Abstract
We have used an inhibiting antibody to determine whether preimmune versus antigen-experienced B cells differ in their requisites for BLyS, a cytokine that controls differentiation and survival. Whereas in vivo BLyS inhibition profoundly reduced naïve B cell numbers and primary immune responses, it had a markedly smaller effect on memory B cells and long-lived plasma cells, as well as secondary immune responses. There was heterogeneity within the memory pools, because IgM-bearing memory cells were sensitive to BLyS depletion whereas IgG-bearing memory cells were not, although both were more resistant than naïve cells. There was also heterogeneity within B1 pools, as splenic but not peritoneal B1 cells were diminished by anti-BLyS treatment, yet the number of natural antibody-secreting cells remained constant. Together, these findings show that memory B cells and natural antibody-secreting cells are BLyS-independent and suggest that these pools can be separately manipulated.
Keywords: B lymphocyte, BAFF, immune memory, mouse
Secondary immune responses and natural antibodies are key elements of protective immunity. During primary immune responses, naïve antigen-reactive B cell clones selectively expand, amplifying their frequency and yielding memory cells (reviewed in ref. 1). Although memory populations are a small proportion of total B cells, they turn over more slowly than their naïve precursors and afford protective immunity upon secondary antigen challenge (2, 3). Primary immune responses also generate long-lived plasma cells (LLPC), which persist indefinitely and maintain systemic antibody levels. Another source of protective antibodies are B1 B cells, some of which constitutively generate “natural” antibodies against endogenous antigens such as phosphorylcholine (PC) (reviewed in ref. 4).
The B lymphocyte stimulator (BLyS) family of cytokines and receptors plays a central role in B cell homeostasis (reviewed in ref. 5). This family includes the ligands BLyS and APRIL and the receptors BR3, TACI, and BCMA. BLyS can bind all three receptors, whereas APRIL binds only TACI and BCMA. Developing B cells begin to express BR3 as they exit the bone marrow and enter the mature follicular (FO) and marginal zone (MZ) pools (6). BLyS regulates these preimmune B cell pools via survival signals delivered through BR3, such that in the absence of either BR3 or BLyS, FO and MZ cells die rapidly (7, 8).
Memory B cells live longer than their naïve precursors (9), raising the question of whether memory B cells still rely on BLyS-BR3 signaling. Furthermore, LLPC express high levels of BCMA (10), which binds either BLyS or APRIL, raising the possibility that either cytokine might afford survival. Similarly, whether natural antibody-forming B1 cells also rely on BLyS is unclear. For example, peritoneal cavity (PerC) B1 cells require neither BLyS nor APRIL for development (11); but some B1 cells express BLyS, and PerC B1 cells also express APRIL (12).
Direct assessment of whether different B lineage subsets vary in their requirements for BLyS has not been possible, because there has been no way to selectively manipulate BLyS availability. Herein we ask whether these B cell subsets rely on BLyS by treating mice with an inhibiting anti-BLyS antibody. Whereas this treatment eliminated preimmune B cells and primary antibody responses, PerC B1 and memory B cell numbers were unaffected. Moreover, both secondary antibody responses and natural antibody production were normal. Finally, BLyS inhibition did not affect established IgG antibody titers or LLPC numbers. Together, these findings demonstrate that natural antibody-forming cells, memory B cells, and LLPC are largely BLyS-independent. Moreover, they suggest that BLyS inhibition may be used to target specific B cell populations.
Results
In Vivo BLyS Inhibition Eliminates Most Primary B Cells.
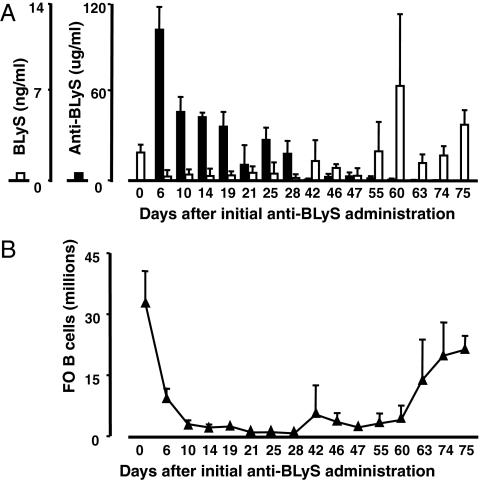
We generated a hamster monoclonal antibody to murine BLyS (10F4) that effectively inhibited BLyS binding to BR3, TACI, and BCMA [supporting information (SI) Fig. S1] and used it for all work described here. Serum anti-BLyS and BLyS levels, as well as splenic FO B cell numbers, were followed after treatment with 100 μg of 10F4 i.p. on days 0 and 5 (Fig. 1 A and B). The half-life of anti-BLyS in vivo was ≈2 weeks, and serum BLyS levels varied reciprocally with anti-BLyS levels. Control hamster IgG1 antibody had no effect on lymphocyte numbers or serum BLyS levels (data not shown).
Fig. 1.
BLyS inhibition in vivo. (A) C57BL/6 mice were treated with 100 μg of anti-BLyS i.p. on days 0 and 5 and subsequently analyzed for serum BLyS (open bars) and anti-BLyS (filled bars). Each bar represents the average ± SD for at least three mice per time point. Untreated mice (n = 34) are shown at day 0. Combined data from three separate experiments are shown. (B) Numbers of splenic follicular (FO) B cells ± SD for the same mice shown in A.
Consistent with their lack of BLyS receptor expression (6, 13), developing bone marrow B cell subsets were unaffected by anti-BLyS treatment (data not shown). In contrast, all preimmune splenic B cell subsets were substantially diminished (Fig. 1B and Fig. S2). Thus, the TR and FO pools were severely reduced after anti-BLyS treatment, and MZ B cells were eliminated. Autoreconstitution began at days 40–45 and mirrored the kinetics observed in other models (14). Transiently elevated BLyS levels were regularly observed at the onset of reconstitution.
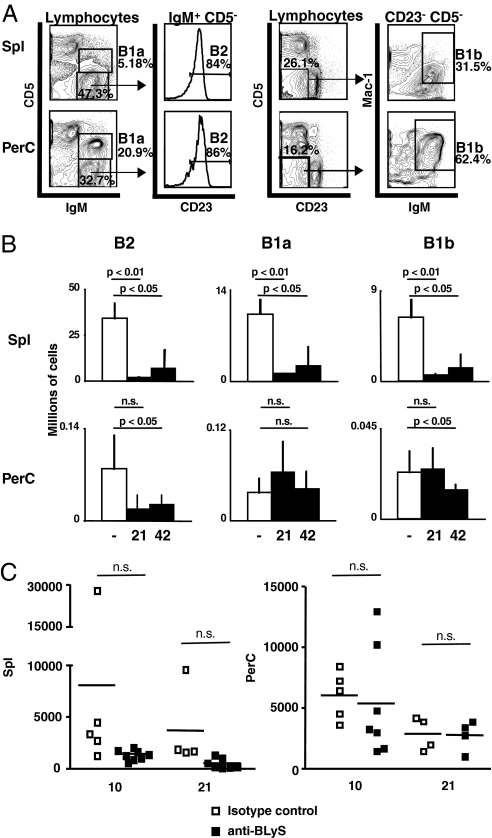
Anti-BLyS treatment ablated splenic but not peritoneal B1 B cells (Fig. 2 A and B), reducing both B1a and B1b subsets in the spleen. To assess B1 function, we measured the number of spontaneous IgM anti-PC antibody-producing cells, because B1 cells are the main producers of such antibodies (15). The numbers of these cells were similar in both spleen and PerC in mice treated with anti-BLyS or isotype control antibody, although variance in the spleen was higher in controls (Fig. 2C).
Fig. 2.
Effects of BLyS inhibition on B1 subsets. (A) Gating scheme for identification of B2, B1a, and B1b cells in spleen (Upper) or peritoneal cavity (Lower). Lymphocyte-gated cells are further identified as B1a (CD5+ IgM+) or B2 (CD5− IgM+) as shown in left plots and histograms; in addition, B1a cells are CD23− (data not shown), whereas B2s are CD23+ and thus include late TR and FO B cells. B1b cells are CD5lo/− CD23− IgM+ Mac1+ as shown in right plots. (B) Numbers of splenic (Upper) and PerC (Lower) B2 and B1 cells. Cell numbers ± SD for three untreated mice (open bars) and three treated mice are shown. n.s., not significant. (C) Numbers of PC-specific antibody-producing cells in spleen and PerC of isotype control-treated (open symbols) or anti-BLyS-treated (filled symbols) mice at 10 and 21 days after treatment. Each symbol represents one mouse.
Memory B Cell Compartments and Responses Resist Anti-BLyS Treatment.
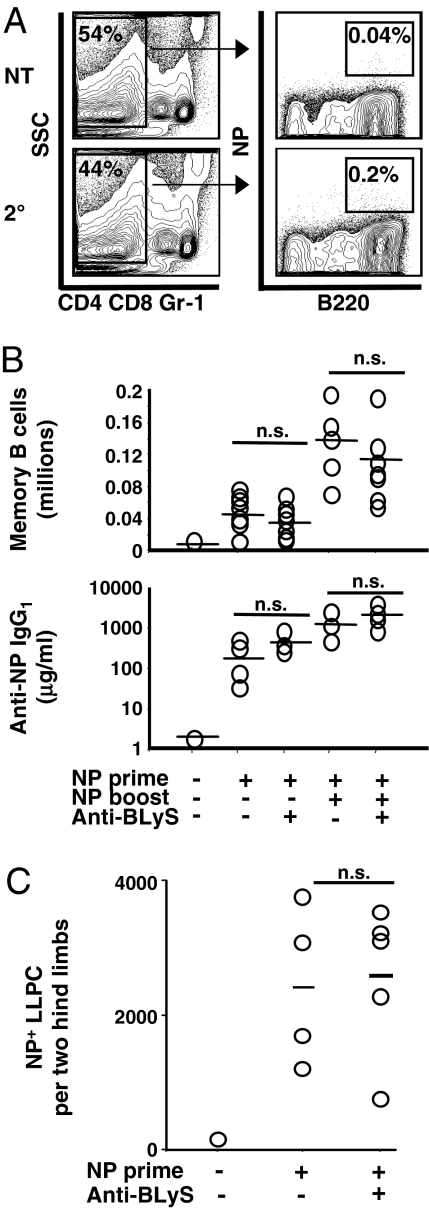
Primary immune responses to both T-dependent (TD) and T-independent (TI) antigens were attenuated in anti-BLyS-treated mice (Fig. S3). To assess the influence of anti-BLyS on NP-specific memory cells and LLPC in wild-type animals, C57BL/6J mice received primary immunizations with NP-CGG, a well characterized TD antigen (Memory System 1) (see refs. 2 and 16 and details in Materials and Methods). Some mice were treated with anti-BLyS ≥8 weeks later, when memory B cells and standing anti-NP titers had been established, but before secondary (booster) immunization with NP-CGG. Neither splenic NP+ memory B cell numbers nor high-affinity IgG1 antibody titers were affected by BLyS inhibition before eliciting a secondary immune response (Fig. 3 A and B). Moreover, the increases in NP+ B cells and NP-specific antibody after booster immunizations were similar in anti-BLyS treated and control mice. Finally, established NP-specific BM LLPC were also unaffected (Fig. 3C).
Fig. 3.
BLyS ablation does not alter NP-reactive memory B cells or LLPC generated in wild-type mice (Memory System 1). (A and B) Memory B cell analysis of C57BL/6 mice immunized with NP-CGG. Primary immunization was at day 0, anti-BLyS treatment was at day 56, and NP booster immunization was at day 77. Memory B cell numbers and IgG titers were assessed at day 84 for all mice including untreated and primed-only controls. NP-reactive memory B cells were identified according to the FACS gating scheme shown in A. After doublet discrimination, DAPI exclusion, and lymphocyte gating, memory B cells were phenotyped as CD4−CD8−Gr-1−B220+NP+. NT, not treated control. NP-reactive memory B cell numbers and high-affinity anti-NP IgG1 antibody titers, as assessed by ELISA, are shown in B. Each symbol in graphs represents an individual mouse, black lines represent means, and combined results from two separate experiments are shown. Symbols at the bottom indicate treatment given (+) or not given (−). (C) Number of NP-specific LLPC in the bone marrow of control mice or anti-BLyS-treated mice at 21 days after anti-BLyS treatment and 77 days after NP-CGG immunization. Phenotyping strategy for LLPC is presented in Fig. S4.
To extend these findings, we evaluated how anti-BLyS affected memory B cell survival in two additional memory systems that were based on adoptive transfer of NP-specific B cells from Vh186.2 transgenic (Tg; System 2) or knockin (KI; System 3) mice into recipients that mount poor endogenous responses to NP-CGG (see refs. 2 and 16 and details in Materials and Methods). These systems produce large numbers of antigen-specific memory B cells, permitting further resolution of memory subsets. In these systems naïve host B cells can serve as internal controls.
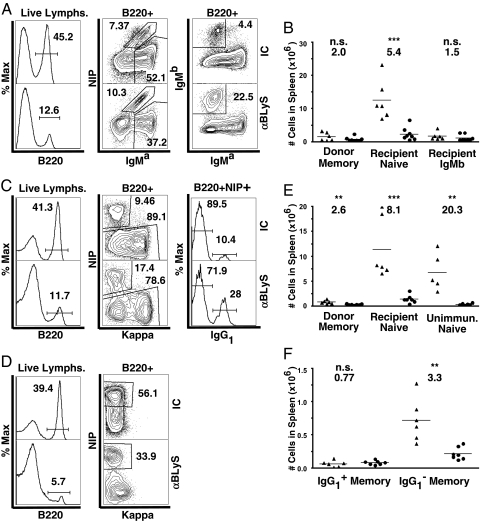
Thirty-three weeks after transfer and immunization, mice from Memory System 2 were treated with anti-BLyS or isotype control given in two doses 5 days apart. After 15 days, naïve recipient B cell numbers were reduced by 5.4-fold (P < 0.001), as predicted (8) (Fig. 4 A and B). In contrast, NP-specific donor-derived IgMa-bearing memory B cells were not as affected by anti-BLyS; they showed only a 2-fold depletion that was not significantly different from control treatment. The host cells that do not express the Tg, and thus are positive for the endogenous IgMb marker, were also not significantly depleted. Although the exact identity of these cells is uncertain, the most likely explanation is that these rare endogenous cells (17) were expanded by environmental antigens and hence are memory cells.
Fig. 4.
Memory B cells generated in adoptive transfer systems are more resistant to BLyS depletion than naïve B cells. Memory B cells were generated from mVh186.2 Tg (Memory System 2; A and B) or Vh186.2 KI (Memory System 3; C, E, and F) donor B cells after adoptive transfer into AM14/Vκ8R double Tg mice and subsequent immunization i.p. with NP-CGG in alum. Naïve controls were unimmunized Vh186.2 KI donor strain (D and E) and recipient IgMa non-NIP-binding (A–C and E) B cells. Recipient mice >12 weeks after immunization and unimmunized donor strain controls were treated with two 100-μg i.p. doses of anti-BLyS (10F4) or hamster IgG isotype control (IC). Fifteen days later, spleens were harvested and analyzed by FACS. (A) Memory System 2. Single, live lymphocytes were identified by FSC and SSC profiles and EMA exclusion. Upper and Lower represent isotype control (IC) versus anti-BLyS-treated animals, respectively. NIP+ IgMa+ B cells were NP-specific donor-derived memory cells. NIP− IgMa+ B cells were recipient AM14/Vκ8R specificity naïve B cells. IgMa− IgMb+ B cells were non-Tg-bearing recipient B cells. This population is expanded in aged recipient mice and may represent endogenous memory B cells. Representative FACS plots are shown. (B) Total numbers of B cells in each subgroup were calculated from live splenocyte counts and FACS-based frequencies as shown in A. Each point represents an individual animal. Average fold depletion in anti-BLyS-treated (circles) versus isotype control-treated (triangles) animals is indicated. One-way Student's t tests were performed. n.s., not significant. **, P ≤ 0.01; ***, P < 0.001. (C) Memory System 3. Live lymphocytes were identified as in A. NIP+ κlow B cells were NP-specific donor-derived memory cells. NIP− B cells were recipient-derived naïve cells. Memory B cells were fractionated into IgG1-switched and nonswitched B cells. (D) Naïve controls for Memory System 3. Spleens were harvested from unimmunized Vh186.2 KI donor-strain mice. Live lymphocytes were gated as described in A. (E and F) Total numbers of B cells from each System 3 subgroup were calculated from live splenocyte counts and FACS-based frequencies.
We next investigated the effects of anti-BLyS in Memory System 3 (Fig. S5), which enabled comparison of class-switched IgG1+ vs. unswitched IgG1− memory B cells. As expected, naïve recipient B cells in immunized mice were reduced 8.1-fold (P < 0.001) after anti-BLyS treatment, as were NP-binding naïve B cells from unimmunized donor mice (Fig. 4 C–F). In contrast, the total donor-derived memory B cell pool was reduced only 2.6-fold (P < 0.01), similar to the extent of reduction seen in System 2 and substantially different from effects on naïve B cells. Among these donor-derived memory B cells, the IgG1− memory cells, which are nearly entirely unswitched IgM-bearing cells (our unpublished observations), were reduced 3.3-fold (P < 0.01). In contrast, the IgG1+ memory B cells were not significantly depleted. Thus, while all memory B cells were relatively resistant to BLyS depletion compared with their naïve precursors, unswitched IgM-bearing memory cells remain somewhat BLyS-dependent, and IgG-bearing memory cells do not.
Discussion
Mature B cells in preimmune pools depend on BLyS for survival, as evidenced by their rapid disappearance after in vivo BLyS inhibition. In contrast, memory B cells resist BLyS depletion, and recall responses are normal. Furthermore, neither LLPC nor standing antibody titers are impacted by BLyS inhibition. Finally, PerC B1 cells and natural antibody-forming cells are resistant to BLyS depletion. Together, these findings indicate that preimmune and memory B cell pools are governed by distinct survival requisites, suggesting that BLyS inhibition, while eliminating naïve B cells and primary responses, will spare most elements of acquired and natural humoral immunity.
The loss of most naïve B cells after BLyS inhibition is consistent with the lack of FO and MZ B cells seen in BLyS- and BR3-deficient mice (13, 18, 19). Similarly, the attenuation of primary TD and TI responses mirrors prior findings in BLyS-deficient mice (20), reflecting the elimination of preimmune subsets. Because the anti-BLyS used herein blocks BLyS binding to its receptors (Fig. S1), the most likely mechanism involves competition for soluble BLyS that blocks the BR3 signaling required for TR, FO, and MZ B cell survival. Indeed, antigen-experienced subsets also express BLyS binding receptors but were selectively spared, making direct cytotoxic effects unlikely.
BLyS inhibition reduced splenic B1a and B1b pools 2-fold, but peritoneal B1 cells were unaffected, suggesting that they are independently regulated. Because splenic B1 cell numbers and turnover rates are normal in BR3 mutant mice (21), BLyS signaling via TACI or BCMA may influence B1 survival or compartmentalization (20). Alternatively, uncompromised splenic architecture may contribute to splenic B1 maintenance (22). Natural antibody production in anti-BLyS-treated mice is consistent with the idea of functionally distinct B1 subsets in spleen and PerC (23) and suggests that BLyS dependence may distinguish these.
The relative resistance of memory and LLPC to BLyS inhibition suggests that they use alternative survival mechanisms. One possibility is a shift to APRIL dependence. Indeed, activated B cell and plasma cell populations up-regulate TACI and BCMA (but not BR3), both of which can bind APRIL more avidly than BLyS and can promote survival (10, 24, 25). Similarly, memory B cells have increased TACI and decreased BR3 expression but do not appear to express BCMA (26). Memory B cells share the expression of several receptors involved in regulating the renewal of stem cell populations (26), so ligands for these receptors may replace BLyS as key survival factors. TLR stimulation is required for activation of human B cells (27) and leads to TACI up-regulation on murine FO and MZ cells (24), suggesting that TLR ligands play a role.
Concurrent studies by Benson et al. (28) have shown that memory B cells require neither BLyS nor APRIL. Our observations confirm these findings and extend them in two ways. First, we have shown that isotype-switched memory cells are more resistant to BLyS depletion than nonswitched IgM-bearing memory cells. This partial dependence of unswitched memory cells is consistent with observations that unswitched human CD27+ memory cells are intermediate in phenotype between naïve and switched memory cells (29, 30) and supports the notion of heterogeneity among memory B cells (2, 31). We also show heterogeneity within the B1 B cell compartment, because BLyS inhibition spared PerC B1 cells but left a residual B1 subset in the spleen that functionally resembles PerC B1 cells.
Our results suggest that targeting the BLyS/BR3 axis (32, 33) will spare memory, LLPC, and natural antibody-producing B cell populations. Because many human B cell lymphomas are likely derived from postgerminal center cells (34) and autocrine BLyS attenuates apoptosis of non-Hodgkin's lymphoma B cells (35), these data provide an important caveat for BLyS/BR3 targeting in B cell malignancies. Similarly, if autoimmune pathology stems from memory or LLPCs (36), BLyS-targeted treatments may prove ineffective. However, in some autoimmune situations self-reactive antibodies may be replenished by activation of extrafollicular foci and short-lived antibody-forming cells (37). In these scenarios, such directed therapies might deplete pathogenic naïve B cells but spare subsets that maintain induced and natural immunity.
Materials and Methods
Mice.
Female C57BL/6 mice (age 6–12 weeks) were obtained from The Jackson Laboratory. The mVh186.2 Tg, Vh186.2 KI (B1.8), and AM14 Vk8R CB17 strains have been described (38–40). mVh186.2 Tg and Vh186.2 KI mice were maintained on the Jh knockout (KO) strain (41) and crossed to Jκ KO mice (42) to increase the frequency of NP-reactive B cells. All procedures were approved by the University of Pennsylvania or the Yale University Institutional Animal Care and Use Committees.
BLyS and Anti-BLyS Assays.
Serum BLyS was measured by ELISA using mBAFFR-Fc (Alexis) as a capture reagent and an anti-murine BLyS monoclonal antibody (16D7; Human Genome Sciences) as a detector. Samples were diluted to a final concentration of 10% matrix on the assay plate. The limit of detection was 0.8 ng/ml. Serum anti-BLyS (10F4) levels were measured by ELISA; the limit of quantification was 0.075 μg/ml, and the limit of detection was 0.039 μg/ml.
In Vivo Inhibition of BLyS.
Monoclonal IgG1 hamster anti-mouse BLyS (clone 10F4; Human Genome Sciences) was diluted in PBS and injected i.p. Purified Armenian hamster IgG1 (clone G235-2356; BD Pharmingen) was the isotype control.
Anti-NP Antibody ELISAs.
Plates were coated with 10 μg/ml NP3BSA in 100 mM bicarbonate buffer and blocked with 2% BSA in PBS, and serum dilutions were incubated for 1 h. NP-specific IgG1 standard was a gift of G. Kelsoe (Duke University, Durham, NC). HRP-conjugated goat anti-mouse IgG1 (Southern Biotechnology Associates) was used for detection with a TMB substrate kit (BD Biosciences). Washes were performed by using PBS plus 0.1% Tween 20.
Anti-PC Antibody ELISPOTS.
Immobilon-P plates (Millipore) were coated with 10 μg/ml PC16-BSA (Biosearch Technologies) and blocked with 2% BSA in PBS. After erythrocyte lysis, splenic or PerC cell suspensions were enumerated, and cell suspensions were diluted 2-fold, then incubated for 4 h. Plates were developed with biotin-conjugated anti-mouse IgM (Southern Biotechnology Associates) followed by ExtrAvidin-Alkaline Phosphatase using NBT/BCIP substrate (Sigma); color development was terminated with 1 M NaH2PO4. Spots were enumerated on CTL-ImmunoSpot reader (Cellular Technologies). The number of background spots obtained with BSA-coated wells was subtracted from the number of spots counted on PC16-coated wells for each mouse.
Generation of TD Primary and Memory Responses in Wild-Type Mice.
For primary responses, female C57BL/6J mice were immunized i.p. with 50–100 μg of NP16-CGG in alum (2, 16). To generate memory B cells and LLPC (Memory System 1), mice were rested ≥8 weeks after primary immunization, then rechallenged with 50 μg of i.p. NP16-CGG. Memory B cells, LLPC, and serum NP+ antibodies were analyzed 7 days after the boost dates.
Generation of Naïve and TD Memory B Cells in Adoptive Transfer Systems.
“Memory System 2” has been described in detail previously (2, 26). Briefly, splenocytes containing 1 × 106 NP-specific naïve B cells from mVh186.2 Ig heavy chain transgenic (Tg) Jh KO mice (39) were adoptively transferred into recipient mice carrying rearranged Ig Tgs of irrelevant specificities (AM14 IgH and Vκ8R, which produce anti-IgG2aa antibody), and which therefore mount poor endogenous responses to NP. Six hours after transfer, recipient mice were immunized i.p. with 50 μg of NP25-CGG in alum. More than 12 weeks after transfer, when recently activated germinal center B cells (3, 39, 43) were undetectable, there was a large and stable donor-derived NP-specific memory B pool, identified as NP-binding IgMa+ B cells. This population was virtually (≈99%) free of “contaminating” donor-derived naïve B cells, because few donor B cells survived in the absence of immunization. The vast majority of recipient-derived B cells were AM14/Vκ8R Ig-bearing naïve cells. There was also a small population of recipient-derived non-Tg-bearing B cells, but, because recipient mice were on the CB.17 background, these were readily distinguished from BALB/c donor B cells by Ig allotype (Igb versus Iga, respectively). A further advantage of this system is that mVh186.2 Tg-derived B cells do not secrete NP-specific antibody (16), which can confound memory cell identification based on NP specificity alone (44, 45). Additionally, because the Vh186.2 Tg prevents class switch, this system models IgM B cell memory.
Memory System 3 (Fig. S5) was identical to System 2, except donor B cells were isolated from Vh186.2 KI (B1.8) mice (38). An advantage of this model is that B cells can undergo isotype class switching. More than 12 weeks after transfer and immunization, NP-specific donor-derived B cells averaged 1.1 million per spleen in comparison to 25,000 per spleen in adjuvant-only-treated animals. Thus, in immunized animals, an average of 2.3% of NP-specific B cells were naïve whereas 97.7% were memory. In System 3, donor-derived memory B cells were identified as NP-binding κlow IgG1+ or IgG1− (virtually all of which are IgM-bearing) cells.
Antibodies and Flow Cytometry.
Splenocytes and bone marrow were harvested and stained as described (ref. 50 and Fig. S2). Splenic and PerC B1 B cells were analyzed by using the following antibodies: FITC-anti-CD5, PE-anti-Mac-1 (CD11b), biotin-anti-CD23, and APC-anti-IgM (all from BD Biosciences). NP-binding B cell analysis in Memory System 1 used the following antibodies: DAPI vital dye (Invitrogen), PE-Cy5-anti-CD4 and anti-CD8, PE-anti-Syndecan-1, FITC-anti-GL7 (BD), PE-Cy5-anti-Gr-1 (eBioscience), biotin-anti-IgD, FITC-anti-lambda, FITC-anti-Igβ (Southern Biotechnology Associates), Streptavidin-Pacific Blue (Invitrogen), PE-Cy7-anti-B220 (eBioscience) and APC-NP (M.P.C. laboratory). Analyses of Systems 2 and 3 used APC-Cy7-anti-B220 (RA3-6B2; BD), FITC-anti-IgG1 (A85-1; BD), and AI488-anti-IgMa (RS3.1), biotinylated anti-IgMb (AF6-78), Pacific Blue anti-κ (187.1), and APC-NIP, all produced in the M.J.S. laboratory. Data were collected on a BD LSR II flow cytometer and analyzed with FlowJo software (Tree Star).
Supplementary Material
Acknowledgments.
This work was supported by U.S. Public Health Service Grants AI054488 (to M.P.C.), AI073939 (to M.P.C.), A143603 (to M.J.S.), and T32 AI-055428 (to J.E.C.); by a sponsored research agreement from Human Genome Sciences, Inc. (M.P.C.); a Goldie Simon Preceptorship from the Lupus Foundation of America (to J.E.C.); and grants from the Dermatology and Arthritis Foundations (to M.M.T.).
Footnotes
Conflict of interest statement: M.P.C. was supported in part by a sponsored research agreement between Human Genome Sciences, Inc., and the University of Pennsylvania.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807841105/DCSupplemental.
References
- 1.Zubler RH. Naive and memory B cells in T-cell-dependent and T-independent responses. Springer Semin Immunopathol. 2001;23:405–419. doi: 10.1007/s281-001-8167-7. [DOI] [PubMed] [Google Scholar]
- 2.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. J Exp Med. 2007;204:2103–2114. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schittek B, Rajewsky K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 1990;346:749–751. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- 4.Baumgarth N, Tung JW, Herzenberg LA. Inherent specificities in natural antibodies: A key to immune defense against pathogen invasion. Springer Semin Immunopathol. 2005;26:347–362. doi: 10.1007/s00281-004-0182-2. [DOI] [PubMed] [Google Scholar]
- 5.Kalled SL, Ambrose C, Hsu YM. The biochemistry and biology of BAFF, APRIL, and their receptors. Curr Dir Autoimmun. 2005;8:206–242. doi: 10.1159/000082105. [DOI] [PubMed] [Google Scholar]
- 6.Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol. 2002;168:5993–5996. doi: 10.4049/jimmunol.168.12.5993. [DOI] [PubMed] [Google Scholar]
- 7.Yan M, et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11:1547–1552. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]
- 8.Schiemann B, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 9.Anderson SM, Tomayko MM, Shlomchik MJ. Intrinsic properties of human and murine memory B cells. Immunol Rev. 2006;211:280–294. doi: 10.1111/j.0105-2896.2006.00398.x. [DOI] [PubMed] [Google Scholar]
- 10.O'Connor BP, et al. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider P, et al. Maturation of marginal zone and follicular B cells requires B cell activating factor of the tumor necrosis factor family and is independent of B cell maturation antigen. J Exp Med. 2001;194:1691–1697. doi: 10.1084/jem.194.11.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu VT, Enghard P, Riemekasten G, Berek C. In vitro and in vivo activation induces BAFF and APRIL expression in B cells. J Immunol. 2007;179:5947–5957. doi: 10.4049/jimmunol.179.9.5947. [DOI] [PubMed] [Google Scholar]
- 13.Gross JA, et al. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. Impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 14.Allman DM, Ferguson SE, Lentz VM, Cancro MP. Peripheral B cell maturation. II. Heat-stable antigen(hi) splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells. J Immunol. 1993;151:4431–4444. [PubMed] [Google Scholar]
- 15.Zhong X, Tumang JR, Gao W, Bai C, Rothstein TL. PD-L2 expression extends beyond dendritic cells/macrophages to B1 cells enriched for V(H)11/V(H)12 and phosphatidylcholine binding. Eur J Immunol. 2007;37:2405–2410. doi: 10.1002/eji.200737461. [DOI] [PubMed] [Google Scholar]
- 16.Anderson SM, Hannum LG, Shlomchik MJ. Memory B cell survival and function in the absence of secreted antibody and immune complexes on follicular dendritic cells. J Immunol. 2006;176:4515–4519. doi: 10.4049/jimmunol.176.8.4515. [DOI] [PubMed] [Google Scholar]
- 17.Shlomchik MJ, Zharhary D, Saunders T, Camper SA, Weigert MG. A rheumatoid factor transgenic mouse model of autoantibody regulation. Int Immunol. 1993;5:1329–1341. doi: 10.1093/intimm/5.10.1329. [DOI] [PubMed] [Google Scholar]
- 18.Harless SM, et al. Competition for BLyS-mediated signaling through Bcmd/BR3 regulates peripheral B lymphocyte numbers. Curr Biol. 2001;11:1986–1989. doi: 10.1016/s0960-9822(01)00598-x. [DOI] [PubMed] [Google Scholar]
- 19.Yan M, et al. Identification of a receptor for BLyS demonstrates a crucial role in humoral immunity. Nat Immunol. 2000;1:37–41. doi: 10.1038/76889. [DOI] [PubMed] [Google Scholar]
- 20.Shulga-Morskaya S, et al. B cell-activating factor belonging to the TNF family acts through separate receptors to support B cell survival and T cell-independent antibody formation. J Immunol. 2004;173:2331–2341. doi: 10.4049/jimmunol.173.4.2331. [DOI] [PubMed] [Google Scholar]
- 21.Lentz VM, Hayes CE, Cancro MP. Bcmd decreases the life span of B-2 but not B-1 cells in A/WySnJ mice. J Immunol. 1998;160:3743–3747. [PubMed] [Google Scholar]
- 22.Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002;195:771–780. doi: 10.1084/jem.20011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tumang JR, Hastings WD, Bai C, Rothstein TL. Peritoneal and splenic B-1 cells are separable by phenotypic, functional, and transcriptomic characteristics. Eur J Immunol. 2004;34:2158–2167. doi: 10.1002/eji.200424819. [DOI] [PubMed] [Google Scholar]
- 24.Treml LS, et al. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J Immunol. 2007;178:7531–7539. doi: 10.4049/jimmunol.178.12.7531. [DOI] [PubMed] [Google Scholar]
- 25.Mantchev GT, Cortesao CS, Rebrovich M, Cascalho M, Bram RJ. TACI is required for efficient plasma cell differentiation in response to T-independent type 2 antigens. J Immunol. 2007;179:2282–2288. doi: 10.4049/jimmunol.179.4.2282. [DOI] [PubMed] [Google Scholar]
- 26.Tomayko MM, et al. Systematic comparison of gene expression between murine memory and naive B cells demonstrates that memory B cells have unique signaling capabilities. J Immunol. 2008;181:27–38. doi: 10.4049/jimmunol.181.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol. 2006;36:810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- 28.Benson MJ, et al. Cutting edge: The dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J Immunol. 2008;180:3655–3659. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- 29.Bryant VL, et al. Cytokine-mediated regulation of human B cell differentiation into Ig-secreting cells: Predominant role of IL-21 produced by CXCR5+ T follicular helper cells. J Immunol. 2007;179:8180–8190. doi: 10.4049/jimmunol.179.12.8180. [DOI] [PubMed] [Google Scholar]
- 30.Good KL, Bryant VL, Tangye SG. Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol. 2006;177:5236–5247. doi: 10.4049/jimmunol.177.8.5236. [DOI] [PubMed] [Google Scholar]
- 31.Sanz I, Wei C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol. 2008;20:67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cambridge G, et al. Circulating levels of B lymphocyte stimulator in patients with rheumatoid arthritis following rituximab treatment: Relationships with B cell depletion, circulating antibodies, and clinical relapse. Arthritis Rheum. 2006;54:723–732. doi: 10.1002/art.21650. [DOI] [PubMed] [Google Scholar]
- 33.Halpern WG, et al. Chronic administration of belimumab, a BLyS antagonist, decreases tissue and peripheral blood B-lymphocyte populations in cynomolgus monkeys: Pharmacokinetic, pharmacodynamic and toxicologic effects. Toxicol Sci. 2006;91:586–599. doi: 10.1093/toxsci/kfj148. [DOI] [PubMed] [Google Scholar]
- 34.Kuppers R, Klein U, Hansmann ML, Rajewsky K. Cellular origin of human B-cell lymphomas. N Engl J Med. 1999;341:1520–1529. doi: 10.1056/NEJM199911113412007. [DOI] [PubMed] [Google Scholar]
- 35.He B, et al. Lymphoma B cells evade apoptosis through the TNF family members BAFF/BLyS and APRIL. J Immunol. 2004;172:3268–3279. doi: 10.4049/jimmunol.172.5.3268. [DOI] [PubMed] [Google Scholar]
- 36.Tiller T, et al. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–213. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Lansford R, Manis JP, Sonoda E, Rajewsky K, Alt FW. Ig heavy chain class switching in Rag-deficient mice. Int Immunol. 1998;10:325–332. doi: 10.1093/intimm/10.3.325. [DOI] [PubMed] [Google Scholar]
- 39.Hannum LG, Haberman AM, Anderson SM, Shlomchik MJ. Germinal center initiation, variable gene region hypermutation, and mutant B cell selection without detectable immune complexes on follicular dendritic cells. J Exp Med. 2000;192:931–942. doi: 10.1084/jem.192.7.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prak EL, Weigert M. Light chain replacement: A new model for antibody gene rearrangement. J Exp Med. 1995;182:541–548. doi: 10.1084/jem.182.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J, et al. Immunoglobulin gene rearrangement in B cell deficient mice generated by targeted deletion of the JH locus. Int Immunol. 1993;5:647–656. doi: 10.1093/intimm/5.6.647. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, et al. B cell development in mice that lack one or both immunoglobulin kappa light chain genes. EMBO J. 1993;12:821–830. doi: 10.1002/j.1460-2075.1993.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi Y, Ohta H, Takemori T. Fas is required for clonal selection in germinal centers and the subsequent establishment of the memory B cell repertoire. Immunity. 2001;14:181–192. doi: 10.1016/s1074-7613(01)00100-5. [DOI] [PubMed] [Google Scholar]
- 44.Wolniak KL, Noelle RJ, Waldschmidt TJ. Characterization of (4-hydroxy-3-nitrophenyl)acetyl (NP)-specific germinal center B cells and antigen-binding B220- cells after primary NP challenge in mice. J Immunol. 2006;177:2072–2079. doi: 10.4049/jimmunol.177.4.2072. [DOI] [PubMed] [Google Scholar]
- 45.Bell J, Gray D. Antigen-capturing cells can masquerade as memory B cells. J Exp Med. 2003;197:1233–1244. doi: 10.1084/jem.20020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.