treatment duration. Other factors that have long been considered potentially correlated with the onset of radionecrosis, such as hypertension or diabetes, were not found to be significant in this large cohort.
The above data are consistent with historical data provided by, for example, Peterson et al.,11 who reported an incidence of radionecrosis of 2.5% at a time interval of 8–31 months after radiotherapy in 200 primary brain tumor patients who underwent radiotherapy followed by chemotherapy, and by Sheline et al.,4 who reported a 3.4% incidence of radionecrosis in patients who underwent radiotherapy alone, with a median time of onset of 21 months. It has therefore long been assumed that chemotherapy can have an additive effect on the development of cerebral necrosis in the setting of radiotherapy,12–15 and the effect of chemotherapy administered concomitantly with radiotherapy has been investigated over the last few years. Brandes et al.16 found that radionecrosis occurred more frequently (7.1%) and earlier when a chemotherapy regimen with carboplatin and teniposide was administered concomitantly with radiotherapy in patients with glioblastoma, although this finding was not of statistical significance (p = 0.1).
A similar trend in the frequency of radionecrosis was found by Glantz et al.17 in their phase I study of 60 patients who were given paclitaxel weekly concurrently with radiotherapy: radionecrosis occurred in 10 (17%) patients, and 8 patients underwent re-resection within 20–173 days (median, 49 days) following primary surgery. Of these eight, five had extensive radiation necrosis, although no tumor was identified; in the other three patients, small nests of “possibly” viable tumor cells were observed, surrounded by large areas of necrosis.
The highest incidence of radionecrosis of the brain to be reported in the literature was found in patients on an experimental protocol who received radiotherapy with concurrent carboplatin, followed by adjuvant procarbazine, CCNU, and vincristine (PCV) chemotherapy.18 Treatment-induced necrosis was documented at surgery or autopsy in 19 cases (21%); 21 patients (23%) had a mixed pattern of necrosis and tumor, and an additional 13 patients (14%) had no surgical or autoptic signs of predominant radiation necrosis but presented signs of radiation necrosis on MRI. Interestingly, histologic data suggest that the rate of radionecrosis may be slightly higher in patients with anaplastic oligodendroglial tumors than in patients with anaplastic astrocytoma (17% vs. 33%); this observation indirectly supports the glial theory for the development of radionecrosis.18 Furthermore, in their postmortem study on 25 patients with gliomas, Burger et al.19 reported three cases of radionecrosis located in the white matter adjacent to the tumor 3 months after combined radiochemotherapy.
Findings reported in the literature suggest that radionecrosis is closely linked to the features of both radiotherapy (dose, volume, etc.) and chemotherapy delivery and that its rapid onset in patients treated with radiotherapy and chemotherapy suggests the presence of damage to both the neuroglia and vasculature.
Now that concurrent TMZ and radiotherapy are used as standard therapy, the postradiotherapy radiological assessment is made earlier than before, when radiotherapy was given alone. However, it can be difficult to interpret the radiological image obtained, as any changes observed may be due to treatment-related pseudoprogression rather than true disease progression. In their recent study of 51 patients treated with radiotherapy and concomitant TMZ, Chamberlain et al.20 reported seven (14%) cases of early necrosis without signs of tumor recurrence; 26 patients had a radiological diagnosis of early disease progression and, of these, 15 underwent re-resection, with 7 (47%) of the 15 having a surgical diagnosis of radionecrosis. These data open a dual scenario: the possibility of a higher incidence of early radionecrosis and the risk of mistaking the latter for disease progression. In a series of 32 glioma patients, de Wit et al.21 observed that the first postradiotherapy MRI showed progressive enhancement in nine cases; in three of these nine cases, MR images showed improvement or stabilization for 6 months without additional treatment being given.
Jefferies et al.22 reported pseudoprogression in 3 of 15 patients given concomitant radiotherapy and TMZ; they suggested that a criterion for identifying pseudo-progression is the absence of correlated symptoms of disease progression, although this proposal has not been accepted worldwide because edema, which often accompanies pseudoprogression, is also symptomatic of tumor.
Diagnosis
Conventional MR techniques, such as T2- and gadolinium-enhanced T1-weighted imaging, have limitations in discriminating tumor recurrence and treatment-induced necrosis. Current physiologic and metabolic MRI techniques allow the analysis of tumor or necrotic tissue properties and provide more accurate information on chemical composition, perfusion, and water mobility within the tissue.
Proton MR spectroscopic imaging (1H MRSI), a technique that can detect proton metabolites in tissue, displays the distribution of these metabolites within tumor tissue as a molecular image.23 Chemical compounds and metabolites commonly detected in brain tissue include compounds containing choline (Cho), creatine (Cre), lactate, lipid, and N-acetylaspartate (NAA). The increased Cho present in tumors indicates augmented cell membrane phospholipids due to tumor cell proliferation and moreover can be taken into account in glioma grading and in differentiating between neoplastic and nonneo-plastic lesions. Specific spectroscopic changes that have been reported in cases of radiation injury include a reduction in NAA and various changes in Cho and Cre levels.24,25 In general, high Cho levels have been found in the presence of disease progression, whereas low Cho levels have been found in radiation necrosis. Unfortunately, in many of the enhancing regions, including those appearing early after concomitant treatment, often both tumor cells and radiation injury are present, and the spectral patterns in these cases are less clear than those observed in cases of pure tumor or pure radiation necrosis.26 A recent study utilizing the two-dimensional proton spectroscopic imaging technique reported a 97% success rate in retrospective differentiation between recurrent tumor and radiation injury, with a significant increase in Cho/ NAA and Cho/Cre ratios in areas of recurrent tumor, compared with areas of radiation injury and normal adjacent brain tissue.27 With the use of a cutoff value of 1.8 for either the Cho/NAA or Cho/Cre ratio for tumor recurrence, 27 of 28 patients were correctly diagnosed retrospectively.27 Also, a ratio of lipid/lactate to Cho of less than 0.75 can distinguish between tumor tissue and pure necrosis.28
Perfusion is considered a useful tool in the diagnosis of recurrence and necrosis. In the presence of glioma growth and infiltration, vascular alterations can also be found, with a consequent increase in the regional cerebral blood volume (rCBV) value in regions with excess vascularization and a decrease in regions with vasogenic edema or necrosis. A recent study of 23 high-grade gliomas found that changes in tumor rCBV occurring during the early radiotherapy course can also be predictive of survival.29 Diffusion MRI, a technique measuring the mobility of water within tissues at the cellular level, is therefore sensitive to microenvironment changes in the tumor and tissue.23 Alterations in the tumor following therapy may include cellular swelling (secondary to loss of cellular water homeostasis) followed by necrotic or apoptotic cell death with high apparent diffusion coefficient (ADC) values, whereas low ADC values reflect high cellularity. Some authors have observed that ADC values are useful in distinguishing between high-grade glioma and normal tissue, although they do not allow differentiation between a high-grade glioma and the surrounding edema.30,31
In GBM, membrane turnover, cell density, and vascularity are increased. Therefore increased membrane turnover (high Cho), high vascularity (high rCBV), and an increased cellularity (low ADC) found by MR spectroscopy (MRS), perfusion, and diffusion should lead to suspicion of the presence of a tumor in the enhancing lesion.31
18F-Fluorodeoxyglucose (FDG) FDG-PET is considered useful in differentiating delayed radiation injury from recurrent high-grade glioma, and sensitivities ranging from 81% to 86% and specificities from 40% to 94% have been reported with this approach.32 However, false-positive FDG uptake can be observed in non-malignant inflammatory processes, subclinical seizure activity, and healing processes up to 3 months after surgery. In particular, radiation injury can activate repair mechanisms or lead to inflammatory activity, which can increase glucose metabolism.33 When this tool is used, however, any attempt to identify a pseudoprogression that occurs early after concomitant treatment provides less reliable findings than MRS.
Clinical Implications
Conventional neuroradiological techniques do not always allow a differentiation between radionecrosis and recurrence,34,35 the former appearing as postradiotherapy radiological deterioration; as a worsening in cerebral edema with an increase in contrast enhancement; and as an image of a mass, with signs similar to those of recurrence.36 These difficulties in discriminating between tumor progression and the effect of treatment can profoundly compromise subsequent patient management. These phenomena, also known as “pseudoprogressions” and already described in the past, occur with or without chemotherapy associated with radiotherapy. Data recently reported in the randomized EORTC/NCIC phase III trial of patients with newly diagnosed GBM given TMZ in addition to radiotherapy have provided a new standard of care for such patients.1 However, while a small, albeit significant, PFS advantage (5 vs. 6.9 months) has been achieved with this approach, a clear-cut and significant benefit was obtained in 2-year overall survival (9% vs. 24%); this type of effect is not frequent in medical oncology, where significant PFS advantages do not often confer an overall survival advantage. There are at least two explanations for the EORTC/NCIC trial findings. One is that concomitant radiochemotherapy followed by adjuvant chemotherapy may be more effective than radiotherapy alone in a particular subset of patients with later disease progression and a longer survival, these advantages being characterized by a molecular hallmark: MGMT promoter methylation. An exclusive but consistent increase in the efficacy of the treatment in a subgroup of patients might result in a small but consistent increase in both PFS (1.9 months) and overall survival (2.5 months). Intriguingly, the results of some studies suggest that the outcomes in glioblastoma patients surviving with radionecrosis may be more favorable than those in patients without this complication18,37,38 and that radiotherapy is more effective when combined with TMZ in patients with high MGMT promoter methylation. The concept of “therapy-induced necrosis” and its radiological manifestations of “pseudoprogression” should replace the outdated term “early radionecrosis.”
Overall, data on combined chemoradiation treatments suggest that the rate and onset of therapy-induced necrosis are higher and earlier, respectively, in these GBM patients, the phenomena being linked to different pathogenic mechanisms (Figs. 1 and 2). In the EORTC/ NCIC trial, 25% of the patients in the radiotherapy and TMZ arm received TMZ retreatment at the time of progression.1 No data on retreatment motivation and response were given. Thus, a third possible explanation for the conversion of a small PFS advantage into a consistent overall survival advantage might lie in the overestimation of disease progression in the TMZ-radiotherapy arm.
Fig. 1.
Clinical course of pseudoprogression in a 65-year-old patient with glioblastoma multiforme. (A) Presurgical MRI scan. (B) Postsurgical MRI scan. (C) MRI scan performed 1 month after combined temozolomide (TMZ)/radiotherapy; adjuvant TMZ was continued. (D) Four months later, during administration of maintenance TMZ. (E) Eight months later, during administration of maintenance TMZ.
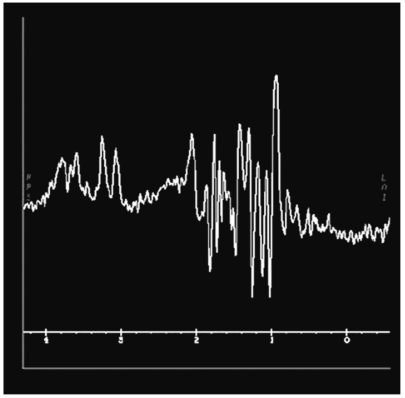
Fig. 2.
MR spectroscopic imaging 10 months after temozolomide plus radiotherapy. Choline:creatine and N-acetylaspartate:choline ratios were 1.3 and 0.92, respectively, suggesting a residual non-neoplastic lesion.
Therapeutic Management
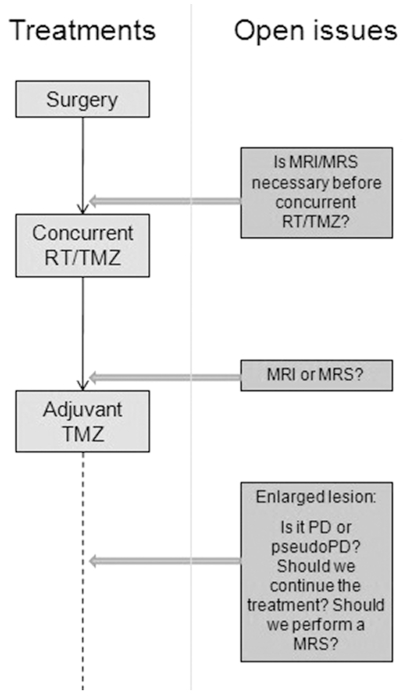
When early radionecrosis is suspected after radiochemotherapy, adjuvant therapy should not be interrupted without a convincing diagnosis of progressive tumor, obtained by MRS with diffusion/perfusion analysis or PET. Although biopsy can discriminate between recurrence and radiation injury, its use is limited by the potential heterogeneity of these lesions. Moreover, it has been postulated that pathologic evaluation of postradiotherapy specimens is not helpful in predicting outcome or in deciding upon further management. The open question is what should be done (Fig. 3): completion of the six, or rather 12, cycles of adjuvant TMZ if a radiologically enhancing lesion persists without the presence of a tumor being confirmed by MRS or PET findings? This may occur because radionecrosis is characterized by a variable clinical course, with spontaneous recovery in some cases and rapid progression to frank necrosis in others. Surgery, if feasible, has long been considered the most effective available approach for the treatment of radionecrosis, because the administration of steroids alone is often followed by an only temporary improvement or by relapse when this treatment is discontinued. However, a few studies have reported long-term improvement in patients receiving steroids, even after completion of this treatment. For early progression presenting immediately after radiochemotherapy, re-reoperation is of limited value because the pathology report will likely find a mixed tissue, if primary radionecrosis and “treated tumor” cells are present, and the role of these cells in causing the relapse is not clear.
Fig. 3.
Open questions in detection and interpretation of suspect lesions after combined radiotherapy/temozolomide (RT/TMZ) and potential implication in treatments. Abbreviations: MRS, MR spectroscopy; PD, progressive disease.
Glantz et al.39 studied heparin and warfarin therapy after unsuccessful steroid administration in a small series of patients treated with radiotherapy for glioma. This approach led to an improvement in five of eight patients. However, no studies on larger series of patients treated with anticoagulant therapy are available in the literature. Some authors advocate the use of hyperbaric oxygen, the rationale for its use hinging on the fact that hyperbaric oxygen increases the tissue pO2 and enhances angiogenesis; results following this approach have been promising.40,41 More recently, in a series of eight patients, bevacizumab, a humanized murine monoclonal antibody against vascular endothelial growth factor, alone and in combination with other agents, was found to be effective against radionecrosis, the mechanism underlying its efficacy probably being an ability to decrease capillary leakage, thus minimizing any associated brain edema.42 MRS may be a useful tool for tracing a flow chart for the treatment of early progressive/pseudoprogressive lesions, and adjuvant chemotherapy should be stopped only in the presence of clear radiological, spectroscopic, diffusion, and perfusion criteria of progression, with non–cross-resistant chemotherapy or surgery being offered to these patients as an alternative approach. Currently, clinical deterioration, improvement, and stabilization are reported in cases of pseudoprogression; it is thus clear that the patient’s clinical status can be misleading in discriminating between tumor progression and radionecrosis. Prospective trials should therefore be undertaken to clarify this issue.
Conclusion
A more reliable strategy for the identification of pseudo-progression in patients with glioblastoma treated with concomitant and adjuvant TMZ is required for the identification of patients unlikely to benefit by switching from effective adjuvant treatment to a potentially less effective and more toxic regimen. New techniques, such as PET43,44 and MRS, may be useful in differentiating recurrent tumor from radiotherapy-induced necrosis, but these modalities still fail to provide a clear-cut answer. Although TMZ therapy has now become the standard of care for patients with newly diagnosed GBM, there are still numerous unanswered questions, and larger randomized trials should be conducted to guide clinical decision making in the management of patients with glioblastoma. There is an urgent need for prospective studies that evaluate clinical, molecular, pathologic, and (above all) functional radio-imaging parameters in the attempt to achieve a more reliable differential diagnosis between neoplasia and radiation injury, thus leading to a more satisfactory outcome for patients.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman WF, Levin VA, Wilson CB. Evaluation of malignant glioma patients during the postirradiation period. J Neurosurg. 1979;50:624–628. doi: 10.3171/jns.1979.50.5.0624. [DOI] [PubMed] [Google Scholar]
- 4.Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215–1228. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 5.Pratt RA, Di Chiro G, Weed JC., Jr Cerebral necrosis following irradiation and chemotherapy for metastatic choriocarcinoma. Surg Neurol. 1977;7:117–120. [PubMed] [Google Scholar]
- 6.Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153:357–370. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 7.Brandes AA, Rigon A, Monfardini S. Radiotherapy of the brain in elderly patients. Contra Eur J Cancer. 2000;36:447–452. doi: 10.1016/s0959-8049(99)00322-6. [DOI] [PubMed] [Google Scholar]
- 8.DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789–796. doi: 10.1212/wnl.39.6.789. [DOI] [PubMed] [Google Scholar]
- 9.Chakravarti A, Erkkinen MG, Nestler U, et al. Temozolomide-mediated radiation enhancement in glioblastoma: a report on underlying mechanisms. Clin Cancer Res. 2006;12:4738–4746. doi: 10.1158/1078-0432.CCR-06-0596. [DOI] [PubMed] [Google Scholar]
- 10.Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65:499–508. doi: 10.1016/j.ijrobp.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Peterson K, Clark HB, Hall WA, Truwit CL. Multifocal enhancing magnetic resonance imaging lesions following cranial irradiation. Ann Neurol. 1995;38:237–244. doi: 10.1002/ana.410380217. [DOI] [PubMed] [Google Scholar]
- 12.Allen JC. The effects of cancer therapy on the nervous system. J Pediatr. 1978;93:903–909. doi: 10.1016/s0022-3476(78)81209-8. [DOI] [PubMed] [Google Scholar]
- 13.Allen JC, Rosen G, Mehta BM, Horten B. Leukoencephalopathy following high-dose iv methotrexate chemotherapy with leucovorin rescue. Cancer Treat Rep. 1980;64:1261–1273. [PubMed] [Google Scholar]
- 14.Di Chiro G, Oldfield E, Wright DC, et al. Cerebral necrosis after radiotherapy and/or intraarterial chemotherapy for brain tumors: PET and neuropathologic studies. AJR Am J Roentgenol. 1988;150:189–197. doi: 10.2214/ajr.150.1.189. [DOI] [PubMed] [Google Scholar]
- 15.Mahaley MS, Jr, Whaley RA, Blue M, Bertsch L. Central neurotoxicity following intracarotid BCNU chemotherapy for malignant gliomas. J Neurooncol. 1986;3:297–314. doi: 10.1007/BF00165578. [DOI] [PubMed] [Google Scholar]
- 16.Brandes AA, Rigon A, Zampieri P, et al. Carboplatin and teniposide concurrent with radiotherapy in patients with glioblastoma multiforme: a phase II study. Cancer. 1998;82:355–361. doi: 10.1002/(sici)1097-0142(19980115)82:2<362::aid-cncr17>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 17.Glantz MJ, Choy H, Kearns CM, et al. Phase I study of weekly outpatient paclitaxel and concurrent cranial irradiation in adults with astrocytomas. J Clin Oncol. 1996;14:600–609. doi: 10.1200/JCO.1996.14.2.600. [DOI] [PubMed] [Google Scholar]
- 18.Levin VA, Yung WK, Bruner J, et al. Phase II study of accelerated fractionation radiation therapy with carboplatin followed by PCV chemotherapy for the treatment of anaplastic gliomas. Int J Radiat Oncol Biol Phys. 2002;53:58–66. doi: 10.1016/s0360-3016(01)02819-x. [DOI] [PubMed] [Google Scholar]
- 19.Burger PC, Mahley MS, Jr, Dudka L, Vogel FS. The morphologic effects of radiation administered therapeutically for intracranial gliomas: a postmortem study of 25 cases. Cancer. 1979;44:1256–1272. doi: 10.1002/1097-0142(197910)44:4<1256::aid-cncr2820440415>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 20.Chamberlain MC, Glantz MJ, Chalmers L, Van Horn A, Sloan AE. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82:81–83. doi: 10.1007/s11060-006-9241-y. [DOI] [PubMed] [Google Scholar]
- 21.de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63:535–537. doi: 10.1212/01.wnl.0000133398.11870.9a. [DOI] [PubMed] [Google Scholar]
- 22.Jefferies S, Burton K, Jones P, Burnet N. Interpretation of early imaging after concurrent radiotherapy and temozolomide for glioblastoma. Clin Oncol (R Coll Radiol) 2007;19:S33. [Google Scholar]
- 23.Cao Y, Sundgren PC, Tsien CI, Chenevert TT, Junck L. Physiologic and metabolic magnetic resonance imaging in gliomas. J Clin Oncol. 2006;24:1228–1235. doi: 10.1200/JCO.2005.04.7233. [DOI] [PubMed] [Google Scholar]
- 24.Schlemmer HP, Bachert P, Henze M, et al. Differentiation of radiation necrosis from tumor progression using proton magnetic resonance spectroscopy. Neuroradiology. 2002;44:216–222. doi: 10.1007/s002340100703. [DOI] [PubMed] [Google Scholar]
- 25.Schlemmer HP, Bachert P, Herfarth KK, Zuna I, Debus J, van Kaick G. Proton MR spectroscopic evaluation of suspicious brain lesions after stereotactic radiotherapy. AJNR Am J Neuroradiol. 2001;22:1316–1324. [PMC free article] [PubMed] [Google Scholar]
- 26.Rock JP, Scarpace L, Hearshen D, et al. Associations among magnetic resonance spectroscopy, apparent diffusion coefficients, and image-guided histopathology with special attention to radiation necrosis. Neurosurgery. 2004;54:1111–1117. doi: 10.1227/01.neu.0000119328.56431.a7. [DOI] [PubMed] [Google Scholar]
- 27.Weybright P, Sundgren PC, Maly P, et al. Differentiation between brain tumor recurrence and radiation injury using MR spectroscopy. AJR Am J Roentgenol. 2005;185:1471–1476. doi: 10.2214/AJR.04.0933. [DOI] [PubMed] [Google Scholar]
- 28.Rock JP, Hearshen D, Scarpace L, et al. Correlations between magnetic resonance spectroscopy and image-guided histopathology, with special attention to radiation necrosis. Neurosurgery. 2002;51:912–919. doi: 10.1097/00006123-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Cao Y, Tsien CI, Nagesh V, et al. Survival prediction in high-grade gliomas by MRI perfusion before and during early stage of RT [corrected] Int J Radiat Oncol Biol Phys. 2006;64:876–885. doi: 10.1016/j.ijrobp.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Castillo M, Smith JK, Kwock L, Wilber K. Apparent diffusion coefficients in the evaluation of high-grade cerebral gliomas. AJNR Am J Neuroradiol. 2001;22:60–64. [PMC free article] [PubMed] [Google Scholar]
- 31.Catalaa I, Henry R, Dillon WP, et al. Perfusion, diffusion and spectroscopy values in newly diagnosed cerebral gliomas. NMR Biomed. 2006;19:463–475. doi: 10.1002/nbm.1059. [DOI] [PubMed] [Google Scholar]
- 32.Chen W. Clinical applications of PET in brain tumors. J Nucl Med. 2007;48:1468–1481. doi: 10.2967/jnumed.106.037689. [DOI] [PubMed] [Google Scholar]
- 33.Ricci PE, Karis JP, Heiserman JE, Fram EK, Bice AN, Drayer BP. Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? . AJNR Am J Neuroradiol. 1998;19:407–413. [PMC free article] [PubMed] [Google Scholar]
- 34.Burger PC, Dubois PJ, Schold SC, Jr, et al. Computerized tomographic and pathologic studies of the untreated, quiescent, and recurrent glioblastoma multiforme. J Neurosurg. 1983;58:159–169. doi: 10.3171/jns.1983.58.2.0159. [DOI] [PubMed] [Google Scholar]
- 35.Dooms GC, Hecht S, Brant-Zawadzki M, Berthiaume Y, N orman D, Newton TH. Brain radiation lesions: MR imaging. Radiology. 1986;158:149–155. doi: 10.1148/radiology.158.1.3940373. [DOI] [PubMed] [Google Scholar]
- 36.Tihan T, Barletta J, Parney I, Lamborn K, Sneed PK, Chang S. Prognostic value of detecting recurrent glioblastoma multiforme in surgical specimens from patients after radiotherapy: should pathology evaluation alter treatment decisions? . Hum Pathol. 2006;37:272–282. doi: 10.1016/j.humpath.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Floyd NS, Woo SY, Teh BS, et al. Hypofractionated intensity- modulated radiotherapy for primary glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2004;58:721–726. doi: 10.1016/S0360-3016(03)01623-7. [DOI] [PubMed] [Google Scholar]
- 38.Forsyth PA, Kelly PJ, Cascino TL, et al. Radiation necrosis or glioma recurrence: is computer-assisted stereotactic biopsy useful? . J Neurosurg. 1995;82:436–444. doi: 10.3171/jns.1995.82.3.0436. [DOI] [PubMed] [Google Scholar]
- 39.Glantz MJ, Burger PC, Friedman AH, Radtke RA, Massey EW, Schold SC., Jr Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44:2020–2027. doi: 10.1212/wnl.44.11.2020. [DOI] [PubMed] [Google Scholar]
- 40.Chuba PJ, Aronin P, Bhambhani K, et al. Hyperbaric oxygen therapy for radiation-induced brain injury in children. Cancer. 1997;80:2005–2012. doi: 10.1002/(sici)1097-0142(19971115)80:10<2005::aid-cncr19>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Leber KA, Eder HG, Kovac H, Anegg U, Pendl G. Treatment of cerebral radionecrosis by hyperbaric oxygen therapy. Stereotact Funct Neurosurg. 1998;70(suppl 1):229–236. doi: 10.1159/000056426. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67:323–326. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 43.Chao ST, Suh JH, Raja S, Lee SY, Barnett G. The sensitivity and specificity of FDG PET in distinguishing recurrent brain tumor from radionecrosis in patients treated with stereotactic radiosurgery. Int J Cancer. 2001;96:191–197. doi: 10.1002/ijc.1016. [DOI] [PubMed] [Google Scholar]
- 44.Langleben DD, Segall GM. PET in differentiation of recurrent brain tumor from radiation injury. J Nucl Med. 2000;41:1861–1867. [PubMed] [Google Scholar]