Abstract
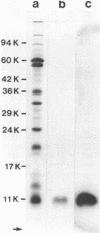
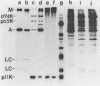
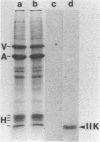
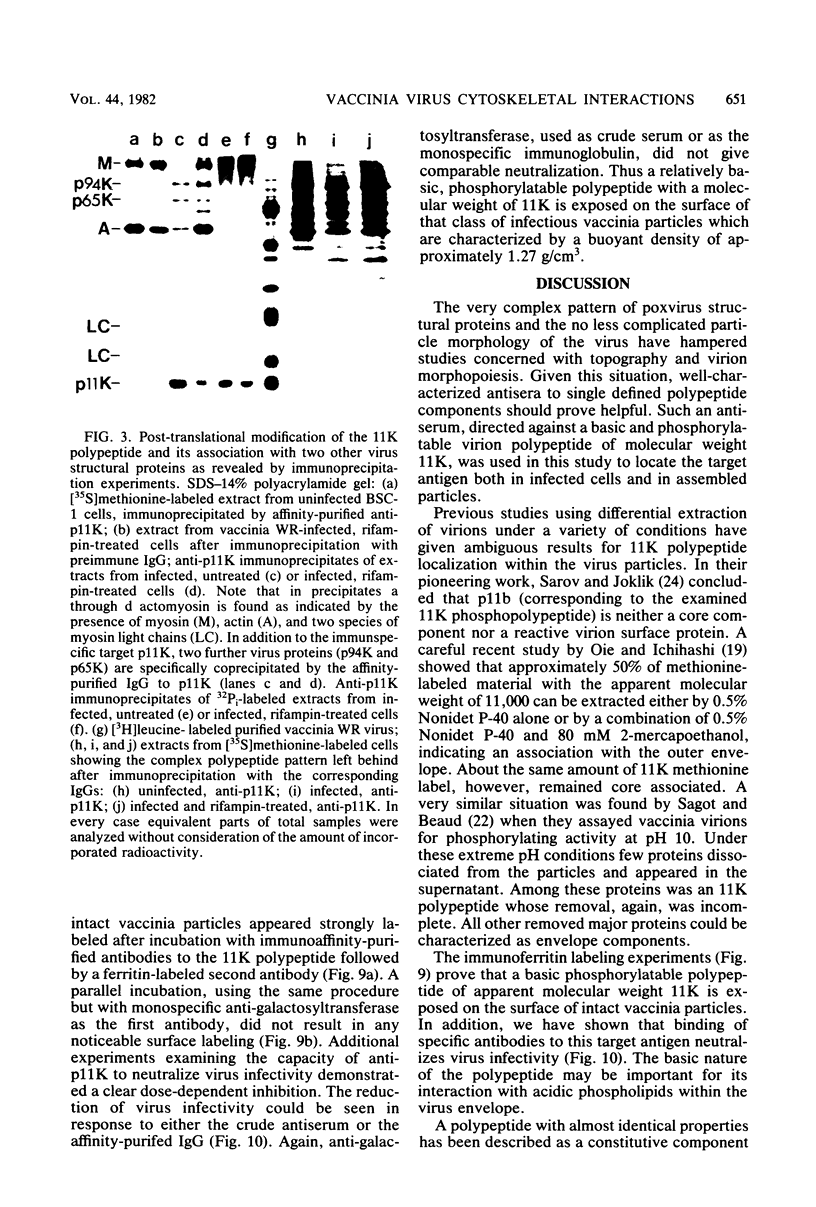
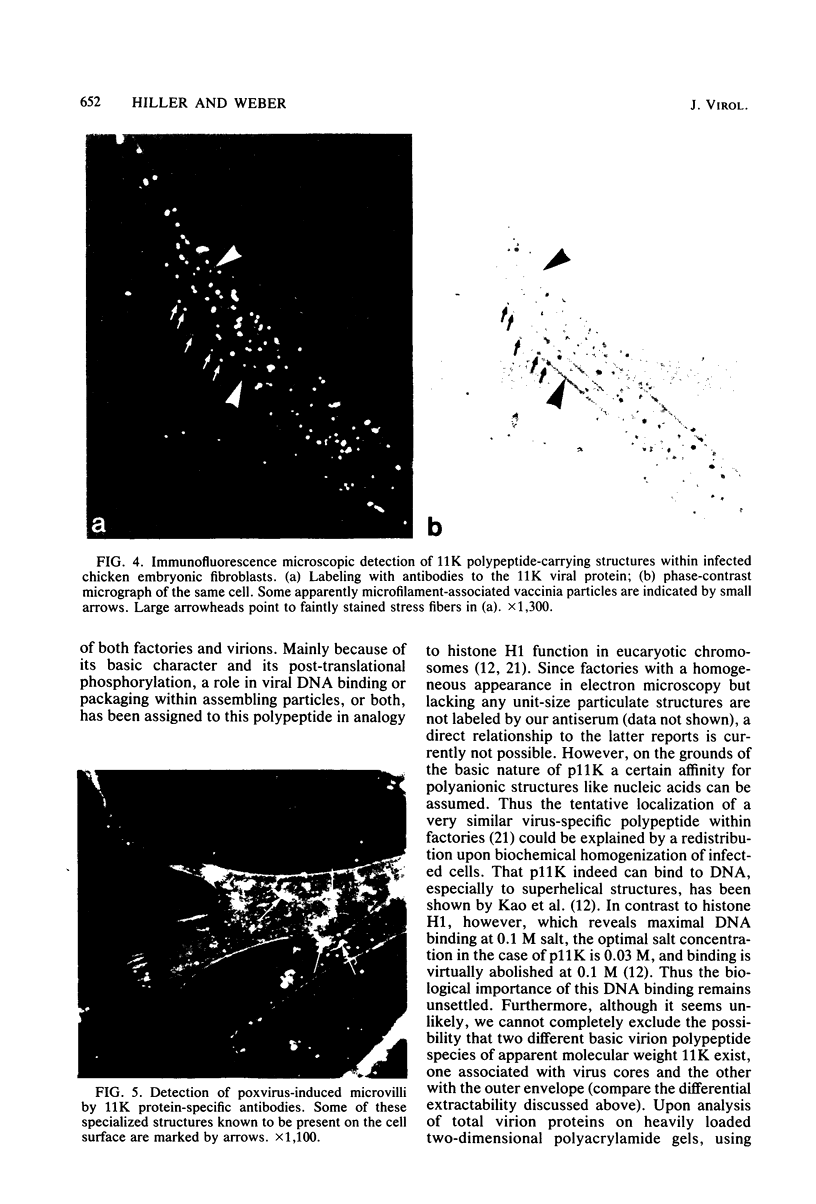
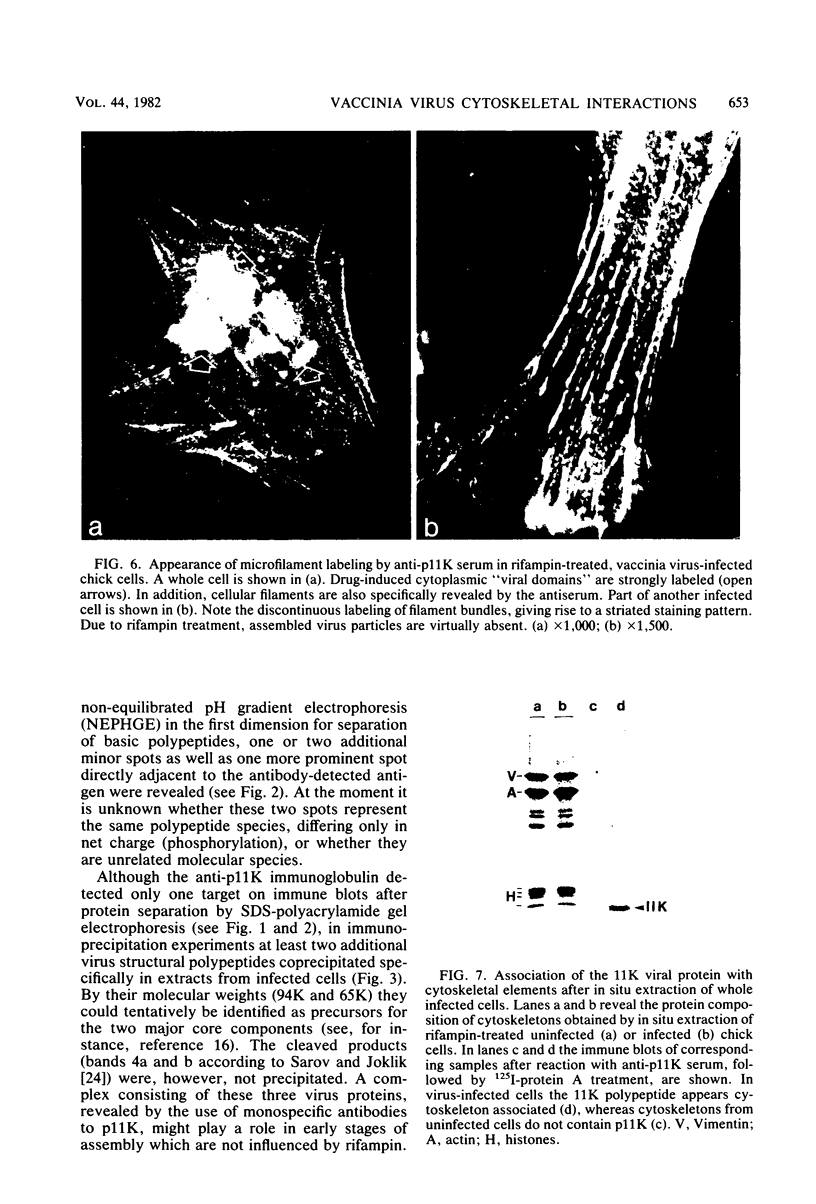
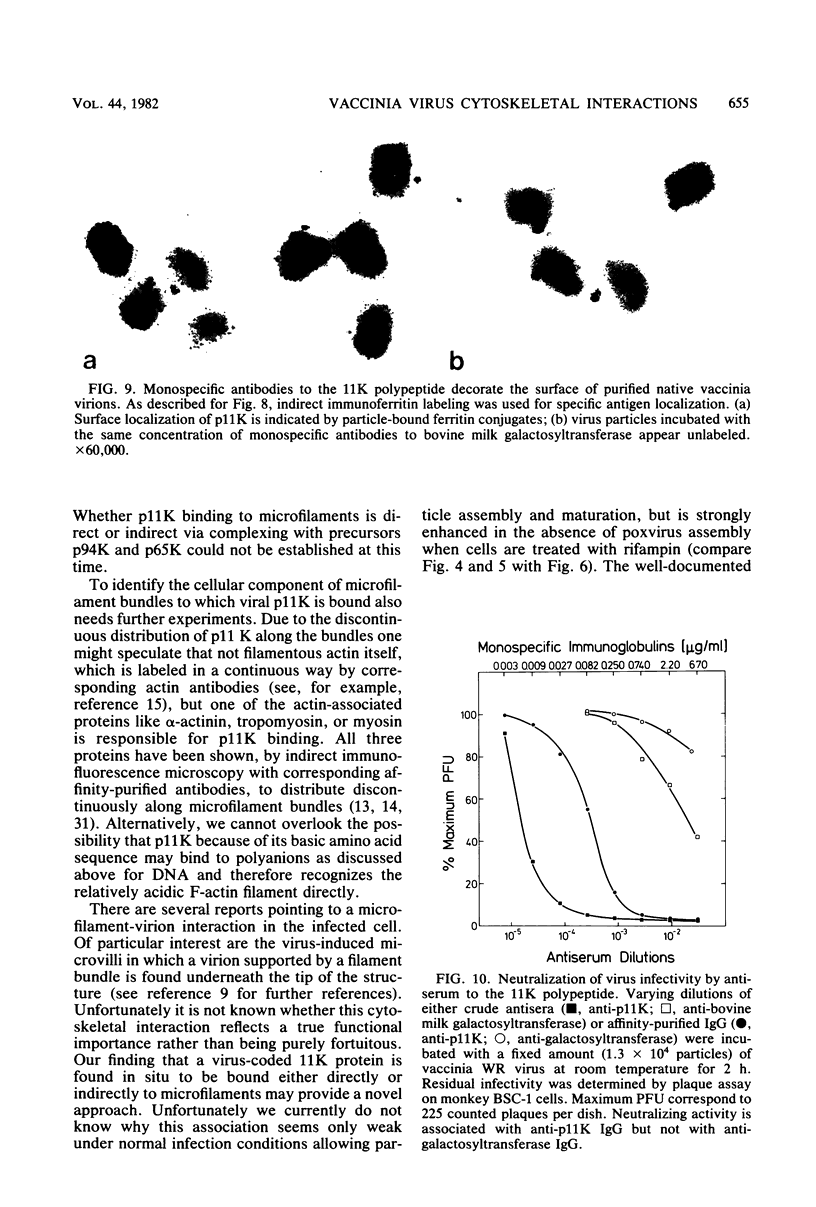
A phosphorylated vaccinia virus structural polypeptide of an apparent molecular weight of 11,000 (p11K) was isolated by preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis and used for antibody induction. After purification by antigen affinity chromatography, the immunoglobulins detected only one target of a rather basic nature in two-dimensional immune blotting procedures of total virion proteins. By use of a combination of biological, biochemical, and microscopic techniques, p11K could be located on the surface of those vaccinia virus particles, with "classical" morphology and a buoyant density of 1.27 g/cm3. Upon immunoprecipitation from radioactively labeled infected cells, p11K appeared to be complexed to two additional virus structural proteins, which could be tentatively identified by their molecular weights as precursors for the two major core constituents. When virus assembly was inhibited by rifampin treatment of infected cells, a great part of p11K, either free or in complexed form, was found associated with actin-containing cytoskeletal elements. The ability of p11K to interact with a not-yet-identified, microfilament-associated cellular protein may be related to previous findings showing that assembled vaccinia particles in situ are found in connection with microfilaments. A possible role for the structures precipitated by p11K-specific antibodies in early stages of particle assembly is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brown S., Levinson W., Spudich J. A. Cytoskeletal elements of chick embryo fibroblasts revealed by detergent extraction. J Supramol Struct. 1976;5(2):119–130. doi: 10.1002/jss.400050203. [DOI] [PubMed] [Google Scholar]
- Cabral F., Solioz M., Rudin Y., Schatz G., Clavilier L., Slonimski P. P. Identification of the structural gene for yeast cytochrome c oxidase subunit II on mitochondrial DNA. J Biol Chem. 1978 Jan 10;253(1):297–304. [PubMed] [Google Scholar]
- DALES S., SIMINOVITCH L. The development of vaccinia virus in Earle's L strain cells as examined by electron microscopy. J Biophys Biochem Cytol. 1961 Aug;10:475–503. doi: 10.1083/jcb.10.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essani K., Dales S. Biogenesis of vaccinia: evidence for more than 100 polypeptides in the virion. Virology. 1979 Jun;95(2):385–394. doi: 10.1016/0042-6822(79)90493-8. [DOI] [PubMed] [Google Scholar]
- Grimley P. M., Rosenblum E. N., Mims S. J., Moss B. Interruption by Rifampin of an early stage in vaccinia virus morphogenesis: accumulation of membranes which are precursors of virus envelopes. J Virol. 1970 Oct;6(4):519–533. doi: 10.1128/jvi.6.4.519-533.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller G., Eibl H., Weber K. Characterization of intracellular and extracellular vaccinia virus variants: N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine interferes with cytoplasmic virus dissemination and release. J Virol. 1981 Sep;39(3):903–913. doi: 10.1128/jvi.39.3.903-913.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller G., Jungwirth C., Weber K. Fluorescence microscopical analysis of the life cycle of vaccinia virus in chick embryo fibroblasts. Virus-cytoskeleton interactions. Exp Cell Res. 1981 Mar;132(1):81–87. doi: 10.1016/0014-4827(81)90085-9. [DOI] [PubMed] [Google Scholar]
- Hiller G., Weber K., Schneider L., Parajsz C., Jungwirth C. Interaction of assembled progeny pox viruses with the cellular cytoskeleton. Virology. 1979 Oct 15;98(1):142–153. doi: 10.1016/0042-6822(79)90533-6. [DOI] [PubMed] [Google Scholar]
- Ichihashi Y. Unit Complex of vaccinia polypeptides linked by disulfide bridges. Virology. 1981 Aug;113(1):277–284. doi: 10.1016/0042-6822(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Kao S. Y., Ressner E., Kates J., Bauer W. R. Purification and characterization of a superhelix binding protein from vaccinia virus. Virology. 1981 Jun;111(2):500–508. doi: 10.1016/0042-6822(81)90352-4. [DOI] [PubMed] [Google Scholar]
- Katz E., Moss B. Formation of a vaccinia virus structural polypeptide from a higher molecular weight precursor: inhibition by rifampicin. Proc Natl Acad Sci U S A. 1970 Jul;66(3):677–684. doi: 10.1073/pnas.66.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Burridge K. Alpha-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell. 1975 Nov;6(3):289–298. doi: 10.1016/0092-8674(75)90180-4. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Tropomyosin antibody: the specific localization of tropomyosin in nonmuscle cells. J Cell Biol. 1975 Jun;65(3):549–561. doi: 10.1083/jcb.65.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Weber K. Actin antibody: the specific visualization of actin filaments in non-muscle cells. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2268–2272. doi: 10.1073/pnas.71.6.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaya A., Pogo B. G., Dales S. Biogenesis of vaccinia: separation of early stages from maturation by means of rifampicin. Virology. 1970 Apr;40(4):1039–1051. doi: 10.1016/0042-6822(70)90150-9. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Oie M., Ichihashi Y. Characterization of vaccinia polypeptides. Virology. 1981 Aug;113(1):263–276. doi: 10.1016/0042-6822(81)90153-7. [DOI] [PubMed] [Google Scholar]
- PETERS D. Morphology of resting vaccinia virus. Nature. 1956 Dec 29;178(4548):1453–1455. doi: 10.1038/1781453a0. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., Katz J. R., Dales S. Biogenesis of poxviruses: synthesis and phosphorylation of a basic protein associated with the DNA. Virology. 1975 Apr;64(2):531–543. doi: 10.1016/0042-6822(75)90130-0. [DOI] [PubMed] [Google Scholar]
- Sagot J., Beaud G. Phosphorylation in vivo of a vaccinia-virus structural protein found associated with the ribosomes from infected cells. Eur J Biochem. 1979 Jul;98(1):131–140. doi: 10.1111/j.1432-1033.1979.tb13169.x. [DOI] [PubMed] [Google Scholar]
- Saltzgaber-Müller J., Schatz G. Heme is necessary for the accumulation and assembly of cytochrome c oxidase subunits in Saccharomyces cerevisiae. J Biol Chem. 1978 Jan 10;253(1):305–310. [PubMed] [Google Scholar]
- Sarov I., Joklik W. K. Isolation and characterization of intermediates in vaccinia virus morphogenesis. Virology. 1973 Mar;52(1):223–233. doi: 10.1016/0042-6822(73)90411-x. [DOI] [PubMed] [Google Scholar]
- Sarov I., Joklik W. K. Studies on the nature and location of the capsid polypeptides of vaccinia virions. Virology. 1972 Nov;50(2):579–592. doi: 10.1016/0042-6822(72)90409-6. [DOI] [PubMed] [Google Scholar]
- Soloski M. J., Cabrera C. V., Esteban M., Holowczak J. A. Studies concerning the structure and organization of the vaccinia virus nucleoid. I. Isolation and characterization of subviral particles prepared by treating virions with guanidine-HCL, nonidet-P40, and 2-mercaptoethanol. Virology. 1979 Dec;99(2):209–217. doi: 10.1016/0042-6822(79)90001-1. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner A., Baxby D. Structural polypeptides of Orthopoxvirus: their distribution in various members and location within the virion. J Gen Virol. 1979 Dec;45(3):537–545. doi: 10.1099/0022-1317-45-3-537. [DOI] [PubMed] [Google Scholar]
- WESTWOOD J. C., HARRIS W. J., ZWARTOUW H. T., TITMUSS D. H., APPLEYARD G. STUDIES ON THE STRUCTURE OF VACCINIA VIRUS. J Gen Microbiol. 1964 Jan;34:67–78. doi: 10.1099/00221287-34-1-67. [DOI] [PubMed] [Google Scholar]
- Weber K., Bibring T., Osborn M. Specific visualization of tubulin-containing structures in tissue culture cells by immunofluorescence. Cytoplasmic microtubules, vinblastine-induced paracrystals, and mitotic figures. Exp Cell Res. 1975 Oct 1;95(1):111–120. doi: 10.1016/0014-4827(75)90615-1. [DOI] [PubMed] [Google Scholar]
- Weber K., Groeschel-Stewart U. Antibody to myosin: the specific visualization of myosin-containing filaments in nonmuscle cells. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4561–4564. doi: 10.1073/pnas.71.11.4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]