Abstract
Decades of studies have shown that eliminating circadian rhythms of mammals does not compromise their health or longevity in the laboratory in any obvious way. These observations have raised questions about the functional significance of the mammalian circadian system, but have been difficult to address for lack of an appropriate animal model. Surgical ablation of the suprachiasmatic nucleus (SCN) and clock gene knockouts eliminate rhythms, but also damage adjacent brain regions or cause developmental effects that may impair cognitive or other physiological functions. We developed a method that avoids these problems and eliminates rhythms by noninvasive means in Siberian hamsters (Phodopus sungorus). The present study evaluated cognitive function in arrhythmic animals by using a hippocampal-dependent learning task. Control hamsters exhibited normal circadian modulation of performance in a delayed novel-object recognition task. By contrast, arrhythmic animals could not discriminate a novel object from a familiar one only 20 or 60 min after training. Memory performance was not related to prior sleep history as sleep manipulations had no effect on performance. The GABA antagonist pentylenetetrazol restored learning without restoring circadian rhythms. We conclude that the circadian system is involved in memory function in a manner that is independent of sleep. Circadian influence on learning may be exerted via cyclic GABA output from the SCN to target sites involved in learning. Arrhythmic hamsters may have failed to perform this task because of chronic inhibitory signaling from the SCN that interfered with the plastic mechanisms that encode learning in the hippocampus.
Keywords: GABA, hippocampus, memory, sleep, suprachiasmatic nucleus
The circadian system provides daily temporal organization for a wide range of biological processes in plants and animals. In mammals, circadian timing is permanently eliminated after ablation of the master circadian clock, the suprachiasmatic nucleus (SCN) (1). Biological events that are ordinarily nocturnal or diurnal before the lesion occur throughout the 24-h day in SCN-lesioned animals (1). Formal longevity studies have not been done in SCN-ablated animals; however, anecdotal evidence accumulated from many laboratories indicates that such animals live long, healthy lives. Although these studies have helped confirm the SCN as a bona fide biological clock, they have also raised questions about the fundamental functional significance of the circadian system. In fact, the good health and longevity of SCN-lesioned animals support the notion that the circadian system is of little consequence to their overall physiology. One notable exception to this trend is reproduction in rodents. Elimination of circadian timing by SCN ablation eliminates estrous cycles and thereby prevents reproduction (for review, see ref. 2).
One barrier to investigating the functional significance of the circadian system has been the lack of an appropriate animal model. Circadian timing can be eliminated by ablation of the SCN, chronic exposure to bright light, or genetic modification, but these techniques have notable limitations for assessing the contribution of the circadian system to normal physiological functioning. Ablation of the SCN reliably eliminates rhythms, but also necessarily damages adjacent hypothalamic tissue and results in the nonspecific synthesis and release of stress and reproductive hormones (3–5). Chronic exposure to bright light, on the other hand, requires several weeks to eliminate rhythms but the effects are transient; daily oscillations resume within a few days after animals are returned to a light–dark (LD) cycle (6–9). Moreover, constant bright light is also a major stressor to the animals. Only a few days of constant light treatment will increase stress hormones to levels that interfere with cognitive performance (10, 11). Knocking out certain combinations of clock genes can eliminate rhythms (12). With this approach, loss of gene function is, however, present in all tissues throughout development, not just in the adult SCN. Furthermore, because most circadian clock genes are expressed throughout the body and are most likely pleiotropic in function, they can be expected to affect other regulatory systems (13, 14). In fact, studies in transgenic mice and Drosophila suggest that circadian clock genes may be directly involved in sleep homeostasis independent of their role in generating circadian rhythms (15–18). For example, mice null for both cryptochrome genes (cry1−/−, cry2−/−) are not only arrhythmic, but also have severe deficits in sleep regulation (18).
We have developed a procedure that renders Siberian hamsters (Phodopus sungorus) arrhythmic by noninvasive means, thus avoiding the confounds of lesions, constant light, or genetic manipulations. This model system makes it possible to assess the function of the circadian system beyond its known role as a biological clock. The circadian system of Phodopus is well characterized because it has been intensively studied for >30 years as a model of seasonal rhythmicity. Circadian rhythms in sleep–wake cycles, body temperature, and locomotor activity can be eliminated within a few days in these hamsters by simple light treatments that need only be administered a single time (19, 20). Since our original finding, other laboratories have reported a similar propensity for arrhythmicity in behavioral and hormonal rhythms in this species (21, 22). This noninvasive method for eliminating rhythms has the advantage of allowing animals to remain undisturbed in the continued presence of their standard LD cycle and in their home cages during the light treatment.
We have used this model of circadian arrhythmia to investigate the contribution of the circadian system to declarative learning and memory. Several studies have already demonstrated that performance on learning tasks is optimal at 24-h intervals from the time of training and is significantly worse at noncircadian intervals (23–26). We were, by contrast, interested in evaluating whether loss of rhythms would lead to more general cognitive impairments in our neurologically intact arrhythmic animals regardless of time of day. We therefore evaluated rhythmic and arrhythmic hamsters in the novel-object recognition (NOR) task, which is a simple hippocampal-mediated task based on the innate tendency of rodents to seek novelty (27). We now show that: (i) loss of circadian organization prevents the formation of episodic memories, and that this loss is not a consequence of disrupted sleep regulation induced by the arrhythmic state, and (ii) learning performance can be rescued in arrhythmic animals by the noncompetitive GABA antagonist pentylenetetrazol (PTZ) without restoring circadian rhythmicity.
Results
Elimination of Circadian Rhythms.
Several previous studies have failed to find any gender differences in circadian organization or in response to the light treatment used in the current experiments (20, 28). Likewise, we found no sex differences in performance on the NOR task at any zeitgeber time (ZT) or testing interval (P > 0.05; data not shown). Thus, equal numbers of male and female hamsters were used in all conditions and at all time points, and these data have been combined.
All animals were stably entrained to the LD cycle for several weeks before they were administered the light treatment to eliminate circadian behavior (e.g., Fig. 1). Approximately 55% of hamsters became arrhythmic within a few days after the light treatment with bouts of motor activity uniformly distributed throughout the light and dark phases of the LD cycle (e.g., Fig. 1). The remaining animals either reentrained (e.g., Fig. 1) or free ran as described (20). Only hamsters that were clearly arrhythmic, as determined by periodogram analysis and visual inspection of the actograms, were used in the NOR task. Control animals consisted of two groups: (i) those that reentrained after the light treatment, and (ii) those that were randomly selected from the colony. Animals from both of these groups were housed singly in conditions identical to those of the arrhythmic animals. There were no differences among the aforementioned two control groups in their performance in the NOR task at any given time point (P > 0.05). Therefore, data from these two groups of control animals were combined.
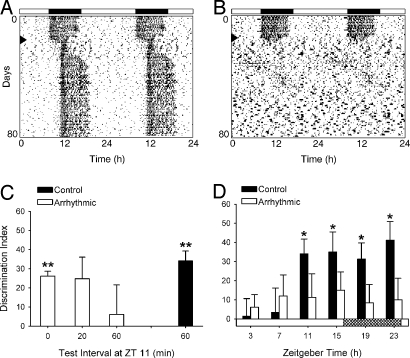
Fig. 1.
Performance on the NOR task. (A) Control hamster that remained rhythmic after the combination light pulse and 3-h phase delay of the LD cycle (arrowhead). Filled bars indicate dark phase before the treatment. (B) An arrhythmic hamster in which circadian locomotor activity rhythms were eliminated within a few days after the treatment (arrowhead). (C) DIs for independent groups of animals tested at ZT 11 (i.e., 11 h after light onset). Positive values indicate more time spent with the novel object than the familiar one; zero indicates equal time spent with each object. Control animals readily detected the novel object after a 60-min test interval (P < 0.001, n = 15). Arrhythmic hamsters could not perform this task after a 60-min test interval (P = 0.71, n = 8) or 20-min test interval (P = 0.07, n = 8), but they performed successfully after a test interval of 0 min (P < 0.001, n = 7). (D) DIs for all animals with a 60-min test interval at different points of the LD cycle (n = 8–10 animals per group at each time point). Stippled bar indicates time of light and dark in the animal room. *, P < 0.01; **, P < 0.001 significantly different from zero.
Performance on the NOR Task.
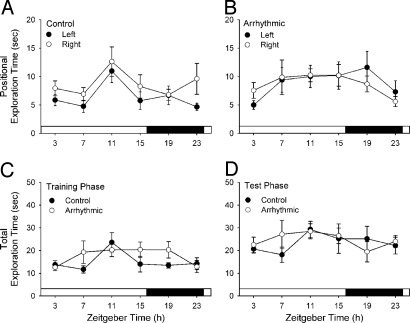
Each animal was tested in the NOR task only once. All experimental groups are thus fully independent. To exclude the possibility that NOR performance might be confounded by a priori spatial or object biases, placement of the novel object was alternated between the left and right corners of the open-field arena. Neither control nor arrhythmic hamsters showed unrelated preferences for exploring objects based on their position in the arena during either the training or testing phases of the experiment (Fig. 2 A and B; P > 0.05 at each time point). Moreover, total object exploration times did not differ among control and arrhythmic animals at any time point (Fig. 2 C and D; P > 0.05).
Fig. 2.
Novel object location and total exploration times had no effect on object recognition. (A and B) Time spent with the novel object was the same at all times of day for both groups whether it was placed in the left or right corner of the chamber (P > 0.05 for object position and time of day, two-way ANOVA for each group of animals). (C and D) There was no significant effect of time of day for time spent with the novel object among control (entrained) and arrhythmic hamsters during either the training phase or the test phase of the NOR task (P > 0.05 for animal group and time of day, two-way ANOVA for each phase).
As has been shown for a variety of learning tasks in other species, there was a robust circadian rhythm in performance on the NOR task among control animals (Fig. 1D). Control (i.e., entrained) hamsters readily detected the novel object after a 60-min test interval during the dark phase and latter part of the light phase, but failed to do so early in the light phase (Fig. 1D). By contrast, arrhythmic hamsters could not discriminate between novel and familiar objects at any point during the LD cycle (Fig. 1D). When tested late in the light phase (ZT 11), control animals readily detected the novel object after a 60-min test interval (Fig. 1C; P < 0.001, n = 15). By contrast, arrhythmic hamsters could not perform this task after a 20-min test interval (P = 0.07, n = 8), or 60-min test interval (P = 0.71, n = 8). They could, however, recall previously seen objects after a minimal delay (i.e., 0 min; P < 0.001, n = 7).
Learning Performance After Sleep Deprivation.
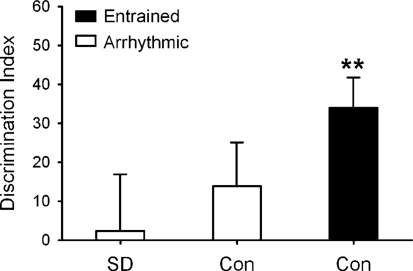
We considered the hypothesis that arrhythmic hamsters performed poorly on the NOR task because they may have been sleeping during the interval between training and testing. Contrary to much of the literature that has described how sleep improves learning (29), there is evidence that short periods of sleep (e.g., 10 min) directly after a learning event can prevent the formation of episodic memories and induce a type of retrograde amnesia (30). It was plausible that arrhythmic hamsters may have slept during the interval between training and testing because of their lack of prolonged periods of wakefulness (19), and this sleep immediately after training might have induced a form of retrograde amnesia. To test this possibility, two groups of arrhythmic animals (n = 10 each) were tested on the NOR task at ZT 11 in the same manner as other animals in the study. A cohort (n = 9) of entrained hamsters served as positive controls. During the 60-min interval between training and testing, one group of arrhythmic hamsters was sleep-deprived by gentle handling as described (19), whereas the other group of arrhythmic animals was left undisturbed.
Sleep deprivation did not improve performance of arrhythmic animals on the NOR task. Entrained animals readily detected the novel object (P < 0.01), whereas sleep-deprived and nondeprived arrhythmic animals could not (P > 0.05; Fig. 3). There were also no differences in spatial patterns of exploration or total exploration times among any of these three groups (P > 0.05; data not shown).
Fig. 3.
Sleep deprivation had no effect on object recognition. Time spent with the novel object at ZT 11 did not differ between arrhythmic hamsters that were sleep-deprived (SD) or used as controls (Con) and allowed to sleep (P > 0.05), whereas entrained controls readily recognized the novel object (**, P < 0.001).
Learning Performance Is Restored by the GABA Antagonist PTZ.
Several GABA antagonists, including PTZ, significantly improve performance on the NOR task in Ts65Dn mice, which have been used as a model of Down's syndrome and intellectual disability (31). Because GABA is the primary neurotransmitter of the SCN, we hypothesized that the normal pattern of GABA output from the SCN may have been altered in arrhythmic hamsters in such a way as to increase inhibitory input at SCN target sites involved in cognition. We therefore tested whether the GABA antagonist PTZ would restore learning performance on the NOR task as it does in Ts65Dn mice.
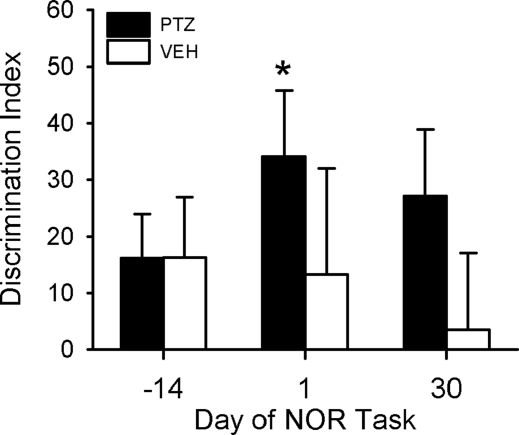
A group of hamsters were made arrhythmic as described in the previous experiments and tested on the NOR task 4–8 weeks later. Arrhythmic hamsters were divided into two groups and matched on their mean discrimination indices (DI), neither of which was significant (Fig. 4, day −14; P > 0.05). Beginning 3 days later, animals were injected daily for 10 days at the same time (i.e., 2 h before lights off) with either PTZ (n = 9) or the vehicle solution (VEH; n = 9). All animals were tested again at ZT 11 on days 1 and 30 after the injections were terminated. PTZ significantly restored performance on the NOR task (P = 0.020) on day 1, but not on day 30 (P = 0.055), to values similar to those obtained in entrained control animals (e.g., Fig. 1). DIs for vehicle-treated animals were not significant at any time point (P > 0.05). PTZ-treated hamsters remained arrhythmic through the end of the study.
Fig. 4.
The GABA inhibitor PTZ restored learning performance in arrhythmic hamsters. Animals treated with PTZ (n = 9) or vehicle (VEH; n = 9) solution were group-matched based on DIs obtained before drug injections (day −14). Day 0 = the last day of injections. DIs increased significantly in PTZ-treated hamsters on day 1 (D1; P = 0.020), but not on day 30 (D30; P = 0.055), after termination of drug treatment. DIs were not significant (P > 0.05) in vehicle-treated animals at any time point.
Discussion
Past studies have shown that cognitive performance is modulated by the circadian system in humans and other animals. The inability of arrhythmic hamsters to perform a simple learning task supports a role for the circadian system in learning and memory that goes beyond that of simply providing temporal organization to memory function. This study suggests that the circadian system is required for normal memory formation in a way that is independent of its role in organizing sleep and wakefulness. Restoration of learning performance by the GABA antagonist PTZ suggests that the SCN may modulate learning in normal animals via cyclic GABA release.
The Circadian System Versus Sleep: Contributions to Object Recognition Memory.
Numerous studies have found that sleep enhances retention of declarative information (for review, see ref. 29). Given this positive relationship, it would be tempting to assume that daily rhythms in memory performance in entrained animals in the present study could be driven by daily changes in sleep architecture. That hypothesis is not supported by a comparison of sleep patterns among entrained and arrhythmic Siberian hamsters (19). If one compares the relative amounts of rapid eye movement (REM) and non-REM (NREM) sleep, as well as slow wave activity (SWA), expressed by Siberian hamsters at times of day when they cannot perform the NOR task (e.g., ZT 7) with times of day when task performance is optimal (e.g., ZT 11), it is evident that sleep state distributions at these two times are indistinguishable from one another (19). Scores on the NOR task at these two time points represent the extremes observed in the overall population of animals, even though the distribution of sleep states is the same across this 4-h interval. Hence, circadian timing and not prior sleep state may account for the naturally occurring cycle in NOR task performance in our entrained hamsters. This situation is similar to that of humans in which forced desynchrony studies have delineated a clear role for the circadian system in modulating cognitive function that is independent of sleep history (32, 33).
Lack of Object Recognition Memory in Arrhythmic Hamsters: Loss of Circadian Function or Retrograde Amnesia Associated with Sleep Onset?
The role of sleep in learning and memory has received a great deal of attention in the last few years. Many studies in humans and other animals have shown that sleep deprivation before or after training on a memory task diminishes subsequent performance, depending on the types of task and memory being tested (29). These findings have interesting implications for the present study because the temporal organization of sleep differs in our animals compared with that of other studies. Entrained and arrhythmic Siberian hamsters express similar amounts of NREM and REM sleep and SWA during the light phase (19). During the dark phase, however, entrained animals spend most of their time awake and have relatively shorter sleep bouts than they do in the light. By contrast, arrhythmic animals sleep as much during the dark phase as they do in the light phase, and sleep bout duration (NREM and REM) does not decrease in the dark (19). Thus, the primary difference in sleep architecture between entrained and arrhythmic hamsters is that arrhythmic animals lack the nighttime period of consolidated wakefulness that is characteristic of entrained animals.
It thus becomes difficult to explain the poor performance of arrhythmic hamsters on the NOR task in terms of sleep processes that promote memory given that they sleep more, not less, than entrained animals. At least one study in humans has shown that a night of enhanced slow wave sleep improved memory performance on a word pair association task (34), and others have shown that a short period of sleep (10 min) immediately after learning disrupts recognition memory in humans (30). We therefore assessed whether the lack of prolonged wakefulness in arrhythmic hamsters might have interfered with memory consolidation by sleep-depriving them during the 60-min interval between training and testing, but the sleep deprivation did not improve memory performance. We conclude that the inability of arrhythmic hamsters to discern object novelty is not a by-product of short-term retrograde amnesia resulting from rapid transitions to sleep after learning experiences as has been observed in humans (30). Taken together, sleep effects cannot explain the daily rhythm in learning among entrained animals nor can they explain the inability of arrhythmic hamsters to perform the NOR learning task.
Circadian Rhythms Are Required for Declarative Learning and Memory.
The most parsimonious explanation for the present results is that the circadian system influences declarative learning and memory by interacting directly or indirectly with the medial temporal lobe (i.e., the hippocampal formation and parahippocampal cortices). We chose the NOR task because it at least partially depends on the hippocampus (35). In our attempts to delineate a more specific relationship between the circadian system and hippocampal-guided behavior, we used the Morris water maze because performance in the maze has been used historically as an index of hippocampal function in rats and mice (36). We quickly found, however, that we could not extend the water maze task to Siberian hamsters. Typically, during water maze training animals regard the water as an aversive stimulus that motivates them to learn the location of a hidden platform submerged just beneath the water's surface so they may escape the water. We found, however, that Siberian hamsters are not averse to water. Despite the high energy expenditure entailed in prolonged swimming, they remained atop the hidden platform for only a few seconds before voluntarily reentering the water. We therefore turned to the NOR task even though the role of the hippocampus in recognition memory has been more controversial than its role in building spatial maps (27). On the other hand, numerous studies have shown that the hippocampus does make important contributions to the ability of animals to discriminate novel objects from familiar ones (37). Thus, it is likely that loss of circadian timing diminished the ability of the hippocampus to encode learned information.
Restoration of Learning by PTZ in Arrhythmic Hamsters.
We hypothesize that the diminished cognitive function of our arrhythmic hamsters might be caused by an overinhibition of synaptic circuits in brain regions critical for learning and memory. This concept has been used recently to explain memory deficits in the Ts65Dn mouse model of Down's syndrome (31). Previous electrophysiological and neuroanatomical data had suggested that poor hippocampal function in these mice could be attributed to excess GABAergic signaling (38, 39), and, commensurate with this suggestion, chronic administration of GABAA receptor antagonists completely restored Ts65Dn object recognition memory and hippocampal long-term potentiation to normal levels (31). A similar mechanism may underlie the cognitive deficits observed in our arrhythmic hamsters. We therefore propose that GABAergic output from the SCN is constant rather than cyclic in arrhythmic animals. The resulting elevations of inhibitory tone in SCN targets reduces synaptic excitability in the hippocampus and thereby attenuates the ability to gather and consolidate information. Restoration of learning performance by PTZ supports the hypothesis that chronically elevated GABA levels may be involved in the learning deficits in arrhythmic hamsters.
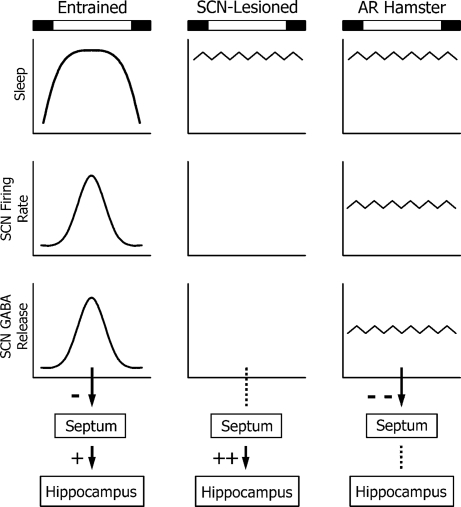
Constant GABA release from the arrhythmic hamster SCN might occur in the following manner. Individual SCN neurons function as autonomous circadian oscillators that are normally in phase with one another, such that the discharge rate from the entire population is high only during the middle of the day and low at other times (40). This situation changes when animals are made arrhythmic by exposure to constant light. Individual neurons continue to oscillate, but are now completely out of phase with one another (41). Consequently, daily peak firing rates are no longer confined to the middle of the day, but instead occur at random times of the day and night so that there is no longer a circadian oscillation in the collective output from the SCN (ref. 41 and Fig. 5). Hence, the discharge rate from the population of SCN neurons is maintained at a constant level that is intermediate between the normal daily peak and trough firing rates observed in normal animals (42, 43). The SCN is a substantial source of GABA in the brain and uses it as its principal neurotransmitter (44–46). Accordingly, the steady output of GABA from the arrhythmic SCN to limbic system structures may hinder synaptic excitability required for memory formation Although the SCN does not project to the hippocampus directly, it does heavily innervate other structures such as the septum that influence hippocampal function (47, 48). GABA agonists injected into the septum disrupt learning of hippocampal-dependent tasks (49–51). Thus, the arrhythmic SCN might elevate GABA tone in the septum to a degree that it depresses excitatory cholinergic input to the hippocampus (Fig. 5).
Fig. 5.
Hypothetical model showing how various manipulations of SCN function might affect learning. (Left) In entrained nocturnal animals, both sleep and SCN neuronal firing rate are high during the day. Presumably, GABA release from the SCN is highest at this time as well. The SCN would thus provide cyclic inhibitory input to structures such as the septum, which would permit normal daily oscillations in the balance of excitatory (+) and inhibitory (−) tone in the hippocampus. (Center) In SCN-lesioned animals, the absence of GABA input to the septum (dashed line) would permit chronic cholinergic excitation of the hippocampus and might explain why learning deficits are not observed in SCN-lesioned animals. (Right) In our arrhythmic (AR) hamsters, the SCN might provide a steady release of GABA to inhibit (−−) the septum because of continuous neuronal activity in the SCN. This, in turn, would reduce septal cholinergic input to the hippocampus, thereby attenuating memory formation there. Black and white rectangles indicate relative times of dark and light, respectively, in the animal facility.
One might predict that complete ablation of the SCN should produce the same learning deficits as reported here. Our hypothesis predicts, however, that complete ablation of the SCN would actually improve learning because of the reduced GABA tone in SCN targets. The few studies that have examined learning in SCN-lesioned animals support this idea. Intact rats exhibit a pronounced circadian rhythm in performance on a passive avoidance learning task (52). In that study, test scores were optimal 24 h after training, but were significantly reduced at 18 or 30 h after training. As expected, this rhythm in performance was eliminated in SCN-lesioned rats, but surprisingly, these animals performed as well at all three time points as did control animals at the 24-h interval (52). In other studies, SCN-lesioned rats learned a T-maze discrimination task faster than intact animals (53) and no learning deficits were found in SCN-lesioned golden hamsters on a conditioned place preference task where time of day was used as the discriminative stimulus (54). Despite the fact that these studies all used different learning tasks and were done with different species, the results were consistent: SCN lesions either improved or had no negative effect on learning.
Summary.
The functional significance of the circadian system is difficult to assess experimentally, largely because the apparent general good health and longevity of arrhythmic animals masks underlying deficits that normally go undetected in the sterile environment of the laboratory. Revealing such deficits requires methods for eliminating circadian rhythms that have a minimal impact on other physiological systems and avoid the confounding secondary effects of brains lesions, constant bright light, and gene knockouts. Hamsters made arrhythmic by our noninvasive method were unable to discriminate a novel object only 20 min after training. Treatment of arrhythmic animals with the noncompetitive GABA antagonist PTZ restored the ability of these animals to perform the NOR task without restoring their circadian rhythms. Some improvement in their cognitive performance was still seen 30 days after the treatment ended. We suggest that the SCN modulates learning and memory functions involving the medial temporal lobe through daily cycling of GABA tone. Because Siberian hamsters can be made arrhythmic without damaging neurological function or altering their genetics, they provide a model for investigating the relative contributions of circadian rhythms and sleep in learning and memory and other physiological functions.
Materials and Methods
Animals and Housing Conditions.
Siberian hamsters (P. sungorus sungorus) were bred in the laboratory in a 16:8-h LD cycle (lights on at 0200 h, PST) at an ambient temperature of 22°C. Animals were housed two to four per cage in the colony room and then individually in white polypropylene cages (30 × 17 × 17 cm; Nalgene) just before the beginning of the study. During experiments, hamsters were maintained in six recording chambers (10 individually caged animals per chamber). Each cage was equipped with its own light source and a photosensor that allowed illumination cycles to be recorded by computer. Animals were provided with cotton batting for nesting material; food (Purina chow 5015) and tap water were available ad libitum. All experimental procedures were approved by Stanford University's Administrative Panel on Laboratory Animal Care and were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Lighting Conditions.
Light fixtures illuminating both the room where light pulses were administered and the activity recording chambers contained two cool white fluorescent tubes (4,100 K; 40 W; Philips) producing an intensity of 10–60 μW/cm2 on cage floors when water bottles, food, and cage lids were in place. Variations in light intensity depended on the position of the light meter photocell (International Light model IL-1405 radiometer system) within the cage. The light sensor was pointed upward from the cage bottom for these measurements.
Activity Recording and Analysis.
Activity was measured by passive infrared motion detectors mounted directly above the tip of the water bottle sipper tube. In this configuration, activity levels primarily reflected drinking behavior and locomotor activity that occurred directly under the sipper tube. These detectors have a temporal resolution of 1–2 s for successive counts of activity. Activity bouts were summed in 10-min intervals and stored on computer. The presence or absence of circadian periodicity in locomotor activity was determined by χ2 periodogram analysis (ClockLab; Actimetrics) on 10-day blocks of data for each animal immediately before training in the NOR task. Peaks in the periodogram were deemed statistically significant if they exceeded the 99.9% confidence interval limit. Animals were considered arrhythmic if there were no significant peaks in the periodogram in the circadian range, activity was distributed throughout the LD cycle, and daily rhythm onsets and offsets could not be identified visually.
Drug Treatment.
PTZ (Sigma) was dissolved in saline and aliquoted into five vials that were frozen (−20°C) until needed. Vials were defrosted on alternate days and refrigerated when not in use. PTZ was injected i.p. (1 mg/kg) daily for 10 consecutive days 2 h before lights off in the animal room.
Experimental Protocol.
Equal numbers of males and females were used in all groups. Experimental animals were separated and housed singly in the same photoperiod as the colony room (LD 16:8, lights on at 0200 PST). Locomotor activity was continuously recorded from this time to until the experiment was terminated. Fourteen days later, lights in the activity recording chambers were turned on for 2 h beginning 5 h after lights off (i.e., 2-h light pulse). The next day, the LD cycle was phase-delayed by 3 h via an extension of the light phase (lights on at 0500 PST). Subsequently, separate groups of hamsters were assessed on the NOR task at different times of day, 4–8 weeks after rhythms were eliminated. Each animal was tested only once in the study so that data from each time point represent independent and naïve subjects.
The NOR task was carried out in a black acrylic open-field arena (58 × 58 × 46 cm) with a video camera mounted overhead. Light intensity at the bottom of the arena was calibrated to match the light intensity of the experimental chambers where the animals were housed before testing (10–60 μW/cm2). These adjustments were made by mounting white poster board baffles over the arena that also served to even out illumination across the open field. For the training phase of the NOR task, hamsters were placed along the center wall of the arena with two identical objects located at adjacent corners. Animals were allowed to freely explore the objects for 5 min and then returned to their home cages for 0, 20, or 60 min. After this interval, one of the objects was replaced by a similarly sized item of different color and shape, and animals were returned to the open field where they were allowed to explore for another 5 min. For the testing phase, placement of the novel object was alternated from the left to right corner from trial to trial to prevent spatial biases in object exploration. The arena was cleaned with 70% ethanol before subsequent animals were tested. Videotape of the trials was scored by two observers (C.H. and C.W.). The total time spent with each object was recorded with two stopwatches. Interobserver reliability was >95%.
The time when animals were tested is given by ZT, when ZT 0 is the time of lights on in the animal room. Preference for the novel object is expressed as a DI, where DI = (time with novel object − time with familiar object)/total exploration time of both objects × 100. Positive DIs indicate a preference for the novel object, whereas a value of zero indicates no preference for either object. A one-sample t test was used to determine whether DIs were statistically significant from zero. The effects of arrhythmicity and time of day on exploration times were evaluated by two-way ANOVA.
Acknowledgments.
We thank Deveroux Ferguson, Jessy Klima, and Shawn Sorrells for help with this project. This research was supported by Howard Hughes Medical Institute Grant 52003745.
Footnotes
The authors declare no conflict of interest.
References
- 1.Rusak B, Zucker I. Neural regulation of circadian rhythms. Physiol Rev. 1979;59:449–526. doi: 10.1152/physrev.1979.59.3.449. [DOI] [PubMed] [Google Scholar]
- 2.Boden MJ, Kennaway DJ. Circadian rhythms and reproduction. Reproduction. 2006;132:379–392. doi: 10.1530/rep.1.00614. [DOI] [PubMed] [Google Scholar]
- 3.Bittman EL, Bartness TJ, Goldman BD, DeVries GJ. Suprachiasmatic and paraventricular control of photoperiodism in Siberian hamsters. Am J Physiol. 1991;260:R90–R101. doi: 10.1152/ajpregu.1991.260.1.R90. [DOI] [PubMed] [Google Scholar]
- 4.Buijs RM, Kalsbeek A, van der Woude TP, van Heerikhuize JJ, Shinn S. Suprachiasmatic nucleus lesion increases corticosterone secretion. Am J Physiol. 1993;264:R1186–R1192. doi: 10.1152/ajpregu.1993.264.6.R1186. [DOI] [PubMed] [Google Scholar]
- 5.Ma YJ, Kelly MJ, Ronnekleiv OK. Pro-gonadotropin-releasing hormone (ProGnRH) and GnRH content in the preoptic area and the basal hypothalamus of anterior medial preoptic nucleus/suprachiasmatic nucleus-lesioned persistent estrous rats. Endocrinology. 1990;127:2654–2664. doi: 10.1210/endo-127-6-2654. [DOI] [PubMed] [Google Scholar]
- 6.Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. III. Heavy water and constant light: Homeostasis of frequency? J Comp Physiol. 1976;106:267–290. [Google Scholar]
- 7.Depres-Brummer P, Levi F, Metzger G, Touitou Y. Light-induced suppression of the rat circadian system. Am J Physiol. 1995;268:R1111–R1116. doi: 10.1152/ajpregu.1995.268.5.R1111. [DOI] [PubMed] [Google Scholar]
- 8.Eastman CL, Rechtschaffen A. Circadian temperature and wake rhythms of rats exposed to prolonged continuous illumination. Physiol Behav. 1983;31:417–427. doi: 10.1016/0031-9384(83)90061-6. [DOI] [PubMed] [Google Scholar]
- 9.Witting W, Boerma D, Koster van Hoffen GC, Swaab DF, Mirmiran M. Light suppresses frequency and endogenous amplitudes of the circadian system in nocturnal animals. Biol Rhythm Res. 1995;26:477–485. [Google Scholar]
- 10.Welberg L, Thrivikraman KV, Plotsky PM. Combined pre- and postnatal enrichment programs the HPA axis differentially in male and female rats. Psychoneuroendocrinology. 2005;31:553–564. doi: 10.1016/j.psyneuen.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Wen-Pei M, et al. Exposure to chronic constant light impairs spatial memory and influences long-term depression in rats. Neurosci Res. 2007;59:224–230. doi: 10.1016/j.neures.2007.06.1474. [DOI] [PubMed] [Google Scholar]
- 12.Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 13.Greenspan RJ. The flexible genome. Nat Rev Genet. 2001;2:383–387. doi: 10.1038/35072018. [DOI] [PubMed] [Google Scholar]
- 14.Meyer-Bernstein EL, Sehgal A. Molecular regulation of circadian rhythms in Drosophila and mammals. Neuroscientist. 2001;7:496–505. doi: 10.1177/107385840100700606. [DOI] [PubMed] [Google Scholar]
- 15.Naylor E, et al. The circadian clock mutation alters sleep homeostasis in the mouse. J Neurosci. 2000;20:8138–8143. doi: 10.1523/JNEUROSCI.20-21-08138.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw PJ, Franken P. Perchance to dream: Solving the mystery of sleep through genetic analysis. J Neurobiol. 2003;54:179–202. doi: 10.1002/neu.10167. [DOI] [PubMed] [Google Scholar]
- 17.Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–291. doi: 10.1038/417287a. [DOI] [PubMed] [Google Scholar]
- 18.Wisor JP, et al. A role for cryptochromes in sleep regulation. BMC Neurosci. 2002;3:20. doi: 10.1186/1471-2202-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Larkin JE, Yokogawa T, Heller HC, Franken P, Ruby NF. Homeostatic regulation of sleep in arrhythmic Siberian hamsters. Am J Physiol. 2004;287:R104–R111. doi: 10.1152/ajpregu.00676.2003. [DOI] [PubMed] [Google Scholar]
- 20.Ruby NF, Barakat MT, Heller HC. Phenotypic differences in reentrainment behavior and sensitivity to nighttime light pulses in Siberian hamsters. J Biol Rhythms. 2004;19:1–12. doi: 10.1177/0748730404268055. [DOI] [PubMed] [Google Scholar]
- 21.Steinlechner S, Stieglitz A, Ruf T. Djungarian hamsters: A species with a labile circadian pacemaker? Arrhythmicity under a light-dark cycle induced by short light pulses. J Biol Rhythms. 2002;17:248–258. doi: 10.1177/074873040201700308. [DOI] [PubMed] [Google Scholar]
- 22.Weinert D, Schottner K. An inbred lineage of Djungarian hamsters with a strongly attenuated ability to synchronize. Chronobiol Intl. 2007;24:1065–1079. doi: 10.1080/07420520701791588. [DOI] [PubMed] [Google Scholar]
- 23.Cain SW, Chou T, Ralph MR. Circadian modulation of performance on an aversion-based place learning task in hamsters. Behav Brain Res. 2004;150:201–205. doi: 10.1016/j.bbr.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Holloway FA, Wansley RA. Multiple retention deficits at periodic intervals after active and passive avoidance learning. Behav Biol. 1973;9:1–14. doi: 10.1016/s0091-6773(73)80164-6. [DOI] [PubMed] [Google Scholar]
- 25.Holloway FA, Wansley RA. Multiple retention deficits at periodic intervals after passive-avoidance learning. Science. 1973;180:208–210. doi: 10.1126/science.180.4082.208. [DOI] [PubMed] [Google Scholar]
- 26.Wincour G, Hasher L. Age and time-of-day effects on learning and memory in a nonmatching-to-sample test. Neurobiol Aging. 2004;25:1107–1115. doi: 10.1016/j.neurobiolaging.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 27.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 28.Barakat MT, O'Hara BF, Cao VH, Heller HC, Ruby NF. Light induces. c-fos and per1 expression in the suprachiasmatic nucleus of arrhythmic hamsters. Am J Physiol. 2005;289:R1381–R1386. doi: 10.1152/ajpregu.00695.2004. [DOI] [PubMed] [Google Scholar]
- 29.Walker MP, Stickgold R. Sleep, memory, and plasticity. Annu Rev Physiol. 2006;57:139–166. doi: 10.1146/annurev.psych.56.091103.070307. [DOI] [PubMed] [Google Scholar]
- 30.Wyatt JK, Bootzin RR, Anthony J, Bazant S. Sleep onset is associated with retrograde and anterograde amnesia. Sleep. 1994;17:502–511. doi: 10.1093/sleep/17.6.502. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez F, et al. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci. 2007;10:411–413. doi: 10.1038/nn1860. [DOI] [PubMed] [Google Scholar]
- 32.Dijk DJ, Czeisler CA. Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett. 1994;166:63–68. doi: 10.1016/0304-3940(94)90841-9. [DOI] [PubMed] [Google Scholar]
- 33.Dijk DJ, Duffy JF, Czeisler CA. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:112–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 34.Gais S, Born J. Low acetylcholine during slow-wave sleep is critical for declarative memory consolidation. Proc Natl Acad Sci USA. 2004;101:2140–2144. doi: 10.1073/pnas.0305404101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci USA. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 37.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: A new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Belichenko PV, et al. Synaptic structural abnormalities in the Ts65Dn mouse model of Down syndrome. J Comp Neurol. 2004;480:281–298. doi: 10.1002/cne.20337. [DOI] [PubMed] [Google Scholar]
- 39.Kleschevnikov AM, et al. Hippocampal long-term potentiation suppressed by increased inhibition in the Ts65Dn mouse, a genetic model of Down syndrome. J Neurosci. 2004;24:8153–8160. doi: 10.1523/JNEUROSCI.1766-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prosser RA. In vitro circadian rhythms of the mammalian suprachiasmatic nuclei: comparison of multi-unit and single-unit neuronal activity recordings. J Biol Rhythms. 1998;13:30–38. doi: 10.1177/074873098128999899. [DOI] [PubMed] [Google Scholar]
- 41.Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci. 2005;8:267–269. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- 42.Margraf RR, Puchalski W, Lynch GR. Absence of daily neuronal rhythm in the suprachiasmatic nuclei of acircadian Djungarian hamsters. Neurosci Lett. 1992;142:175–178. doi: 10.1016/0304-3940(92)90367-g. [DOI] [PubMed] [Google Scholar]
- 43.Mason R. The effects of continuous light exposure on Syrian hamster suprachiasmatic (SCN) neuronal discharge activity in vitro. Neurosci Lett. 1991;123:160–163. doi: 10.1016/0304-3940(91)90920-o. [DOI] [PubMed] [Google Scholar]
- 44.Belenky MA, Yarom Y, Pickard GE. Heterogenous expression of γ-aminobutyric acid and γ-associated receptors and transporters in the rat suprachiasmatic nucleus. J Comp Neurol. 2008;506:708–732. doi: 10.1002/cne.21553. [DOI] [PubMed] [Google Scholar]
- 45.Castel M, Morris JF. Morphological heterogeneity of the GABAergic network in the suprachiasmatic nucleus, the brain's circadian pacemaker. J Anat. 2000;196:1–13. doi: 10.1046/j.1469-7580.2000.19610001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moore RY, Speh JC. GABA is the principal neurotransmitter of the circadian system. Neurosci Lett. 1993;150:112–116. doi: 10.1016/0304-3940(93)90120-a. [DOI] [PubMed] [Google Scholar]
- 47.Morin LP, Goodless-Sanchez N, Smale L, Moore RY. Projections of the suprachiasmatic nuclei, subparaventricular zone, and retrochiasmatic area in the golden hamster. Neuroscience. 1994;61:391–410. doi: 10.1016/0306-4522(94)90240-2. [DOI] [PubMed] [Google Scholar]
- 48.Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport Phaseolus vulgaris leucoagglutinin in the rat. J Comp Neurol. 1987;258:204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- 49.Parent MB, Laurey PT, Wilkniss S, Gold PE. Intraseptal infusions of muscimol impair spontaneous alternation performance: Infusions of glucose into the hippocampus, but not the medial septum, reverse the deficit. Neurobiol Learn Mem. 1997;68:75–85. doi: 10.1006/nlme.1997.3769. [DOI] [PubMed] [Google Scholar]
- 50.Degroot A, Parent MB. Infusions of physostigmine into the hippocampus or the entorhinal cortex attenuate avoidance retention deficits produced by intra-septal infusions of the GABA agonist muscimol. Brain Res. 2001;920:10–18. doi: 10.1016/s0006-8993(01)02798-6. [DOI] [PubMed] [Google Scholar]
- 51.Krebs DL, Parent MB. Hippocampal infusions of pyruvate reverse the memory-impairing effects of septal muscimol infusions. Eur J Pharmacol. 2005;520:91–109. doi: 10.1016/j.ejphar.2005.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stephan FK, Kovacevic NS. Multiple retention deficit in passive avoidance in rats is eliminated by suprachiasmatic lesions. Behav Biol. 1978;22:456–462. doi: 10.1016/s0091-6773(78)92565-8. [DOI] [PubMed] [Google Scholar]
- 53.Mistlberger RE, de Groot MHM, Bossert JM, Marchant EG. Discrimination of circadian phase in intact and suprachiasmatic nuclei-ablated rats. Brain Res. 1996;739:12–18. doi: 10.1016/s0006-8993(96)00466-0. [DOI] [PubMed] [Google Scholar]
- 54.Ko CH, McDonald RJ, Ralph MR. The suprachiasmatic nucleus is not required for temporal gating of performance on reward-based learning and memory task. Biol Rhythm Res. 2003;34:177–192. [Google Scholar]