Abstract
Previous studies revealed that synaptotagmin 1 is the major Ca2+ sensor for fast synchronous transmitter release at excitatory synapses. However, the molecular identity of the Ca2+ sensor at hippocampal inhibitory synapses has not been determined. To address the functional role of synaptotagmin 1 at identified inhibitory terminals, we made paired recordings from synaptically connected basket cells (BCs) and granule cells (GCs) in the dentate gyrus in organotypic slice cultures from wild-type and synaptotagmin 1-deficient mice. As expected, genetic elimination of synaptotagmin 1 abolished synchronous transmitter release at excitatory GC–BC synapses. However, synchronous release at inhibitory BC–GC synapses was maintained. Quantitative analysis revealed that elimination of synaptotagmin 1 reduced release probability and depression but maintained the synchrony of transmitter release at BC–GC synapses. Elimination of synaptotagmin 1 also increased the frequency of both miniature excitatory postsynaptic currents (measured in BCs) and miniature inhibitory postsynaptic currents (recorded in GCs), consistent with a clamping function of synaptotagmin 1 at both excitatory and inhibitory terminals. Single-cell reverse-transcription quantitative PCR analysis revealed that single BCs coexpressed multiple synaptotagmin isoforms, including synaptotagmin 1–5, 7, and 11–13. Our results indicate that, in contrast to excitatory synapses, synaptotagmin 1 is not absolutely required for synchronous release at inhibitory BC–GC synapses. Thus, alternative fast Ca2+ sensors contribute to synchronous release of the inhibitory transmitter GABA in cortical circuits.
Keywords: GABAergic interneurons, basket cells, hippocampus, Ca2+ sensor
GABAergic interneurons of the basket cell subtype (BCs) play a key role in neuronal network function. These interneurons control the average activity level in principal neurons via fast feed-forward and feedback inhibition (1) and are involved in the generation of network oscillations in the γ-frequency range (2). BCs receive a fast excitatory synaptic input, which allows them to detect coincident activity of principal neurons (3). Furthermore, BCs generate rapid inhibitory output signals in their target cells (4). Previous studies revealed that transmitter release at BC output synapses is exclusively mediated by P/Q-type Ca2+ channels (5, 6) and that these Ca2+ channels are tightly coupled to the Ca2+ sensors of exocytosis, with coupling distances of 10–20 nm (7). Tight coupling contributes to fast signaling, increasing the speed and temporal precision of transmitter release.
Expression of specialized Ca2+ sensors of exocytosis may also contribute to the speed and temporal precision of synaptic transmission. However, the synaptic Ca2+ sensors that mediate glutamatergic BC input and GABAergic BC output have not yet been identified. At several synapses, synaptotagmin 1 is essential for fast transmitter release (8–11). Genetic elimination of synaptotagmin 1 abolishes synchronous release in both glutamatergic and GABAergic synapses in culture (12–16). Furthermore, mutations in the C2A Ca2+-binding domain of synaptotagmin 1 induce parallel changes in the affinity of Ca2+ binding and the Ca2+ sensitivity of transmitter release (17, 18). Synaptotagmins represent a highly diverse protein family, comprising 15 isoforms in rats and mice (9). The functional role of the other synaptotagmins has remained unclear. Synaptotagmins are differentially expressed in central neurons (16, 19, 20) and differ in kinetic properties (21). Thus, it is possible that differential expression of synaptotagmins determines the time course of transmitter release and the proportion of synchronous and asynchronous release at different synapses (6, 22–24). However, this hypothesis has not been directly tested.
To examine the functional contribution of synaptotagmin 1 to both excitatory input synapses and inhibitory output synapses of interneurons, we compared synaptic transmission at excitatory granule cell–basket cell (GC–BC) synapses and inhibitory basket cell–granule cell (BC–GC) synapses in the dentate gyrus between wild-type and synaptotagmin 1-deficient mice. Because the synaptotagmin 1-deficient mice die within 48 h after birth (14), experiments were performed in organotypic slice culture (25). We found that synaptotagmin 1 is essential for fast transmitter release at excitatory GC–BC synapses but not at inhibitory BC–GC synapses.
Results
Synaptotagmin 1 Is Essential for Synchronous Transmitter Release at Excitatory GC–BC, but Not Inhibitory BC–GC, Synapses.
To assess the role of synaptotagmin 1 at identified excitatory and inhibitory synapses, we made paired recordings between fast spiking, parvalbumin-expressing BCs and GCs in the dentate gyrus of organotypic slice cultures (25). BCs were identified by the fast spiking action potential (AP) phenotype (26), the location of the axon in the granule cell layer as revealed by biocytin staining, and, in a subset of cells, the immunoreactivity for the Ca2+-binding protein parvalbumin [supporting information (SI) Fig. S1]. In organotypic slice culture from wild-type mice, the input resistances of BCs and GCs were similar to those in acute slices (59.3 ± 2.1 MΩ and 250 ± 37 MΩ, respectively; 136 BCs and 10 GCs in which QX-314 was omitted from the pipette solution; see ref. 27). Furthermore, the basic functional properties of synaptic transmission at both excitatory GC–BC and inhibitory BC–GC synapses in synaptically connected pairs of neurons were preserved. In particular, the short latency, the fast 20–80% rise time, the large peak amplitude, the small proportion of failures of synaptic transmission, and the different decay time constants of excitatory postsynaptic currents (EPSCs) and inhibitory postsynaptic currents (IPSCs) were maintained in organotypic slices (Fig. S2 and Table S1) (3, 4).
To examine the contribution of synaptotagmin 1 to excitatory and inhibitory synaptic transmission, we compared the functional properties of GC–BC and BC–GC synapses in synaptotagmin 1-deficient mice with those in wild-type animals (Fig. 1). As originally reported for excitatory synapses between dissociated cultured neurons (14), genetic elimination of synaptotagmin 1 abolished synchronous transmitter release at excitatory GC–BC synapses (Fig. 1 A and B). In synaptotagmin 1-deficient mice, single APs in the presynaptic GC failed to evoke synchronous EPSCs in the postsynaptic BC, whereas 50-Hz trains of 10 APs evoked a series of asynchronous EPSCs, resulting in a slowly rising and decaying average current (Fig. 1B). The synaptic connection probability between GCs and BCs was identical in wild-type and knockout mice (36% in both cases, 23 of 64 pairs versus 8 of 22 pairs connected), suggesting that the lack of synchronous release was not caused by abnormal connectivity in synaptotagmin 1-deficient mice. These results show that synaptotagmin 1 is essential for synchronous glutamate release at an identified principal neuron–interneuron synapse, as reported previously for other glutamatergic synapses (14, 28).
Fig. 1.
Synaptotagmin 1 is required for synchronous transmitter release at excitatory GC–BC synapses but not inhibitory BC–GC synapses. (A) Evoked EPSCs at the excitatory GC–BC synapse from wild-type mice. (Left) EPSCs evoked by a single presynaptic AP. Upper trace, presynaptic APs; lower traces, 10 consecutive EPSCs. Average EPSC is shown in green. (Right) Average EPSCs evoked by a 50-Hz train of 10 APs. (B) Evoked EPSCs at the excitatory GC–BC synapse from synaptotagmin 1-deficient mice. (Left) EPSCs evoked by a single presynaptic AP. Upper trace, presynaptic APs; lower traces, 10 consecutive EPSC trials. Average trace is shown in green. (Right) Upper trace, presynaptic APs; second trace, average EPSC from 50 individual sweeps; bottom three traces, single trials. (C and D) Evoked IPSCs at the inhibitory BC–GC synapse from wild-type (C) or synaptotagmin 1-deficient (D) mice. (Left) IPSCs evoked by a single presynaptic AP. Upper traces, presynaptic APs; lower traces, 10 consecutive IPSCs. Average trace is shown in green. (Right) Average IPSCs evoked by 50-Hz trains of 10 APs.
In contrast, genetic elimination of synaptotagmin 1 did not abolish synchronous transmitter release at inhibitory BC–GC synapses (Fig. 1 C and D). In synaptotagmin 1-deficient mice, both single APs and 50-Hz trains of 10 APs evoked synchronous IPSCs in the postsynaptic GC with short latency (Fig. 1 C and D). As observed for GC–BC pairs, the synaptic connection probability was similar in wild-type and knockout mice (59%, 22 of 37 pairs versus 48%, 28 of 58 pairs connected). These results show that synaptotagmin 1 is not absolutely required for synchronous transmitter release at inhibitory BC–GC synapses.
Elimination of Synaptotagmin 1 Reduces Release Probability and Multiple-Pulse Depression but Maintains Synchrony of Release at Inhibitory BC–GC Synapses.
We next compared the properties of synaptic transmission at inhibitory BC–GC synapses between wild-type and synaptotagmin 1-deficient mice (Fig. 2 A and B). The mean amplitude of evoked IPSCs (including failures) was significantly different between wild-type (688 ± 142 pA) and knockout synapses (455 ± 92 pA; 22 and 28 pairs; P < 0.05). To assess whether the difference was generated presynaptically, we examined the number of failures and the coefficient of variation. The percentage of failures was 1 ± 1% in wild-type synapses but 5 ± 1% in synaptotagmin 1-deficient synapses (P < 0.005; Fig. 2A). Likewise, the coefficient of variation of the IPSC peak amplitude (defined as the standard deviation divided by the mean) was 0.29 ± 0.03 in wild-type synapses but 0.53 ± 0.04 in knockout mice (P < 0.001; Fig. 2A). To distinguish between a difference in the number of release sites and the probability of transmitter release, we further examined the skewness of the IPSC amplitude distribution in the two conditions. The skewness of the IPSC peak amplitude was −0.16 ± 0.11 in wild-type cultures versus 0.28 ± 0.08 in knockout cultures. This shift from a left-skewed to a right-skewed amplitude distribution was consistent with a reduction in release probability (P < 0.005; Fig. 2A). Taken together, these results indicate that genetic elimination of synaptotagmin 1 leads to a reduction in the probability of synchronous transmitter release at inhibitory BC–GC synapses.
Fig. 2.
Reduction in release probability and depression but maintenance of synchronous transmitter release at inhibitory BC–GC synapses in synaptotagmin 1-deficient mice. (A) Summary bar graphs of the unitary IPSC amplitude (including failures), percentage of failures, coefficient of variation (CV), and skewness of IPSC peak amplitudes in wild-type (left bar, open circles) and synaptotagmin 1-deficient mice (right bar, filled circles). Bars represent mean values, circles show data from individual experiments. Data are from 22 and 28 pairs, respectively. (B) Onset of multiple-pulse depression of IPSCs during a 50-Hz train of 10 APs. (Upper) Representative average IPSC traces from a wild-type and a knockout synapse. (Lower) Plot of normalized IPSC amplitude (IPSCn/IPSC1) against IPSC number for wild-type (open circles) and knockout (filled circles) synapses. Inset shows measurement of peak amplitude of second and subsequent IPSCs after iterative subtraction of fitted decay phases of preceding IPSCs. Data are from 10 and 12 pairs. (C) (Left) BC–GC IPSCs in reduced Ca2+ to Mg2+ concentration ratio in the bath. Upper traces, presynaptic APs; lower traces, 10 consecutive IPSCs. Average trace is shown in green. (Right) Histogram of first quantal latency (open bars) and corresponding time course of release (filled bars), calculated by using the method of Barrett and Stevens (see SI Text). Data from two pairs (wild-type, Upper; knockout, Lower). Number of events was 151 and 228, respectively; [Ca2+] = 0.5 mM/[Mg2+] = 2.5 mM in wild-type synapses and [Ca2+] = 1 mM/[Mg2+] = 2 mM in knockout synapses to obtain a similar proportion of failures (24% in both cases). (D) Average time course of release. Data were horizontally aligned to the peak release rate for each cell, normalized vertically, and averaged. Data are from five and four pairs. Red curve, single exponential function fitted to the decay.
Furthermore, we examined whether the genetic elimination of synaptotagmin 1 alters synaptic dynamics during repetitive stimulation at inhibitory BC–GC synapses (Fig. 2B). In wild-type synapses, a 50-Hz train of 10 APs induced robust depression with a rapidly decaying component, followed by a more sustained component, as shown previously in acute slices (4). On average, the ratio of peak amplitudes of IPSC10 over IPSC1 in the train was 0.19 ± 0.02 (10 pairs). In contrast, in synaptotagmin 1-deficient mice, the same paradigm led to less profound and more variable depression; the average ratio of IPSC10 over IPSC1 was 0.54 ± 0.05 (12 pairs; P < 0.002). Thus, genetic elimination of synaptotagmin 1 significantly reduced fast depression at inhibitory BC–GC synapses.
Finally, we made a quantitative comparison of the synchrony of transmitter release at inhibitory BC–GC synapses between wild-type and synaptotagmin 1-deficient mice (Fig. 2 C and D). To accurately measure the time course of release, we reduced release probability by reducing the Ca2+/Mg2+ concentration ratio in the bath solution. We measured the distribution of latencies to the onset of the first IPSC in each trace and subsequently converted it into the time course of release (see Methods). On average, the IPSC latency was almost identical, 1.41 ± 0.08 ms in wild-type mice (five pairs) and 1.47 ± 0.15 ms in synaptotagmin 1-deficient mice (four pairs; P > 0.5). To further analyze the shape of the time course of release, data were horizontally aligned to the peak release rate for each cell and averaged across cells. The decay was fitted with a single exponential function, which gave time constants of 0.33 ms in wild-type synapses and 0.28 ms in knockout synapses (P < 0.05). Thus, the high synchrony of transmitter release at the inhibitory BC–GC synapse was preserved, or even slightly enhanced, after genetic elimination of synaptotagmin 1.
Effects of Synaptotagmin 1 Elimination on Synchronous and Asynchronous Release at Excitatory and Inhibitory Terminals.
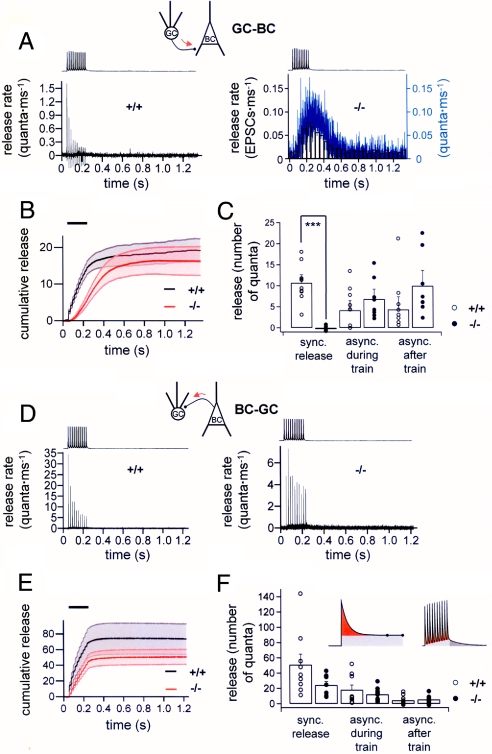
Synaptotagmins have been proposed to act either as triggers (14) or synchronizers (28, 29) of transmitter release. To distinguish between these possibilities, we quantified the amount of synchronous and asynchronous release during and after 50-Hz trains of 10 APs by deconvolution (6, 30, 31) (Fig. 3).
Fig. 3.
Genetic elimination of synaptotagmin 1 preferentially decreases synchronous release but has little effect on asynchronous release. (A) Release rate from a GC–BC pair in a wild-type (Left) and a synaptotagmin 1-deficient (Right) mouse. Black and blue curves represent release rate obtained by deconvolution (filtered digitally at 0.4 kHz); histogram is release rate obtained by direct counting of EPSCs. (B) Average cumulative release in GC–BC pairs (black curve, wild-type; red curve, knockout; stepwise increases in wild-type corresponding to synchronous release components). (C) Total amount of synchronous release, asynchronous release during the train, and asynchronous release after the train at excitatory GC–BC synapses in wild-type (open circles) and synaptotagmin 1-deficient (filled circles) mice; 10 and 8 pairs, respectively. (D) Release rate obtained by deconvolution (filtered digitally at 4 kHz) from a BC–GC pair in a wild-type (Left) and a synaptotagmin 1-deficient (Right) mouse. (E) Average cumulative release in BC–GC pairs (black curve, wild-type; red curve, knockout). (F) Total amount of synchronous release, asynchronous release during the train, and asynchronous release after the train at inhibitory BC–GC synapses in wild-type (open circles) and synaptotagmin 1-deficient (filled circles) mice (9 pairs per condition). Insets schematically illustrate the quantification of synchronous release (red), asynchronous release during train (light gray; interval for quantification indicated by points), and asynchronous release after train (dark gray). Fifty–Hertz trains of 10 presynaptic APs were used in all experiments (traces on top in A and D, horizontal bars in B and E).
For excitatory GC–BC synapses in wild-type mice, the quantal content for synchronous release for a 50-Hz train of 10 APs was 10.7 ± 1.9, whereas the quantal content for asynchronous release was 4.2 ± 2.3 during the train and 4.4 ± 3.0 after the train (10 pairs). For excitatory GC–BC synapses in synaptotagmin 1-deficient mice, the number of synchronously released quanta for the AP train was ≈0 (−0.3 ± 0.4), whereas the number of asynchronously released quanta was 6.8 ± 2.3 during the train and 10.0 ± 3.6 after the train (eight pairs; Fig. 3 A–C). Thus, elimination of synaptotagmin 1 abolished synchronous release at excitatory GC–BC synapses (P < 0.001), whereas asynchronous release during and after the train were not significantly changed (P > 0.4 and P > 0.1, respectively).
For inhibitory BC–GC synapses in wild-type mice, the quantal content for synchronous release for a 50-Hz train of 10 APs was 51.0 ± 13.8, whereas the quantal content for asynchronous release was 18.8 ± 6.3 during the train and 4.5 ± 2.0 after the train (nine pairs). For inhibitory BC–GC synapses in synaptotagmin 1-deficient mice, the number of synchronously released quanta for the AP train was 21.9 ± 4.1, whereas the number of asynchronously released quanta was 11.7 ± 2.4 during the train and 6.9 ± 1.9 after the train (nine pairs; Fig. 3 D–F). Asynchronous release both during and after the train was not significantly affected at knockout synapses (P > 0.5). These results may argue against a synchronizing function and are more consistent with a trigger function of synaptotagmin 1.
Increase in Miniature EPSC and IPSC Frequency in Synaptotagmin 1-Deficient Mice.
Synaptotagmins have been proposed to act as “fusion clamps,” providing inhibitory control of spontaneous transmitter release (32). To address this possibility at identified synapses, we examined the amplitude and frequency of miniature EPSCs (mEPSCs) in BCs and miniature IPSCs (mIPSCs) in GCs after blocking APs with 1 μM tetrodotoxin (TTX) (Fig. 4). For both mEPSCs and mIPSCs, the kinetic parameters of the template used for detection were chosen to mimic the kinetics of evoked synaptic events to maximize the likelihood that evoked and miniature postsynaptic currents (PSCs) were generated at the same synapses. Whereas the mean peak amplitude of mEPSCs and mIPSC was not significantly different between wild type and knockout (150.4 ± 10.9 pA versus 161.4 ± 12.3 pA for mEPSCs; 108.8 ± 8.1 pA versus 133.2 ± 8.3 pA for mIPSCs; P > 0.5), the frequency of both types of events was markedly altered. The frequency of mEPSCs in BCs was ≈3.1-fold larger in synaptotagmin 1-deficient than in wild-type mice (3.43 ± 0.76 Hz in wild type versus 10.57 ± 0.91 Hz in knockout; 6 and 10 cells, respectively; P < 0.005; Fig. 4 A–C). Furthermore, the frequency of mIPSCs in GCs was ≈12.3-fold larger (0.73 ± 0.25 Hz in wild type versus 8.99 ± 0.67 Hz in knockout; 7 and 8 cells, respectively; P < 0.005; Fig. 4 D–F). Thus, genetic elimination of synaptotagmin 1 markedly increased the frequency of miniature PSCs at both excitatory and inhibitory synapses, consistent with the hypothesis that synaptotagmin 1 acts as a clamp for spontaneous fusion.
Fig. 4.
Increase of mEPSC and mIPSC frequency in synaptotagmin 1-deficient mice. (A) Overlay of 20 consecutive original traces of mEPSCs recorded in a BC at −70 mV under control conditions in a wild-type (upper traces) and a synaptotagmin 1-deficient mouse (lower traces). Inset shows single representative mEPSC from wild-type culture (Scale bars: 50 pA, 5 ms.) (B) Cumulative histograms of mEPSC interevent interval (black curve, wild-type; red curve, knockout). Data in A and B were obtained from the same cells. (C) Summary bar graph for mean frequency and peak amplitude of mEPSCs in wild-type (open circles) and knockout (filled circles) mice. Data are from 6 and 10 BCs, respectively. Bars represent mean values, circles show data from individual experiments. In all experiments, 1 μM TTX and 10 μM bicuculline were added to the bath solution. (D–F) Original traces of mIPSCs recorded in a GC at −80 mV (D), histogram of interevent interval and peak amplitude (E), and summary bar graphs for mean frequency and peak amplitude of mIPSCs (F). Data from 7 and 8 GCs, respectively. Inset in D shows single representative mIPSC from wild-type culture (Scale bars: 50 pA, 20 ms). In all experiments, 1 μM TTX, 10 μM CNQX, and 20 μM D-AP5 were added to the bath solution.
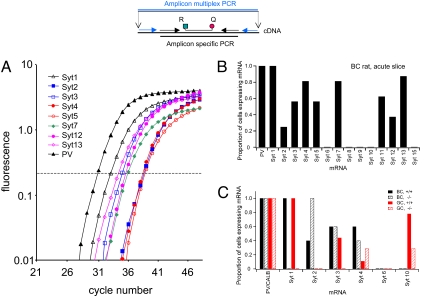
Reverse-Transcription Quantitative PCR (RT-qPCR) Analysis Reveals That Multiple Synaptotagmin Isoforms Are Coexpressed in Single BCs.
Our results indicate that synaptotagmin 1 is essential for synchronous transmitter release at excitatory GC–BC synapses but is not at inhibitory BC–GC synapses, where another Ca2+ sensor appears to be involved. To identify molecular candidates for such an alternative Ca2+ sensor, we analyzed the expression of several synaptotagmins in parvalbumin-expressing BCs in acute slices by single-cell RT-qPCR analysis (Fig. 5). RT-qPCR analysis revealed that single BCs coexpressed multiple synaptotagmins. In addition to synaptotagmin 1, subpopulations of BCs expressed synaptotagmins 2, 3, 4, 5, 7, 11, 12, and 13 (16 cells; Fig. 5 A and B). To link the analysis of expression pattern and synaptic function more directly, we also tested the expression of selected synaptotagmin isoforms in organotypic cultures from wild-type and knockout animals. RT-qPCR analysis confirmed that synaptotagmin 1 was present in both BCs and GCs from wild-type cultures (five and nine cells, respectively) but was missing from knockout cultures (five BCs and seven GCs). The expression level of the other synaptotagmins was similar between wild type and knockout, except for synaptotagmin 2, which was detected in all BCs of the knockout but only in a fraction of wild-type BCs. In contrast, neither wild-type nor knockout GCs were found to express synaptotagmin 2. Thus, our results suggest that BCs coexpress multiple synaptotagmins that may act as alternative Ca2+ sensors at inhibitory BC–GC synapses. For further details, see Tables S3 and S4.
Fig. 5.
Single-cell RT-qPCR analysis reveals that multiple synaptotagmin isoforms are coexpressed in single BCs. (A) Semilogarithmic plot of fluorescence versus cycle number in the qPCR, showing coexpression of several synaptotagmins and parvalbumin (PV) in a single BC from an acute rat brain slice. Horizontal dashed line indicates threshold used for quantification. (B and C) Bar graph of the proportion of cells expressing mRNA for several synaptotagmin isoforms in acute hippocampal slices of rats (B shows data from 16 BCs) and in organotypic slice cultures obtained from wild-type and synaptotagmin 1-deficient mice (C shows data from 5 +/+ BCs, 5 −/− BCs, 9 +/+ GCs, and 7 −/− GCs). Cells were only included if they expressed the specific marker (PV for BCs and calbindin, CALB, for GCs). Inset on top shows location of primers for multiplex PCR (blue), primers for qPCR (black), and fluorescent probe for qPCR (R, reporter dye; Q, quencher).
Discussion
Here, we examined the functional role of synaptotagmin 1 in transmitter release from identified excitatory and inhibitory synapses in the hippocampus. Analysis of identified synapses is particularly important for inhibitory synapses, which markedly differ in functional properties, depending on the presynaptic interneuron subtype (6). The main finding is that the excitatory input and the inhibitory output of BCs depend differentially on synaptotagmin 1 (Table S1 and Fig. S3).
Our results show that synaptotagmin 1 is not absolutely required for fast transmitter release at GABAergic synapses. However, deletion of synaptotagmin 1 increased the proportion of failures, increased the coefficient of variation, and increased the skewness of BC–GC IPSCs, consistent with a reduction in release probability (Fig. 2A). Thus, synaptotagmin 1 is involved in fast transmitter release, although it is not the only Ca2+ sensor at this synapse.
Single-cell RT-qPCR analysis revealed multiple candidates for alternative Ca2+ sensors in BCs. In addition to synaptotagmin 1, BCs expressed synaptotagmin 2, 3, 4, 5, 7, 11, 12, and 13 (Fig. 5). Several results may be consistent with the hypothesis that synaptotagmin 2 is the alternative Ca2+ sensor at the BC–GC synapse. First, synaptotagmin 2 mRNA was detected in 30/40% of BCs in wild-type animals (rats and mice, respectively) and in 100% of BCs in synaptotagmin 1-deficient mice. Second, recombinant expression of synaptotagmin revealed that synaptotagmin 2 can act as a fast Ca2+ sensor and functionally replace synaptotagmin 1 (16). Finally, expression of synaptotagmin 2 mRNA and protein was previously demonstrated in scattered neurons in the hilus (20, 33) and immunolabeling with the antibody znp-1 (presumably recognizing synaptotagmin 2) was found in presynaptic terminals located on somata of CA1 hippocampal pyramidal neurons (34).
However, it is possible that other synaptotagmins form the alternative Ca2+ sensor in BCs. As a single synaptic vesicle contains ≈15 synaptotagmin molecules (35), the formation of heterooligomeric assemblies may be an additional complication. Finally, we cannot exclude that an entirely different protein triggers release at the BC–GC synapse in knockout mice, as recently suggested for auditory hair cells (36) and the calyx of Held (37).
Genetic elimination of synaptotagmin 1 markedly reduced synaptic depression at the inhibitory BC–GC synapse (Fig. 2B). One possible explanation is that this effect on depression was indirect, arising as a consequence of the reduction in release probability. However, this is unlikely, because reduction of release probability by change of the extracellular Ca2+ concentration has only subtle effects on depression at this synapse (4). Another possibility is that the marked increase in the frequency of mIPSCs from ≈1 Hz to ≈9 Hz leads to a partial depression of transmitter release, such that the depression of evoked release became partially occluded. However, this hypothesis is incompatible with divergence and convergence in BC–GC microcircuits (38). If the dentate gyrus contains ≈20,000 BCs and ≈1,000,000 GCs, and if a BC contacts ≈2,500 GCs, a GC would receive convergent input from ≈50 BCs. Thus, the mIPSC frequency of ≈10 Hz observed in the synaptotagmin 1-deficient mice would correspond to only ≈0.2 Hz per synaptic connection. With a time constant of recovery from depression of ≈2 s (at 34°C) (4), this provides sufficient time for recovery from depression.
An alternative explanation for the reduced synaptic depression in the synaptotagmin 1 knockout mouse is that the readily releasable pools of synaptic vesicles in BC terminals consist of two subpools, which correspond to the two phases of synaptic depression (4). In this scenario, the first pool depresses rapidly and depends on synaptotagmin 1. In contrast, the second pool depresses slowly and is independent of synaptotagmin 1, but depends on another Ca2+ sensor. A similar scenario was reported in chromaffin cells, in which genetic elimination of synaptotagmin 1 eliminated release from the readily releasable pool of dense-core vesicles but left release from the slowly releasable pool unaffected (39). Likewise, in the calyx of Held, a fast and a slow vesicular pool can be distinguished. Both positional heterogeneity [i.e., heterogeneity in the distance between Ca2+ source and Ca2+ sensor (40)] and intrinsic heterogeneity [i.e., heterogeneity in the sensitivity of the Ca2+ sensor (41)] may contribute to the distinct properties of the two pools. Whether fast and slow pools at the calyx of Held depend on different synaptotagmin isoforms, however, remains to be determined.
How synaptotagmins contribute to synchronous transmitter release has remained enigmatic. According to one view, synaptotagmins are positive regulators of exocytosis, acting as triggers for vesicle fusion. This view is supported by the original observation that genetic elimination of synaptotagmin 1 selectively abolished synchronous release, but left asynchronous release unchanged (14, 15). This would be consistent with a model in which synaptotagmin 1 triggers synchronous release, whereas another Ca2+ sensor triggers asynchronous release. However, other studies suggested that synaptotagmin synchronizes release rather than controlling the total amount of release, leading to a redistribution between early and late release phases after an AP (28, 29). We found that the amount of asynchronous release at excitatory GC–BC synapses was only slightly increased, whereas the amount of asynchronous release at inhibitory BC–GC synapses was slightly reduced in synaptotagmin 1-deficient mice, with none of the differences being statistically significant with the nonparametric tests used (Fig. 3). Thus, our results may be more consistent with a trigger than with a synchronizing function.
It was also suggested that synaptotagmins are negative regulators of exocytosis, acting as “clamps” that prevent the spontaneous fusion of primed vesicles. Our results clearly show a marked increase in the frequency of both mEPSCs and mIPSCs in knockout mice (Fig. 4). Because the synaptic connection probability was comparable between wild-type and synaptotagmin 1-deficient mice, it is unlikely that this increase in miniature PSC frequency is caused by a change in synaptic connectivity. The origin of the miniature PSCs is unknown. However, it is likely that a large subset of events were generated at GC–BC and BC–GC synapses, respectively, because templates with kinetic parameters that mimicked those of evoked PSCs were used for detection. Thus, our results suggest that at both excitatory GC–BC synapses and inhibitory BC–GC synapses, synaptotagmin 1 has both a trigger function for evoked release and a clamp function for spontaneous release. The clamp function of synaptotagmin could be direct or indirect. For example, synaptotagmin may alter binding of complexins to the SNARE complex (32).
Our results suggest that the effects of synaptotagmin 1 elimination on evoked and spontaneous transmitter release are not strictly parallel. At excitatory GC–BC synapses, elimination of synaptotagmin 1 may have more dramatic effects on synchronous evoked release than on miniature release. In contrast, at inhibitory BC–GC synapses, deletion of synaptotagmin 1 may have stronger effects on miniature release than on synchronous evoked release. This would imply that the remaining non-synaptotagmin-1 sensors have a weaker clamping efficacy than synaptotagmin 1 itself (Fig. S3).
Previous studies revealed that several molecular specializations converge on rapid signaling in BC output synapses. First, BCs exclusively use P/Q-type Ca2+ channels to initiate transmitter release (6). Because P/Q-type Ca2+ channels activate and deactivate very rapidly (42), this specialization will contribute to the high speed of synaptic transmission. Second, Ca2+ channels and Ca2+ sensors of exocytosis are tightly coupled at BC output synapses, leading to a fast and temporally precise local Ca2+ transient at the sensor (7). Finally, as shown in the present article, BC output synapses use alternative Ca2+ sensors in addition to synaptotagmin 1. Measurements of synaptotagmin–liposome disassembly kinetics with fluorescence resonance energy transfer indicated that among all synaptotagmins, synaptotagmin 1, 2, and 3 have fast Ca2+-unbinding rates (21). Furthermore, experiments on transfected neurons in culture revealed that synaptotagmin 1, 2, and 5 can support fast transmitter release, with synaptotagmin 2 generating the fastest time course (16). Because synaptotagmin 2, 3, and 5 are expressed in BCs, these results are consistent with the idea that alternative, non-synaptotagmin-1 Ca2+ sensors may contribute to the high speed of transmission at BC output synapses and, hence, to the rapid time course of feed-forward and feedback inhibition in the hippocampus (1).
Methods
Details of the methods are specified in SI Text. In brief, Stoppini-type interface organotypic slice cultures (25) were prepared from the brains of wild-type or synaptotagmin 1 knockout newborn mice (14) and examined after 16–29 days in vitro. Evoked PSCs were studied by using paired recordings from BCs and GCs in the dentate gyrus (3, 4). Miniature PSCs were recorded in BCs and GCs in the presence of 1 μM TTX. The recording temperature was ≈24°C. Single-cell expression analysis was performed by using reverse transcription, followed by a multiplex PCR and quantitative PCR (RT-qPCR) (27). Evoked PSCs were analyzed as described previously for synapses in acute slices (3, 4, 6). Data are given as mean ± SEM. Statistical significance was assessed using nonparametric tests.
Supplementary Material
Acknowledgments.
We thank J. Bischofberger, J. Behrends, and L. Li for critically reading the manuscript; R. Jahn (Max Planck Institute for Biophysical Chemistry, Göttingen, Germany) and T. Südhof (HHMI Department of Neuroscience, University of Texas Southwestern Medical Center, Dallas, TX) for providing antibodies; M. Heckmann for help with the slice culture; B. Fakler for support; and S. Becherer, I. Koeva, M. Northemann, L. Thirimanna, S. Thomsen, and K. Winterhalter for technical assistance. This work was supported by Deutsche Forschungsgemeinschaft Grants SFB 505, SFB 780, GRK 843, and the Leibniz Program.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800621105/DCSupplemental.
References
- 1.Pouille F, Scanziani M. Routing of spike series by dynamic circuits in the hippocampus. Nature. 2004;429:717–723. doi: 10.1038/nature02615. [DOI] [PubMed] [Google Scholar]
- 2.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 3.Geiger JRP, Lübke J, Roth A, Frotscher M, Jonas P. Submillisecond AMPA receptor-mediated signaling at a principal neuron–interneuron synapse. Neuron. 1997;18:1009–1023. doi: 10.1016/s0896-6273(00)80339-6. [DOI] [PubMed] [Google Scholar]
- 4.Kraushaar U, Jonas P. Efficacy and stability of quantal GABA release at a hippocampal interneuron–principal neuron synapse. J Neurosci. 2000;20:5594–5607. doi: 10.1523/JNEUROSCI.20-15-05594.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- 6.Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron–principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- 7.Bucurenciu I, Kulik A, Schwaller B, Frotscher M, Jonas P. Nanodomain coupling between Ca2+ channels and Ca2+ sensors promotes fast and efficient transmitter release at a cortical GABAergic synapse. Neuron. 2008;57:536–545. doi: 10.1016/j.neuron.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Brose N, Petrenko AG, Südhof TC, Jahn R. Synaptotagmin: A calcium sensor on the synaptic vesicle surface. Science. 1992;256:1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- 9.Südhof TC. Synaptotagmins: Why so many? J Biol Chem. 2002;277:7629–7632. doi: 10.1074/jbc.R100052200. [DOI] [PubMed] [Google Scholar]
- 10.Chapman ER. Synaptotagmin: A Ca2+ sensor that triggers exocytosis? Nat Rev Mol Cell Biol. 2002;3:498–508. doi: 10.1038/nrm855. [DOI] [PubMed] [Google Scholar]
- 11.Koh TW, Bellen HJ. Synaptotagmin I, a Ca2+ sensor for neurotransmitter release. Trends Neurosci. 2003;26:413–422. doi: 10.1016/S0166-2236(03)00195-4. [DOI] [PubMed] [Google Scholar]
- 12.Littleton JT, Stern M, Schulze K, Perin M, Bellen HJ. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca2+ activated neurotransmitter release. Cell. 1993;74:1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- 13.Nonet ML, Grundahl K, Meyer BJ, Rand JB. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell. 1993;73:1291–1305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- 14.Geppert M, et al. Synaptotagmin I: A major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 15.Maximov A, Südhof TC. Autonomous function of synaptotagmin 1 in triggering synchronous release independent of asynchronous release. Neuron. 2005;48:547–554. doi: 10.1016/j.neuron.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, Mashimo T, Südhof TC. Synaptotagmin-1, -2, and -9: Ca2+ sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–581. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Chacón R, et al. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 18.Rhee JS, et al. Augmenting neurotransmitter release by enhancing the apparent Ca2+ affinity of synaptotagmin 1. Proc Natl Acad Sci USA. 2005;102:18664–18669. doi: 10.1073/pnas.0509153102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ullrich B, et al. Functional properties of multiple synaptotagmins in brain. Neuron. 1994;13:1281–1291. doi: 10.1016/0896-6273(94)90415-4. [DOI] [PubMed] [Google Scholar]
- 20.Marquèze B, et al. Cellular localization of synaptotagmin I, II, and III mRNAs in the central nervous system and pituitary and adrenal glands of the rat. J Neurosci. 1995;15:4906–4917. doi: 10.1523/JNEUROSCI.15-07-04906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui E, et al. Three distinct kinetic groupings of the synaptotagmin family: candidate sensors for rapid and delayed exocytosis. Proc Natl Acad Sci USA. 2005;102:5210–5214. doi: 10.1073/pnas.0500941102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goda Y, Stevens CF. Two components of transmitter release at a central synapse. Proc Natl Acad Sci USA. 1994;91:12942–12946. doi: 10.1073/pnas.91.26.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atluri PP, Regehr WG. Delayed release of neurotransmitter from cerebellar granule cells. J Neurosci. 1998;18:8214–8227. doi: 10.1523/JNEUROSCI.18-20-08214.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu T, Trussell LO. Inhibitory transmission mediated by asynchronous transmitter release. Neuron. 2000;26:683–694. doi: 10.1016/s0896-6273(00)81204-0. [DOI] [PubMed] [Google Scholar]
- 25.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 26.Rudy B, McBain CJ. Kv3 channels: Voltage-gated K+ channels designed for high-frequency repetitive firing. Trends Neurosci. 2001;24:517–526. doi: 10.1016/s0166-2236(00)01892-0. [DOI] [PubMed] [Google Scholar]
- 27.Aponte Y, Lien CC, Reisinger E, Jonas P. Hyperpolarization-activated cation channels in fast-spiking interneurons of rat hippocampus. J Physiol (London) 2006;574:229–243. doi: 10.1113/jphysiol.2005.104042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishiki T, Augustine GJ. Synaptotagmin I synchronizes transmitter release in mouse hippocampal neurons. J Neurosci. 2004;24:6127–6132. doi: 10.1523/JNEUROSCI.1563-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshihara M, Littleton JT. Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron. 2002;36:897–908. doi: 10.1016/s0896-6273(02)01065-6. [DOI] [PubMed] [Google Scholar]
- 30.Diamond JS, Jahr CE. Asynchronous release of synaptic vesicles determines the time course of the AMPA receptor-mediated EPSC. Neuron. 1995;15:1097–1107. doi: 10.1016/0896-6273(95)90098-5. [DOI] [PubMed] [Google Scholar]
- 31.Neher E, Sakaba T. Combining deconvolution and noise analysis for the estimation of transmitter release rates at the calyx of Held. J Neurosci. 2001;21:444–461. doi: 10.1523/JNEUROSCI.21-02-00444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giraudo CG, Eng WS, Melia TJ, Rothman JE. A clamping mechanism involved in SNARE-dependent exocytosis. Science. 2006;313:676–680. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- 33.Pang ZP, et al. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J Neurosci. 2006;26:13493–13504. doi: 10.1523/JNEUROSCI.3519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox MA, Sanes JR. Synaptotagmin I and II are present in distinct subsets of central synapses. J Comp Neurol. 2007;503:280–296. doi: 10.1002/cne.21381. [DOI] [PubMed] [Google Scholar]
- 35.Takamori S, et al. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 36.Roux I, et al. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 37.Sun J, et al. A dual-Ca2+-sensor model for neurotransmitter release in a central synapse. Nature. 2007;450:676–682. doi: 10.1038/nature06308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patton PE, McNaughton B. Connection matrix of the hippocampal formation: I. The dentate gyrus. Hippocampus. 1995;5:245–286. doi: 10.1002/hipo.450050402. [DOI] [PubMed] [Google Scholar]
- 39.Voets T, et al. Intracellular calcium dependence of large dense-core vesicle exocytosis in the absence of synaptotagmin I. Proc Natl Acad Sci USA. 2001;98:11680–11685. doi: 10.1073/pnas.201398798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wadel K, Neher E, Sakaba T. The coupling between synaptic vesicles and Ca2+ channels determines fast neurotransmitter release. Neuron. 2007;53:563–575. doi: 10.1016/j.neuron.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 41.Wölfel M, Lou X, Schneggenburger R. A mechanism intrinsic to the vesicle fusion machinery determines fast and slow transmitter release at a large CNS synapse. J Neurosci. 2007;27:3198–3210. doi: 10.1523/JNEUROSCI.4471-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li L, Bischofberger J, Jonas P. Differential gating and recruitment of P/Q-, N-, R-type Ca2+ channels in hippocampal mossy fiber boutons. J Neurosci. 2007;27:13420–13429. doi: 10.1523/JNEUROSCI.1709-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.