Abstract
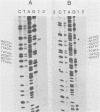
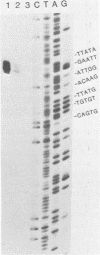
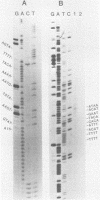
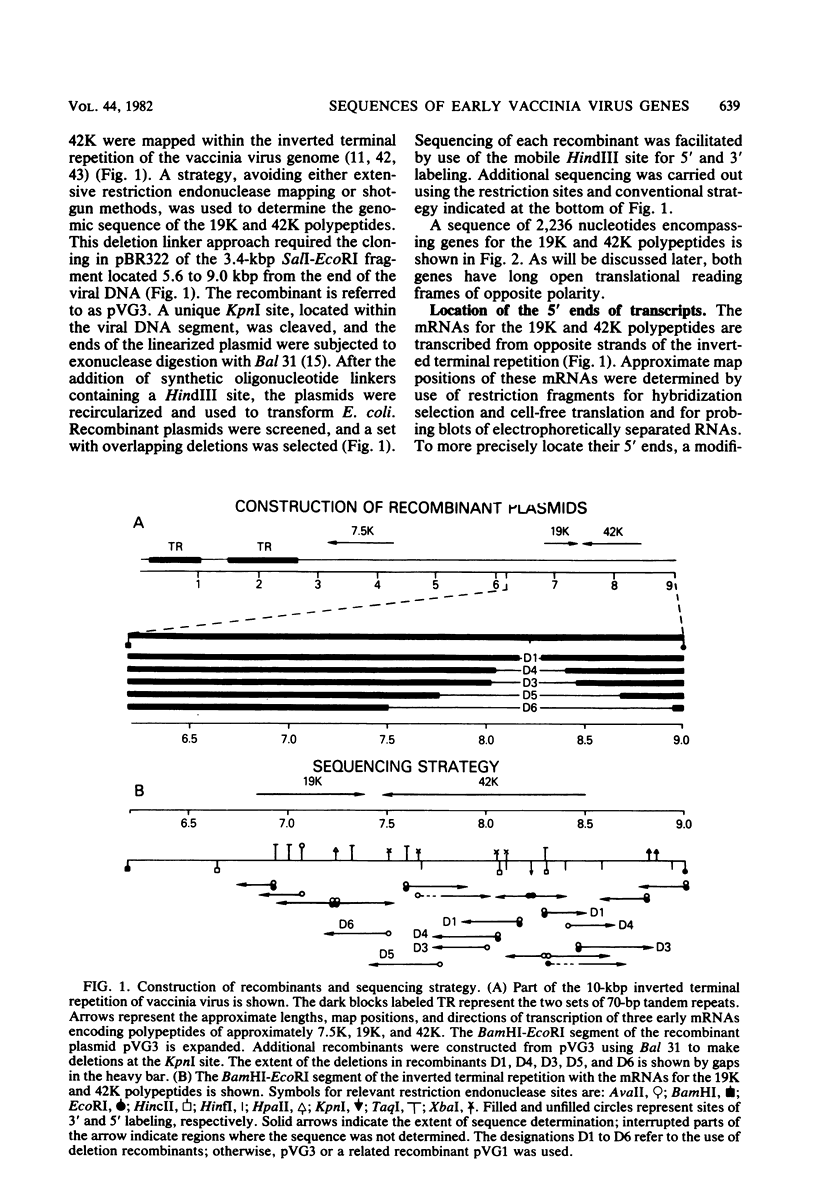
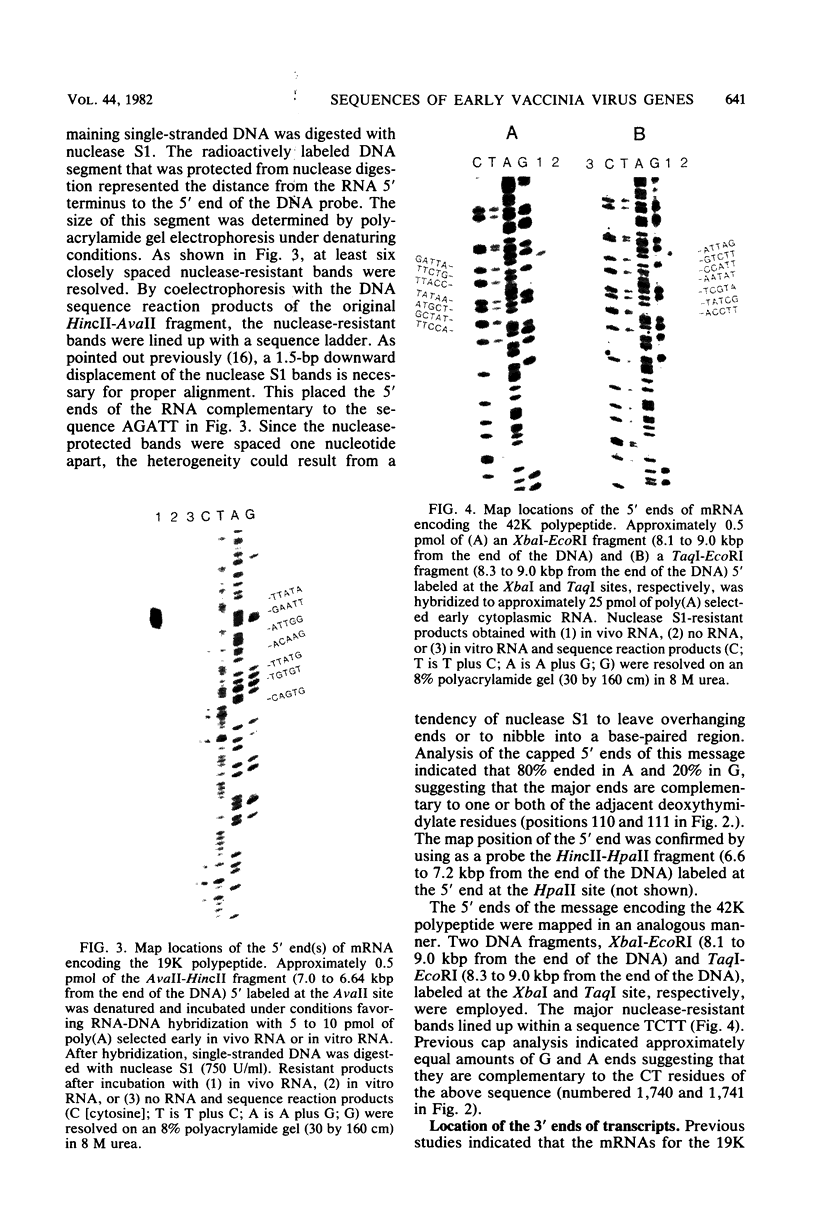
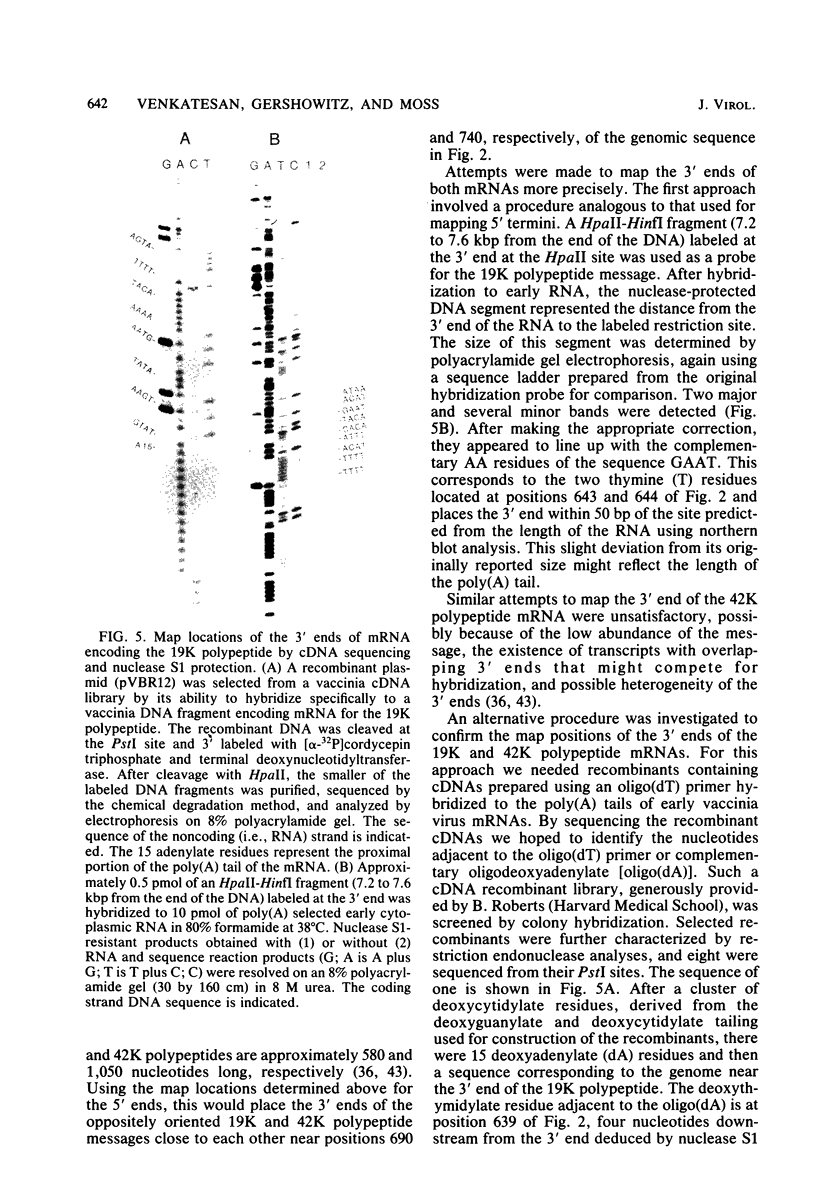
The proximal part of the 10,000-base pair (bp) inverted terminal repetition of vaccinia virus DNA encodes at least three early mRNAs. A 2,236-bp segment of the repetition was sequenced to characterize two of the genes. This task was facilitated by constructing a series of recombinants containing overlapping deletions; oligonucleotide linkers with synthetic restriction sites provided points for radioactive labeling before sequencing by the chemical degradation method of Maxam and Gilbert (Methods Enzymol. 65:499-560, 1980). The ends of the transcripts were mapped by hybridizing labeled DNA fragments to early viral RNA and resolving nuclease S1-protected fragments in sequencing gels, by sequencing cDNA clones, and from the lengths of the RNAs. The nucleotide sequences for at least 60 bp upstream of both transcriptional initiation sites are more than 80% adenine · thymine rich and contain long runs of adenines and thymines with some homology to procaryotic and eucaryotic consensus sequences. The gene transcribed in the rightward direction encodes an RNA of approximately 530 nucleotides with a single open reading frame of 420 nucleotides. Preceding the first AUG, there is a heptanucleotide that can hybridize to the 3′ end of 18S rRNA with only one mismatch. The derived amino acid sequence of the protein indicated a molecular weight of 15,500. The gene transcribed in the leftward direction encodes an RNA 1,000 to 1,100 nucleotides long with an open reading frame of 996 nucleotides and a leader sequence of only 5 to 6 nucleotides. The derived amino acid sequence of this protein indicated a molecular weight of 38,500. The 3′ ends of the two transcripts were located within 100 bp of each other. Although there are adenine · thymine-rich clusters near the putative transcriptional termination sites, specific AATAAA polyadenylic acid signal sequences are absent.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroudy B. M., Moss B. Purification and characterization of a DNA-dependent RNA polymerase from vaccinia virions. J Biol Chem. 1980 May 10;255(9):4372–4380. [PubMed] [Google Scholar]
- Baroudy B. M., Venkatesan S., Moss B. Incompletely base-paired flip-flop terminal loops link the two DNA strands of the vaccinia virus genome into one uninterrupted polynucleotide chain. Cell. 1982 Feb;28(2):315–324. doi: 10.1016/0092-8674(82)90349-x. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Berkner K. L., Folk W. R. Polynucleotide kinase exchange reaction: quantitave assay for restriction endonuclease-generated 5'-phosphoroyl termini in DNA. J Biol Chem. 1977 May 25;252(10):3176–3184. [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Boone R. F., Moss B. Sequence complexity and relative abundance of vaccinia virus mRNA's synthesized in vivo and in vitro. J Virol. 1978 Jun;26(3):554–569. doi: 10.1128/jvi.26.3.554-569.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Cabrera C. V., Esteban M., McCarron R., McAllister W. T., Holowczak J. A. Vaccinia virus transcription: hybridization of mRNA to restriction fragments of vaccinia DNA. Virology. 1978 May 1;86(1):102–114. doi: 10.1016/0042-6822(78)90011-9. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Wittek R., Moss B. Hybridization selection and cell-free translation of mRNA's encoded within the inverted terminal repetition of the vaccinia virus genome. J Virol. 1981 Jan;37(1):284–294. doi: 10.1128/jvi.37.1.284-294.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon C. F., Barbosa E., Moss B. Visualization of an inverted terminal repetition in vaccinia virus DNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4863–4867. doi: 10.1073/pnas.75.10.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady L. J., Paoletti E. Molecular complexity of vaccinia DNA and the presence of reiterated sequences in the genome. Virology. 1977 Jun 15;79(2):337–341. doi: 10.1016/0042-6822(77)90361-0. [DOI] [PubMed] [Google Scholar]
- Gray H. B., Jr, Ostrander D. A., Hodnett J. L., Legerski R. J., Robberson D. L. Extracellular nucleases of Pseudomonas BAL 31. I. Characterization of single strand-specific deoxyriboendonuclease and double-strand deoxyriboexonuclease activities. Nucleic Acids Res. 1975 Sep;2(9):1459–1492. doi: 10.1093/nar/2.9.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Roeder R. G. Definition of a novel promoter for the major adenovirus-associated virus mRNA. Cell. 1980 Nov;22(1 Pt 1):231–242. doi: 10.1016/0092-8674(80)90171-3. [DOI] [PubMed] [Google Scholar]
- Hagenbüchle O., Santer M., Steitz J. A., Mans R. J. Conservation of the primary structure at the 3' end of 18S rRNA from eucaryotic cells. Cell. 1978 Mar;13(3):551–563. doi: 10.1016/0092-8674(78)90328-8. [DOI] [PubMed] [Google Scholar]
- Isle H. B., Venkatesan S., Moss B. Cell-free translation of early and late mRNAs selected by hybridization to cloned DNA fragments derived from the left 14 million to 72 million daltons of the vaccinia virus genome. Virology. 1981 Jul 15;112(1):306–317. doi: 10.1016/0042-6822(81)90636-x. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J., Beeson J. Ribonucleic acid synthesis in vaccinia virus. II. Synthesis of polyriboadenylic acid. J Mol Biol. 1970 May 28;50(1):19–33. doi: 10.1016/0022-2836(70)90101-4. [DOI] [PubMed] [Google Scholar]
- Kaverin N. V., Varich N. L., Surgay V. V., Chernos V. I. A quantitative estimation of poxvirus genome fraction transcribed as "early" and "late" mRNA. Virology. 1975 May;65(1):112–119. doi: 10.1016/0042-6822(75)90011-2. [DOI] [PubMed] [Google Scholar]
- Land H., Grez M., Hauser H., Lindenmaier W., Schütz G. 5'-Terminal sequences of eucaryotic mRNA can be cloned with high efficiency. Nucleic Acids Res. 1981 May 25;9(10):2251–2266. doi: 10.1093/nar/9.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Grace J. T., Jr RNA polymerase activity in purified infectious vaccinia virus. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2280–2287. doi: 10.1073/pnas.58.6.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Isolation and properties of the vaccinia virus DNA-dependent RNA polymerase. J Biol Chem. 1977 Oct 10;252(19):6930–6938. [PubMed] [Google Scholar]
- Nevins J. R., Joklik W. K. Poly (A) sequences of vaccinia virus messenger RNA: nature, mode of addition and function during translation in vitra and in vivo. Virology. 1975 Jan;63(1):1–14. doi: 10.1016/0042-6822(75)90365-7. [DOI] [PubMed] [Google Scholar]
- Oda K. I., Joklik W. K. Hybridization and sedimentation studies on "early" and "late" vaccinia messenger RNA. J Mol Biol. 1967 Aug 14;27(3):395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Queen C. L., Korn L. J. Computer analysis of nucleic acids and proteins. Methods Enzymol. 1980;65(1):595–609. doi: 10.1016/s0076-6879(80)65062-9. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Spencer E., Shuman S., Hurwitz J. Purification and properties of vaccinia virus DNA-dependent RNA polymerase. J Biol Chem. 1980 Jun 10;255(11):5388–5395. [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Tu C. P., Cohen S. N. 3'-end labeling of DNA with [alpha-32P]cordycepin-5'-triphosphate. Gene. 1980 Jul;10(2):177–183. doi: 10.1016/0378-1119(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Baroudy B. M., Moss B. Distinctive nucleotide sequences adjacent to multiple initiation and termination sites of an early vaccinia virus gene. Cell. 1981 Sep;25(3):805–813. doi: 10.1016/0092-8674(81)90188-4. [DOI] [PubMed] [Google Scholar]
- Venkatesan S., Moss B. In vitro transcription of the inverted terminal repetition of the vaccinia virus genome: correspondence of initiation and cap sites. J Virol. 1981 Feb;37(2):738–747. doi: 10.1128/jvi.37.2.738-747.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei C. M., Moss B. Methylated nucleotides block 5'-terminus of vaccinia virus messenger RNA. Proc Natl Acad Sci U S A. 1975 Jan;72(1):318–322. doi: 10.1073/pnas.72.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Bajszár G., Moss B. Mapping of the vaccinia virus thymidine kinase gene by marker rescue and by cell-free translation of selected mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1210–1214. doi: 10.1073/pnas.79.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winberg G., Hammarskjöld M. L. Isolation of DNA from agarose gels using DEAE-paper. Application to restriction site mapping of adenovirus type 16 DNA. Nucleic Acids Res. 1980 Jan 25;8(2):253–264. doi: 10.1093/nar/8.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Barbosa E., Cooper J. A., Garon C. F., Chan H., Moss B. Inverted terminal repetition in vaccinia virus DNA encodes early mRNAs. Nature. 1980 May 1;285(5759):21–25. doi: 10.1038/285021a0. [DOI] [PubMed] [Google Scholar]
- Wittek R., Cooper J. A., Barbosa E., Moss B. Expression of the vaccinia virus genome: analysis and mapping of mRNAs encoded within the inverted terminal repetition. Cell. 1980 Sep;21(2):487–493. doi: 10.1016/0092-8674(80)90485-7. [DOI] [PubMed] [Google Scholar]
- Wittek R., Moss B. Colinearity of RNAs with the vaccinia virus genome: anomalies with two complementary early and late RNAs result from a small deletion or rearrangement within the inverted terminal repetition. J Virol. 1982 May;42(2):447–455. doi: 10.1128/jvi.42.2.447-455.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Moss B. Tandem repeats within the inverted terminal repetition of vaccinia virus DNA. Cell. 1980 Aug;21(1):277–284. doi: 10.1016/0092-8674(80)90135-x. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Hidaka S., Miura K. Relationship between structure of the 5' noncoding region of viral mRNA and efficiency in the initiation step of protein synthesis in a eukaryotic system. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1012–1016. doi: 10.1073/pnas.79.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R. C., Wu R. BK virus DNA sequence coding for the amino-terminus of the T-antigen. Virology. 1979 Jan 30;92(2):340–352. doi: 10.1016/0042-6822(79)90139-9. [DOI] [PubMed] [Google Scholar]