Abstract
The existence of ancient deep-water lakes provides an opportunity to study the independent adaptation of aquatic organisms to pelagic, benthic, and rocky shore habitats. With improving resolution of their phylogenetic relationships, the many cichlid fish species endemic to the African Great Lakes Malawi, Tanganyika, and Victoria provide a significant resource for the comparative study of such evolutionary processes. Here, we show that cichlid lineages colonizing rocky shores and pelagic habitats in the different lakes have independently evolved larger eggs and lower fecundities than benthic lineages, suggesting parallel adaptive life-history evolution. By contrast, other pelagic teleost fishes in both marine and freshwater habitats, including African lakes, typically produce large numbers of very small eggs. Our results also suggest that decreased fecundity and increased egg size not only occurred independently in each lake but occurred independently in the colonization of rocky and pelagic habitats.
Keywords: parallelism, phylogenetically controlled comparative analysis, phylogeny
Elucidating whether similar ecological specializations in closely related lineages are the result of unique or repeated evolutionary events is of particular interest in the understanding of adaptive evolution (1, 2). The demonstration of parallel evolution of closely related yet phylogenetically distinct lineages in similar environments provides strong evidence of evolution driven by natural selection because genetic drift is unlikely to produce repeated evolution in the same direction (3, 4). Instances of parallelism abound in animal evolution (5), but most cases refer to morphological traits such as in cave amphipods (6–8), Anolis lizards (9), or lake whitefish (10). Examples of repeated evolution of the same life-history traits are scarcer, although a convincing case has been made for independent adaptations to predator-mediated mortality in several populations of Trinidadian guppies (11). To build such a case, one needs to demonstrate that multiple populations or species exposed to similar environments have evolved similar traits independently in different localities and evidence that the similarities among populations are the product of the same sort of natural selection (11).
The cichlid fishes of the East African Great Lakes represent an ideal model for the study of parallel evolution. An estimated 660–1,319 species of cichlid fishes have been recorded from Lakes Malawi, Tanganyika, and Victoria, most endemic to a single lake catchment (12). These species flocks are so rich that, collectively, they provide the best example of rapid adaptive radiation in vertebrates (13–16). The independent radiations (14, 15) in the three lakes have produced very similar communities. Many of these species are habitat specialists, with most of them confined either to rocky shores or to sand/mud bottoms (benthic), whereas a few are found in open water (pelagic) habitats (17). There are several well documented examples of parallel evolution of morphology associated with independent colonization of similar habitats (18–20) as well as parallel evolution of coloration (21, 22). Here, we show that parallel evolution of habitat specialization in East African Great Lake cichlids is further accompanied by hitherto undocumented parallel evolution of life-history traits and by a reproductive strategy exceptional for pelagic teleost fish. Unusually, all known cichlid species provide parental care of eggs and young. Many species are biparental substrate-spawners, whose eggs and young are guarded by both parents. Many other species are maternal mouthbrooders, in which females pick up and brood eggs and larvae for a few weeks, with some species even allowing independently feeding young to return to the mouth when they are threatened with predation (17, 23). All cichlid species from Lake Victoria and all except one from Lake Malawi are maternal mouthbrooders. Although nearly every type of parental care is represented in Lake Tanganyika, all pelagic species and many of the rocky shore and benthic species are mouthbrooders [supporting information (SI) Table S1 and SI Text]. Mouthbrooders typically produce fewer, larger eggs than substrate spawners (17), and therefore, to avoid the confounding effect of the type of parental care on the ecological correlates of life-history strategies, all species included in this study are mouthbrooders. We compared batch fecundities and egg sizes of cichlid fishes from pelagic, benthic, and rocky-shore habitats in all three lakes using new and published data. We adopted a two-step approach for data analysis: first, testing for significant effects on the life-history traits with linear models and then assessing whether significant effects were confounded by phylogenetic nonindependence among species (24). The second step required the estimation of a phylogeny for the species included in our dataset. The phylogeny of the Victorian cichlids is still poorly resolved (25), and DNA sequences were available for few species for which we had life-history data. For Lakes Malawi and Tanganyika, we used comparative methods to account for the effect of phylogenetic relationships on life-history correlations (26, 27) and Markovian models to reconstruct evolutionary transitions among habitats (28).
Results
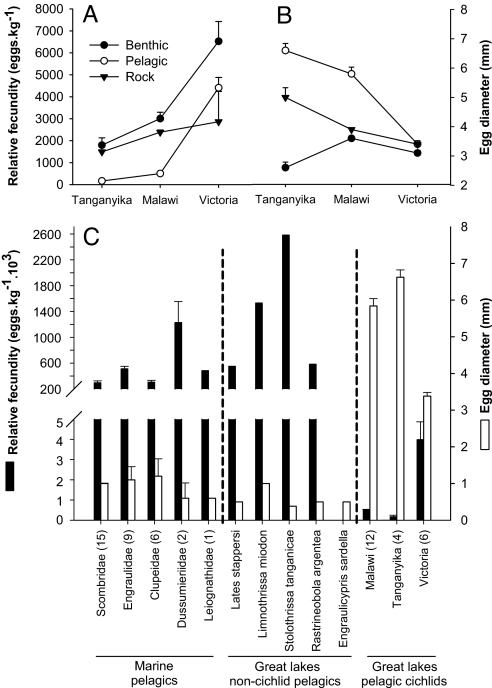
Fecundities differed significantly among habitats and lakes (Fig. 1A and Table 1), but the lack of a significant interaction indicated similar contrasts among habitats in all three lakes. Controlling for phylogeny (Fig. 2A), the difference among lakes was no longer significant, which was expected because the haplochromine species flocks of lakes Malawi and Victoria represent independent colonizations (14), but the habitat effect remained significant. In the analysis without phylogenetic correction, egg diameter varied significantly among both habitats and lakes (Fig. 1B and Table 1), but again, the difference among lakes was confounded with phylogeny (Fig. 2B). A significant interaction term, even after phylogenetic correction (Fig. 2B), indicated that the contrast among habitats was different between lakes. Egg size differences among habitats were greatest in Tanganyika and least in Victoria (Fig. 1B).
Fig. 1.
Pelagic cichlid fishes have lower fecundities (A) and larger eggs (B) than benthic and rocky shore cichlid fishes from the same lake, and (C) pelagic fishes of other families. Shown are means and standard errors, with the number of species in brackets. Data sources are in SI Text.
Table 1.
Cichlid fecundities and egg diameters differed significantly between habitats and lakes
| Effect | Number of eggs |
Egg diameter |
||||
|---|---|---|---|---|---|---|
| F | df | P | F | df | P | |
| Habitat | 38.61 | 2, 123 | < 0.001 | 15.711 | 2, 115 | < 0.001 |
| Lake | 16.06 | 2, 123 | < 0.001 | 24.243 | 2, 115 | < 0.001 |
| Lake × habitat | 1.55 | 4, 119 | 0.193 | 16.437 | 4, 115 | < 0.001 |
| Body mass | 120.8 | 1, 119 | < 0.001 | 6.450 | 1, 115 | 0.012 |
The relationships between egg diameter and habitat varied between lakes (analyses of covariance).
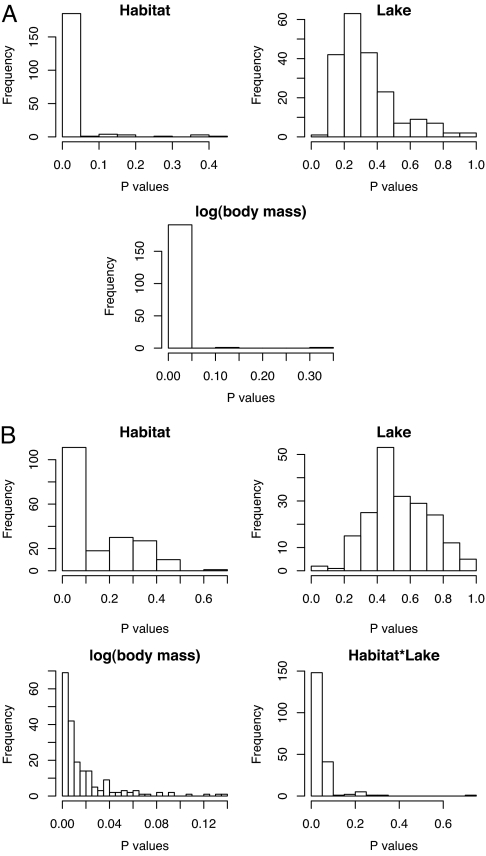
Fig. 2.
Histograms of the P values from the GEE-based phylogenetically controlled comparative analyses for clutch size (A) and oocyte diameter (B). The comparative analyses were repeated for the 200 bootstrap trees to assess whether their results were sensitive to a particular phylogeny. The effects tested were those found significant in the ANCOVA analysis (Table 1). For each of the 200 trees, the comparative analysis was run, and the P values of the tested effects were stored. Most P values for the effect of habitat, and the interaction lake–habitat in the case of egg size, were <0.05, showing that these effects were not affected by the use of a particular phylogeny. However, the P values of the effect of lake were mostly distributed around 0.5, indicating that this effect was mostly because of the phylogenetic relationships among species.
In all three lakes, pelagic species had the lowest fecundities and largest eggs, whereas benthic species had the highest fecundities and smallest eggs (Fig. 1 A and B). Rock-dwelling species showed intermediate characteristics. Within habitats, Tanganyikan species had the lowest fecundities and largest eggs (except for benthic species), Victorian species had the highest fecundities and smallest eggs, and Malawian species had intermediate characteristics.
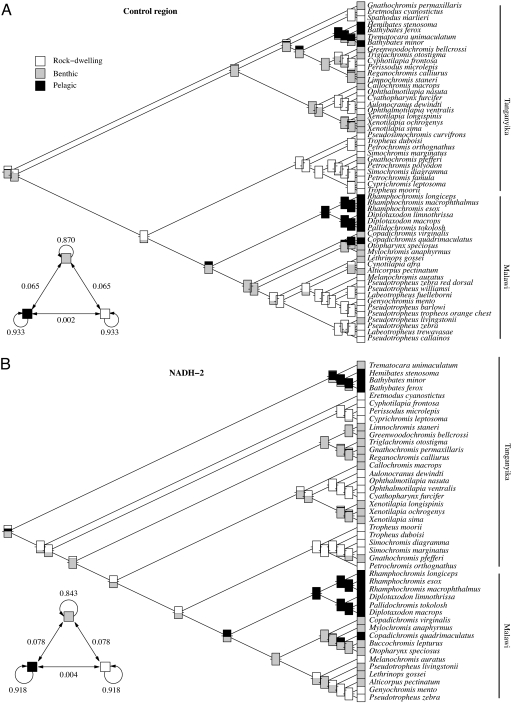
Because habitat is important for the life history evolution of these African cichlids, the rates of transition among habitats during evolution were estimated by using the reconstructed phylogenies and Markovian models. The best fitting model assumed equal rates of transition between benthic and pelagic habitats and between the benthic and rocky ones but no direct transitions between the pelagic and rocky habitats (Fig. 3). Maximum-likelihood estimation of ancestral states suggested that the most recent common ancestors of both Tanganyikan and Malawian endemic cichlids were benthic species and that pelagic and rocky habitats were independently colonized from benthic ancestors in each lake, with no transition between rocky and pelagic habitats. These results suggest that decreased fecundity and increased egg size not only occurred independently in each lake but occurred independently in the colonization of rocky and pelagic habitats.
Fig. 3.
Reconstruction of ancestral character states for habitat in Lake Tanganyika and Lake Malawi using phylogenies estimated from control region (A) and NADH-2 (B). In both cases, the model selected by the AIC assumed equal transition rates between rock-dwelling and benthic species and between pelagic and benthic ones, whereas the transition rate between rock-dwelling and pelagic was assumed to be zero. The Inset below each tree shows typical probabilities of transitions among the three habitats after exponential transformation of the rate matrix for a time scale typical of the trees estimated (0.01). Transition between rock-dwelling and pelagic habitats is made possible via the benthic habitat. Both neighbor-joining trees are in agreement with recent phylogenetic analyses of the Great Lakes cichlids (14).
Strikingly, in all three lakes, pelagic cichlids have independently evolved an exceptional reproductive strategy for pelagic teleosts. Whereas most marine pelagic teleosts produce large numbers of small eggs that develop as pelagic larvae (29–31), pelagic cichlids evolved the opposite strategy by producing only small quantities of large eggs and mouthbrooding both eggs and fry for long periods. It is notable that pelagic species of other fish families are found in the same lakes, and these have high fecundities, small eggs, and no parental care. These include clupeids (Stolothrissa tanganicae, Limnothrissa miodon) and centropomids (Lates spp.) in Lake Tanganyika and cyprinids in Lakes Malawi (Engraulicypris sardella) and Victoria (Rastrineobola argentea) (Fig. 1C).
Discussion
It appears that in Lakes Malawi and Tanganyika and perhaps also in Lake Victoria, the benthic cichlid fish independently colonized rocky and pelagic habitats. The ancestors of lacustrine cichlids are likely to have come from rivers (14), where pelagic zones are nonexistent, and rocky habitats are generally transient or confined to areas of rapid water flow very different from the still waters of lakes. In each lake, a consistent tendency for increased egg diameter and reduction of clutch size was observed as benthic species adapted to either rocky or pelagic habitats.
Life-history theory is dominated by the concept of tradeoffs (32). Our data do not permit us to consider directly the overall level of lifetime or per-clutch reproductive investment nor to estimate postzygotic investment but only to consider, for a given level of total prezygotic investment in oocyte production, the within-clutch tradeoff between offspring number and per-offspring investment (33). Such a tradeoff is well known among fishes, from comparisons between species (34), populations (35), and even siblings (36).
Physical factors may favor the production of few, large eggs by pelagic mouthbrooding cichlids, at least some of which spawn off the substrate in Lakes Malawi (37), Tanganyika (38, 39), and Victoria (40). Midwater-spawning females spin round to catch their eggs, and the laying of fewer eggs at a time may increase retrieval rates and reduce predation risk. However, this advantage does not apply to rocky-shore species that lay their eggs on the bottom.
All known cichlid fish provide parental care to their offspring (23). All of the species in our study are maternal mouthbrooders, but there may be differences between species in the nature and duration of parental care. Riverine maternal mouthbrooding cichlids, like their biparental substrate-spawning relatives, typically care for free-swimming independently feeding fry for periods lasting weeks or months. Although information is patchy and remains to be thoroughly reviewed, many benthic cichlid species in both lakes (and a few rocky-shore species in Malawi) guard free-swimming fry, as apparently do all of the Lake Victoria species irrespective of habitat. Fry guarding may favor the production of larger numbers of smaller offspring, because parental care may increase the survival rates of smaller fry. On rocky shores in Malawi and Tanganyika, fry of many maternal mouthbrooding species are immediately independent on first release, after absorption of the yolk sac, and attempt to establish territories in refuges among rocks (41). As far as we know, there has been little recognition or discussion of the scarcity of parental care of free-swimming fry on rocky shores: The high density of possible predators, the abundance of cover for their approach, and the difficulty in moving a free-swimming brood among the high density of territorial fish may all reduce the benefits of parental care. Notably, the majority of species in Lake Malawi that do provide care for free-swimming fry over rocky habitats are large, often predatory, species that might be expected to be particularly effective guarders (42). Thus, it may be that the principal advantage to the production of large eggs for rocky-shore cichlids lies in the larger size at hatching of the offspring. Larger offspring are likely to be less vulnerable to a certain component of the predator community or vulnerable for a shorter period and be better competitors over food and space (43, 44). It has been suggested that there is high predation pressure on young fish in the crowded rocky shores of the African Great Lakes (17). Less is known about parental strategies of pelagic cichlid fish. Females of the Malawian Rhamphochromis longiceps migrate inshore to release fry in lagoons (45), and mouthbrooding female Rhamphochromis esox are often found over rocks (46), but it is thought that most other species remain offshore throughout their breeding cycle. It is not known whether pelagic cichlids guard free-swimming fry, but juveniles of some species are retained in their mothers' mouths until they are >30 mm and have started exogeneous feeding (47, 48). Prolonged mouthbrooding in pelagic cichlids is probably an adaptation to increase juvenile survival, because increased mouthbrooding duration was positively related to predation risk in other mouthbrooding cichlids (49). Perhaps high-predation risks in the open pelagic zone select against fry guarding and favor large offspring size, but this remains speculative.
Most pelagic marine and freshwater teleost fishes produce many small eggs, whereas pelagic cichlids produce few large eggs. The existence within the same lake of divergent reproductive strategies among fishes living in the same habitat suggests that the direction a particular lineage evolves toward may depend on its starting point (50). Although we lack sufficient information to say which direction they have evolved in since colonizing the lakes, the endemic noncichlid pelagic fishes retain the high fecundity–small eggs pattern typical of the families centropomidae, clupeidae, and cyprinidae (51). Clearly, the pelagic cichlids have not evolved toward this kind of life-history strategy. These cichlids have likely arisen from ancestors that, like all known cichlids, were batch spawners with low fecundities and large eggs. Mouthbrooding cichlids (as in this study) lay even fewer, larger eggs than others (17, 23). Phylogenetic analysis suggests that there have been few, if any, reversals from mouthbrooding to substrate spawning in the evolution of African cichlid parental care (52) and none at all within the Great Lakes. Nevertheless, pelagic cichlids might be expected to have relatively high fecundities and small eggs among mouthbrooding cichlids, rather than the fewer, larger eggs indicated by our analysis.
Low fecundity and large egg size are indications of high levels of parental investment per offspring. Large-scale patchiness of spatial and temporal variability in resource availability is believed to favor production of large numbers of small offspring as a bet-hedging strategy for pelagic teleosts (29, 31). However, reduction of the risk of starvation in prey-poor environments could also select for small broods of large offspring (29). This might apply to cichlids because the pelagic zone of the African Great Lakes is relatively oligotrophic compared with the productive inshore habitats (53, 54). If so, this would suggest that the evolution of large offspring size had different causes among rocky-shore and pelagic cichlids. The small fry of pelagic cyprinids and clupeids initially feed on unicellular algae (55–57), whereas the much larger fry of cichlid fish in all habitats feed mainly on crustacean zooplankton (55, 58, 59). Differential spatiotemporal patchiness of zooplankton and phytoplankton could lead to differing selection pressures among the fish families in the pelagic zones of the African lakes, simply from the initial divergence in offspring size and trophic level.
A comparable approach to offspring size and fecundity in marine species may be illuminating. Although marine pelagic teleosts produce many small offspring, it has been suggested that, among elasmobranchs, transition to a pelagic lifestyle is associated with the evolution of viviparity (60), although this relationship has yet to be quantitatively tested with phylogenetically controlled methods. Elasmobranchs, even more than cichlids, typically produce few, large offspring, and viviparous species have larger offspring (61), although the evidence for reduced fecundity is equivocal (61, 62). Thus, there are intriguing hints that phylogenetically constrained multiple adaptive life-history peaks also exist in the marine pelagic zone.
Our results indicate that an increase in per-egg investment occurred not only in pelagic cichlids but also to a lesser extent in the evolution of specialized rocky-shore cichlids. There are few specialized rocky-shore fishes among the other fish families in the African Great Lakes, but there is a high incidence of parental care among fishes of marine rocky shores (63). This may indicate a common selection pressure with rocky-shore cichlids favoring high per-offspring investment. There are two main life-histories groups among coral reef fishes, each of approximately the same diversity and abundance. The first group mainly consists of species with large body size, long life, and late maturation, which release huge numbers of tiny pelagic eggs (64), often in communal spawning aggregations (65). The other group is mainly made up of small cryptic species that provide parental care for small numbers of benthic eggs (64). So, again, there are interesting hints of a double adaptive peak.
The independent and parallel evolution of similar reproductive traits within the same mouthbrooding mode of parental care in the pelagic, rock-dwelling, and benthic cichlid fishes inhabiting the three lakes strongly suggests that these reproductive strategies are adaptive to the colonization of these habitats. Our results also suggest that decreased fecundity and increased egg size not only occurred independently in each of the three African Great Lakes but occurred independently in the colonization of rocky and pelagic habitats. With finer phylogenetic resolution and more life-history data, future analyses may show further parallel transitions in egg size, fecundity, and perhaps other life-history traits associated with independent transitions between the same habitats by different lineages within the same lake. Additional phylogenetically controlled analysis of life-history traits may reveal parallel evolution and multiple adaptive peaks within other aquatic habitats.
Materials and Methods
Categorization of Taxa.
We analyzed the species most strongly associated with the pelagic zone in each lake and compared them with more rocky-shore- or bottom-living (benthic) cichlids from the same lake. This classification was based on knowledge of the distribution, diet, breeding habits, morphology, and phylogenetic affinities of species. For example, in Lake Malawi, the genera Diplotaxodon and Rhamphochromis comprise at least 20 species of silvery, countershaded, streamlined, midwater-feeding zooplanktivores and piscivores, of which at least 5 species are abundant throughout all open water habitats, even where the bottom depth exceeds 250 m and is deoxygenated. Pallidochromis tokolosh is a deep-water species that feeds on benthic fish (66) but is morphologically similar to Rhamphochromis and has recently been shown to be a member of the Diplotaxodon clade (67, 68). Copadichromis quadrimaculatus is a laterally compressed zooplanktivore, morphologically and genetically similar to other inshore-living species presently classified in Copadichromis. It breeds in shallow rocky and sandy areas, but large adult specimens are an important part of the open-water catches (47, 69). Therefore, C. quadrimaculatus and P. tokolosh were classified as pelagic. Shallow-water, rocky-shore species were distinguished from benthic species occupying sandy or muddy bottoms. For lakes Tanganyika and Victoria, categorization of taxa followed the sources (see Tables S2 and S3).
Sources of Data.
Species identification features for Lake Malawi fish were described elsewhere (70), and nomenclature follows Turner (69) for benthic and offshore species and Ribbink et al. (71) for rock-dwelling species. Benthic and pelagic fish species were collected by monthly trawl catches in the north of the Southwest arm of Lake Malawi. Rock-dwelling species were caught by gill nets in the same area (70). Fish were measured (standard length) to the nearest millimeter and weighed to the nearest 0.1 g. Ripe gonads were fixed in 5% formalin for later determination of gonadosomatic index (GSI = gonad weight/total body weight × 100), fecundity, and mean oocyte weight. Based on gonads in the final preovulation maturation stage (stage 4), fecundity was estimated as the number of oocytes belonging to the size class of the greatest diameter. This group was clearly distinguished by eye and essentially corresponds to the oocytes that would be released in the next spawning bout. Thus, fecundity was measured as the number of oocytes to be released at the next spawn (i.e., batch fecundity). Oocyte weight measurements were carried out on samples preserved for 3 weeks in 5% formalin. The average oocyte weight per female was determined by weighing 50 oocytes belonging to the pool of oocytes considered for fecundity estimates. To allow comparison of oocyte weights of different species, measurements were made at the same vitellogenic stage, when growth has been completed. To determine the GSI threshold above which oocyte weights no longer increase significantly, the mean oocyte weights of females of each species were plotted against their GSI. The GSI corresponding to the beginning of the asymptotic part of the curve (GSIa) was visually determined, and any fish whose GSI was lower than the GSIa were removed. The final GSI threshold was reached when no correlation remained between the mean oocyte weight and the GSI (72).
Data from Lake Malawi species were obtained mainly from our own surveys as well as from published sources (12 pelagic, 34 benthic, and 23 rock-dwelling species, Table S1). Data for species from Lake Tanganyika (4 pelagic, 19 benthic, and 17 rock-dwelling species, Table S2) and Lake Victoria (6 pelagic, 10 benthic, and 3 rock-dwelling species, Table S3) were obtained from the literature. For comparison, we also compiled published data for pelagic species of families other than Cichlidae from East African Great Lakes (5 species) and from several marine pelagic taxa (33 species, Table S4 in SI).
Most of the reproductive data collected in the bibliography refer only to body length not body weight. As body shapes of cichlid fishes within a given habitat are similar in the three lakes (17, 18), the weights corresponding to the published lengths of Lake Tanganyika and Victoria species were estimated by using Lake Malawi per-habitat length–weight (L/Wt) relationships: Benthic: Wt (g) = 0.000155 SL (mm)3.13, n = 11 222, r2 = 0.957.
Rock: Wt (g) = 0.000616 SL (mm)2.85, n = 3 667, r2 = 0.865.
Pelagic: Wt (g) = 0.0000813 SL (mm)2.73, n = 3 172, r2 = 0.880.
For the Malawi species, we used actual weights, except for R. macrophthalmus, R.“gray,” and R. esox, where weight was estimated by using the Malawian L/Wt. For the pelagic L/Wt relationships, we added individual L/Wt raw data for Rhamphochromis spp. (provided by A. B. Thompson) to augment the sample sizes and get a better accuracy for the large fish.
For Lakes Tanganyika and Victoria, “egg size” refers to oocyte maximum diameter. For Lake Malawi, we had egg weights for every species, but only a few egg diameters were measured. For the sake of data homogeneity among the three lakes, oocyte maximum diameter was then used for comparison of reproductive investment. For Lake Malawi, oocyte diameter was estimated from a regression of mean oocyte weight versus mean length of the largest oocyte diameter of 13 Lake Malawi cichlid species (73), from http://cichlidresearch.com/eggtab3.html#Malawi and from data collected in the present study, giving oocyte diameter (mm) = 0.055 weight (mg) + 2.886, r2 = 0.7542. The trend of between-habitat differences in egg length was similar to that observed using egg weight.
Fecundity and egg size in cichlids are generally positively correlated with body mass (74, 75). Such correlations were observed at the intra- and interspecific level for fecundity but only at the interspecific level for egg diameter. Details on the relationships between fecundity or egg diameter and body mass for all combinations of habitat and lakes are presented in Figs. S1 and S2, respectively). Batch fecundity and oocyte diameter were compared by using standard analyses of covariance (ANCOVA, with lake, habitat, and body mass as covariates) performed with R for cichlid fishes occupying pelagic, benthic, and rocky habitats in the three lakes. For a clearer graphic representation of the fecundity and egg diameter contrasts between lakes and habitats among Great Lakes cichlids and between pelagic cichlids and other pelagic teleost fishes (Fig. 1), relative fecundity (number of eggs per kilogram of female) was used.
Phylogenetic and Comparative Analyses.
Given that no published phylogenies of African cichlids include a representative number of species in our dataset, we built a phylogeny using DNA sequences from GenBank. We initially considered five genes (mitochondrial control region, cytochrome b, NADH-2, and nuclear RAG1-exon 3, and RAG1-intron 2), but after alignment, only the control region (581 sequences of 126 species) and the NADH-2 (134 of 79) datasets were further considered.
Alignment was performed with Clustal X with default parameters and then visually inspected. The accession numbers of the 581 sequences of control region and of the 134 sequences of the NADH-2 are listed in SI Text. An examination of the alignment of the control region revealed that the number of sequences per species was highly biased, and many sequences were very short (53.3% of the data were alignment gaps on 1,008 sites). We removed some sequences to homogenize this dataset. This led us to keep 252 sequences representing 125 species (43.3% of alignment gaps). All 134 aligned NADH-2 sequences were used in subsequent analyses (79 species, 1,048 sites, 1.9% of alignment gaps).
The estimation of the species phylogeny was difficult because many species did not appear as monophyletic with these mitochondrial sequences (usually owing to introgression through past hybridization (76). To take all available information for a given species into account, we used the distance-based neighbor-joining method [NJ (77)] where, in a first step, we computed the distances among all aligned sequences, and, in a second step, we computed the distances between each pair of species as the mean of the distances between the sequences belonging to these two species. The NJ tree was estimated from this interspecies distance matrix. To assess which substitution model best fitted the data, we first fitted different models by maximum likelihood using PHYML. We found that the GTR and Tamura-Nei models were very close as best models. We selected the latter to compute the distances between pairs of sequences because there is no closed formula for the former. The estimated trees by NJ are displayed in SI Text (Fig. S3 and Fig. S4 for the control region and the NADH-2 dataset, respectively). We also estimated trees by NJ and by maximum likelihood (ML) with PHYML from the aligned sequences (78). From the between-sequences distance matrix, we computed the between-species distances by averaging all distances from sequences belonging to a pair of species. This was done independently for the control region and NADH-2 data as a way to assess the reliability of our results with respect to a particular molecular phylogeny. The trees were rooted with Boulengerochromis microlepis (14). Before doing the comparative analyses, the trees were trimmed to keep only the species for which we had life-history data (55 and 45 species for the control region and the NADH-2 trees, respectively).
To assess phylogenetic uncertainty, we performed a bootstrap analysis on both datasets by resampling 100 times the aligned sequences and repeating the procedure described above: We ended up with 200 trees. We repeated the comparative analyses with these 200 trees. The comparative analyses were done with a generalized estimating equations (GEE)-based method (24), which allowed us to fit a generalized linear model (that is similar to the ANCOVA performed previously) taking phylogenetic correlation into account. We plotted histograms of the 200 P values (for each tree) for each GEE-based test to assess whether the comparative analyses were influenced by a particular tree.
Evolutionary transitions among habitats were analyzed with Markovian models fitted by maximum likelihood (26). We built several models with different parameterizations of the transition rates among habitats. For instance, the most general model assumes that all rates of habitat change are different and so has six parameters, whereas the simplest model has one parameter (all rates are equal). These different models were compared by likelihood ratio tests and the Akaike information criteria (AIC) when they were not nested. All comparative analyses were done with APE (27).
Supplementary Material
Acknowledgments.
We thank Drs. Rosemary Lowe-McConnell, Frans Witte, Philippe Cury, Dominique Ponton, and Thierry Oberdorff for their comments and suggestions on earlier versions of the manuscript; Drs. Rosanna Robinson and Tony Thompson for allowing us to use some unpublished data on Rhamphochromis reproductive characteristics and length-weight data, respectively; and Dr. Jan Wanink for helping us find information about fecundity and egg size for the African Great Lakes freshwater noncichlid pelagic fishes. This work was supported by the Southern African Development Conference/Global Environment Facility Lake Malawi/Nyasa Biodiversity Conservation Project. We thank the Global Environmental Facility, the World Bank, and the Department for International Development (U.K.) for their support of this part of the project.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802343105/DCSupplemental.
References
- 1.Schluter D. The Ecology of Adaptive Radiation. New York: Oxford Univ Press; 2000. [Google Scholar]
- 2.Schluter D, Clifford EA, Nemethy M, McKinnon JS. Parallel evolution and inheritance of quantitative traits. Am Nat. 2004;163:809–822. doi: 10.1086/383621. [DOI] [PubMed] [Google Scholar]
- 3.Rundle HD, Nagel L, Wenrick Boughman J, Schluter D. Natural selection and parallel speciation in sympatric sticklebacks. Science. 2000;287:306–308. doi: 10.1126/science.287.5451.306. [DOI] [PubMed] [Google Scholar]
- 4.Schluter D, Nagel LM. Parallel speciation by natural selection. Am Nat. 1995;146:292–301. [Google Scholar]
- 5.Futuyma DJ. Evolutionary Biology. 3rd Ed. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- 6.Culver DC, et al. Morphology of cave organisms: Is it adaptive? Mem Biospeol. 1990;17:13–26. [Google Scholar]
- 7.Jones R, Culver DC, Kane TC. Are parallel morphologies of cave organisms the result of similar selection pressures? Evolution. 1992;46:353–365. doi: 10.1111/j.1558-5646.1992.tb02043.x. [DOI] [PubMed] [Google Scholar]
- 8.Kane TC, Culver DC, Jones RT. Genetic structure of morphologically differentiated populations of the Amphipod Gammarus minus. Evolution. 1992;46:272–278. doi: 10.1111/j.1558-5646.1992.tb02002.x. [DOI] [PubMed] [Google Scholar]
- 9.Losos JB, Jackman TR, Larson A, de Queiroz K, Rodriguez-Schettino L. Contingency and determinism in replicated adaptive radiations of island lizards. Science. 1998;279:2115–2118. doi: 10.1126/science.279.5359.2115. [DOI] [PubMed] [Google Scholar]
- 10.Pigeon D, Chouinard A, Bernatchez L. Multiple modes of speciation involved in the parallel evolution of sympatric morphotypes of lake whitefish (Coregonus clupeaformis, Salmonidae) Evolution (Lawrence, Kans) 1997;51:196–205. doi: 10.1111/j.1558-5646.1997.tb02401.x. [DOI] [PubMed] [Google Scholar]
- 11.Reznick DN, Rodd FH, Cardenas M. Life- history evolution in guppies (Poecilia reticulata, Poeciliidae). 4. Parallelism in life-history phenotypes. Am Nat. 1996;147:319–338. [Google Scholar]
- 12.Genner MJ, et al. How does the taxonomic status of allopatric populations influence species richness within African cichlid fish assemblages? J Biogeogr. 2004;31:93–102. [Google Scholar]
- 13.Kocher TD. Adaptive evolution and explosive speciation: the cichlid fish model. Nat Rev Genet. 2004;5:288–298. doi: 10.1038/nrg1316. [DOI] [PubMed] [Google Scholar]
- 14.Salzburger W, Mack T, Verheyen E, Meyer A. Out of Tanganyika: Genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes. BMC Evol Biol. 2005;5:17. doi: 10.1186/1471-2148-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seehausen O. African cichlid fish: A model system in adaptive radiation research. Proc R Soc London Ser B. 2006;273:1987–1998. doi: 10.1098/rspb.2006.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Won Y-J, Sivasundar A, Wang Y, Hey J. On the origin of Lake Malawi cichlid species, a population genetic analysis of divergence. Proc Natl Acad Sci USA. 2005;102:6581–6586. doi: 10.1073/pnas.0502127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fryer G, Iles TD. The Cichlid Fishes of the Great Lakes of Africa. London: Oliver & Boyd; 1972. [Google Scholar]
- 18.Kocher TD, Conroy JA, McKaye KR, Stauffer JR. Similar morphologies of cichlid fishes in Lakes Tanganyika and Malawi are due to convergence. Mol Phyl Evol. 1993;2:158–165. doi: 10.1006/mpev.1993.1016. [DOI] [PubMed] [Google Scholar]
- 19.Kassam D, Seki S, Horic M, Yamaoka K. Nuclear markers reveal that inter-lake cichlids' similar morphologies do not reflect similar genealogy. Mol Phyl Evol. 2006;40:383–388. doi: 10.1016/j.ympev.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Rüber L, Verheyen E, Meyer A. Replicated evolution of trophic specializations in an endemic cichlid fish lineage from Lake Tanganyika. Proc Natl Acad Sci USA. 1999;96:10230–10235. doi: 10.1073/pnas.96.18.10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seehausen O, Mayhew PJ, Van Alphen JJM. Evolution of colour patterns in East African cichlid fishes. J Evol Biol. 1999;12:514–534. [Google Scholar]
- 22.Allender CJ, Seehausen O, Knight ME, Turner GF, Maclean N. Divergent selection during speciation of Lake Malawi cichlid fishes inferred from parallel radiations in nuptial coloration. Proc Natl Acad Sci USA. 2003;100:14074–14079. doi: 10.1073/pnas.2332665100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barlow GW. The Cichlid Fishes. Cambridge, MA: Perseus; 2000. [Google Scholar]
- 24.Felsenstein J. Phylogenies and the comparative method. Am Nat. 1985;125:1–15. [Google Scholar]
- 25.Verheyen E, Salzburger W, Snoeks J, Meyer A. Origin of the superflock of cichlid fishes from Lake Victoria, East Africa. Science. 2003;300:325–329. doi: 10.1126/science.1080699. [DOI] [PubMed] [Google Scholar]
- 26.Paradis E, Claude J. Analysis of comparative data using generalized estimating equations. J Theor Biol. 2002;218:175–185. doi: 10.1006/jtbi.2002.3066. [DOI] [PubMed] [Google Scholar]
- 27.Paradis E, Claude J, Strimmer K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- 28.Pagel MD. Detecting correlated evolution on phylogenies: A general method for the comparative analysis of discrete characters. Proc R Soc London Ser B. 1994;255:37–45. [Google Scholar]
- 29.Winemiller KO, Rose KA. Why do most fish produce so many tiny offspring? Evidence from a size-based model. Am Nat. 1993;142:585–603. doi: 10.1086/285559. [DOI] [PubMed] [Google Scholar]
- 30.Wootton RJ. Life histories as sampling devices: Optimum egg size in pelagic fishes. J Fish Biol. 1994;45:1067–1077. [Google Scholar]
- 31.Cury P, Pauly D. Patterns and propencities in reproduction and growth of marine fishes. Ecol Res. 2000;15:101–106. [Google Scholar]
- 32.Stearns SC. The Evolution of Life Histories. New York: Oxford Univ Press; 1992. [Google Scholar]
- 33.Smith CC, Fretwell SD. The optimal balance between size and number of offspring. Am Nat. 1974;108:499–506. [Google Scholar]
- 34.Elgar MA. Evolutionary compromise between a few large and many small eggs: Comparative evidence in teleost fish. Oikos. 1990;59:283–287. [Google Scholar]
- 35.Gregersen F, Haugen TO, Larsen ON. Egg size differentiation among sympatric demes of brown trout: Possible effects of density-dependent interactions among fry. Ecol Freshwater Fish. 2006;15:237–246. [Google Scholar]
- 36.Einum S, Fleming IA. Highly fecund mothers sacrifice offspring survival to maximize fitness. Nature. 2000;405:565–567. doi: 10.1038/35014600. [DOI] [PubMed] [Google Scholar]
- 37.Eccles DH, Lewis DSC. Midwater spawning in Haplochromis chrysonotus (Boulenger) (Teleostei: Cichlidae) in Lake Malawi. Environ Biol Fish. 1981;6:201–202. [Google Scholar]
- 38.Konings A. The open water column. In: Konings A, editor. Tanganyika cichlids. Zevenhuizen, The Netherlands: Cichlid; 1988. [Google Scholar]
- 39.Ochi H. Mating systems of two midwater-spawning cichlids, Cyprichromis microlepidotus and Paracyprichromis brieni, in Lake Tanganyika. Ichthyol Res. 1996;43:239–246. [Google Scholar]
- 40.Seehausen O. Lake Victoria rock cichlids: taxonomy, ecology and distribution. Zevenhuizen, The Netherlands: Verduyn Cichlids; 1996. [Google Scholar]
- 41.Trendall J. The distribution and dispersal of introduced fish at Thumbi West Island in Lake Malawi, Africa. J Fish Biol. 1988;33:357–369. [Google Scholar]
- 42.Ribbink AJ. Alternative life history styles of some African cichlid fishes. Environ Biol Fish. 1990;28:87–100. [Google Scholar]
- 43.Kamler E. Parent–egg–progeny relationships in teleost fishes: An energetics perspective. Rev Fish Biol Fish. 2005;15:399–421. [Google Scholar]
- 44.Sargent RC, Taylor PD, Gross MR. Parental care and the evolution of egg size in fishes. Am Nat. 1987;129:32–46. [Google Scholar]
- 45.Genner MJ, et al. Genetic homogeneity among breeding ground and nursery areas of an exploited Lake Malawi cichlid fish. Freshwater Biol. 2008;53:1823–1831. [Google Scholar]
- 46.Konings A. Malawi Cichlids in Their Natural Habitat. 4th Ed. El Paso, TX: Cichlid; 2007. [Google Scholar]
- 47.Thompson AB, Allison EH, Ngatunga BP. Distribution and breeding biology of offshore cichlids in Lake Malawi/Niassa. Environ Biol Fish. 1996;47:235–254. [Google Scholar]
- 48.Turner GF, Robinson RL, Ngatunga BP, Shaw PW, Carvalho GR. Coleman RM, editor. Pelagic fishes of lake Malawi/Nyasa. Cichlid Research State of the Art. 2001;9:287–302. J Aquariculture Aquat Sci Special issue. [Google Scholar]
- 49.Taborsky B, Foerster K. Female mouthbrooders adjust incubation duration to perceived risk of predation. Anim Behav. 2004;68:1275–1281. [Google Scholar]
- 50.Blomberg SP, Garland TJ. Tempo and mode in evolution: phylogenetic inertia, adaptation and comparative methods. J Evol Biol. 2002;15:899–910. [Google Scholar]
- 51.Lévêque C, Paugy D, Duponchelle F. La reproduction. In: Lévêque C, Paugy D, editors. Les poissons des Eaux Continentales Africaines. Diversité, Écologie, Utilisation par l'Homme. Paris: IRD Editions; 2006. pp. 147–175. [Google Scholar]
- 52.Goodwin NB, Balshine-Earn S, Reynolds JD. Evolutionary transitions in parental care in cichlid fish. Proc R Soc London Ser B. 1998;265:2265–2272. [Google Scholar]
- 53.Bootsma HA, Hecky RE. Initial measurements of benthic photosynthesis in Lake Malawi. In: Munawar M, Hecky RE, editors. The Great Lakes of the World (GLOW): Food-Web, Health and Integrity. Leiden, The Netherlands: Backhuys; 2001. pp. 31–45. [Google Scholar]
- 54.Irvine K, Waya R. Spatial and temporal patterns of zooplankton standing biomass and production in Lake Malawi. Hydrobiologia. 1999;407:191–205. [Google Scholar]
- 55.Allison EH, Irvine K, Thompson AB, Ngatunga BP. Diets and food consumption rates of pelagic fish in Lake Malawi, Africa. Freshwater Biol. 1996;35:489–515. [Google Scholar]
- 56.Coulter GW. Lake Tanganyika and Its Life. Oxford: Oxford Univ Press; 1991. [Google Scholar]
- 57.Thompson AB, Irvine K. Diet-shifts and food-dependent survival in Eugraulicypris sardella (Cyprinidae) larvae from Lake Malawi, Africa. J Plankton Res. 1997;19:287–301. [Google Scholar]
- 58.Lowe-McConnell RH. Fish fauna of the African Great Lakes: Origins, diversity, and vulnerability. Conservation Biol. 1993;7:634–643. [Google Scholar]
- 59.McKaye KR, Makwinja RD, Menyani WW, Mhone OK. On the possible introduction of nonindigenous zooplankton-feeding fishes into Lake Malawi, Africa. Biol Conservation. 1985;33:289–307. [Google Scholar]
- 60.Wourms JP. Reproduction and development in chondrichthyan fishes. Am Zool. 1977;17:379–410. [Google Scholar]
- 61.Goodwin NB, Dulvy NK, Reynolds JD. Life-history correlates of the evolution of live bearing in fishes. Proc R Soc London Ser B. 2002;357:259–267. doi: 10.1098/rstb.2001.0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wourms JP, Lombardi J. Reflections on the evolution of piscine viviparity. Am Zool. 1992;32:276–293. [Google Scholar]
- 63.Almada VC, Santos RS. Parental care in the rocky intertidal: A case study of adaptation and exaptation in Mediterranean and Atlantic blennies. Rev Fish Biol Fish. 1995;5:23–37. [Google Scholar]
- 64.Claydon J. Spawning aggregations of coral reef fishes: Characteristics, hypotheses, threats and management. Oceanogr Mar Biol. 2005;42:265–301. [Google Scholar]
- 65.Depczynski M, Bellwood DR. Extremes, plasticity, and invariance in vertebrate life history traits: insights from coral reef fishes. Ecology. 2006;87:3119–3127. doi: 10.1890/0012-9658(2006)87[3119:epaiiv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 66.Turner GF. Pallidochromis tokolosh, a new genus and species of deep water cichlid (Telostei: Cichlidae) from lake Malawi, Africa. Ichthyol Explor Freshwater. 1994;5:377–383. [Google Scholar]
- 67.Shaw PW, Turner GF, Idid MR, Robinson RL, Carvalho GR. Genetic population structure indicates sympatric speciation of Lake Malawi pelagic cichlids. Proc R Soc London Ser B. 2000;267:2273–2280. doi: 10.1098/rspb.2000.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Turner GF, Robinson RL, Ngatunga BP, Shaw PW, Carvalho GR. Pelagic cichlid fishes of Lake Malawi/Nyasa: Biology, management and conservation. In: Cowx I, editor. Management and Ecology of Lake and River Fisheries. Blackwell Science, Oxford: Fishing New Books; 2002. [Google Scholar]
- 69.Turner GF. Offshore Cichlids of Lake Malawi. Lauenau, Germany: Cichlid Press; 1996. [Google Scholar]
- 70.Duponchelle F, Ribbink AJ. Fish Ecology Report. SADC/GEF Lake Malawi/Nyasa Biodiversity Conservation Project, Final Report. Salima, Malawi: Southern African Development Conference/Global Environment Facility; 2000. available online at http://malawicichlids.com/mw14001.htm#DR00. [Google Scholar]
- 71.Ribbink AJ, Marsh BJ, Marsh AC, Ribbink AC, Sharp BJ. A preliminary survey of the cichlid fishes of the rocky habitats of Lake Malawi. S Afr J Zool. 1983;18:149–310. [Google Scholar]
- 72.Duponchelle F, Cecchi P, Corbin D, Nunez J, Legendre M. Variations in fecundity and egg size of female Nile tilapia, Oreochromis niloticus, populations from man-made lakes of Côte d'Ivoire. Environ Biol Fish. 2000;57:155–170. [Google Scholar]
- 73.Marsh BA, Marsh AC, Ribbink AJ. Reproductive seasonality in a group of rock-frequenting cichlid fishes in Lake Malawi. J Zool London A. 1986;209:9–20. [Google Scholar]
- 74.Kolm N, Goodwin NB, Balshine S, Reynolds JD. Life history evolution in cichlids 2: Directional evolution of the trade-off between egg number and egg size. J Evol Biol. 2006;19:76–84. doi: 10.1111/j.1420-9101.2005.00987.x. [DOI] [PubMed] [Google Scholar]
- 75.Kolm N, Goodwin NB, Balshine S, Reynolds JD. Life history evolution in cichlids 1: Revisiting the evolution of life histories in relation to parental care. J Evol Biol. 2006;19:66–75. doi: 10.1111/j.1420-9101.2005.00984.x. [DOI] [PubMed] [Google Scholar]
- 76.Rüber L, Meyer A, Sturmbauer C, Verheyen E. Population structure in two sympatric species of the Lake Tanganyika cichlid tribe Eretmodini: Evidence for introgression. Mol Ecol. 2001;10:1207–1225. doi: 10.1046/j.1365-294x.2001.01259.x. [DOI] [PubMed] [Google Scholar]
- 77.Saitou N, Nei M. The neighbour-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 78.Guindon S, Gascuel O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol. 2003;52:696–704. doi: 10.1080/10635150390235520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.