Abstract
Toxoplasma gondii is a member of the phylum Apicomplexa, a diverse group of intracellular parasites that share a unique form of gliding motility. Gliding is substrate dependent and occurs without apparent changes in cell shape and in the absence of traditional locomotory organelles. Here, we demonstrate that gliding is characterized by three distinct forms of motility: circular gliding, upright twirling, and helical rotation. Circular gliding commences while the crescent-shaped parasite lies on its right side, from where it moves in a counterclockwise manner at a rate of ∼1.5 μm/s. Twirling occurs when the parasite rights itself vertically, remaining attached to the substrate by its posterior end and spinning clockwise. Helical gliding is similar to twirling except that it occurs while the parasite is positioned horizontally, resulting in forward movement that follows the path of a corkscrew. The parasite begins lying on its left side (where the convex side is defined as dorsal) and initiates a clockwise revolution along the long axis of the crescent-shaped body. Time-lapse video analyses indicated that helical gliding is a biphasic process. During the first 180o of the turn, the parasite moves forward one body length at a rate of ∼1–3 μm/s. In the second phase, the parasite flips onto its left side, in the process undergoing little net forward motion. All three forms of motility were disrupted by inhibitors of actin filaments (cytochalasin D) and myosin ATPase (butanedione monoxime), indicating that they rely on an actinomyosin motor in the parasite. Gliding motility likely provides the force for active penetration of the host cell and may participate in dissemination within the host and thus is of both fundamental and practical interest.
INTRODUCTION
Toxoplasma gondii is an obligate intracellular parasite that belongs to the phylum Apicomplexa. Most closely related to dinoflagelates and ciliates (Gajadhar et al., 1991), this diverse and ancient phylum consists mainly of intracellular parasites, many of which are important causative agents of human or animal diseases. Although they infect a wide range of different hosts, they share prominent morphological features that are related to their apical specializations for cell attachment and entry. Included in this assemblage are a unique polar microtubular organizing center (Russell and Burns, 1984; Nichols and Chiappino, 1987; Morrissette et al., 1997) and a system of three distinct sets of secretory organelles that are sequentially discharged during invasion (Dubremetz et al., 1993; Carruthers and Sibley, 1997).
Unlike phagocytic uptake, invasion by Toxoplasma occurs by active penetration of the host cell and is dependent on the actinomyosin cytoskeleton of the parasite (Dobrowolski and Sibley, 1996; Dobrowolski et al., 1997a). The entry of host cells is accompanied by invagination of the host cell plasma membrane (Suss-Toby et al., 1996), yet the force for this event appears to be largely independent of the host cell and to rely instead on forward motility by the parasite (Morisaki et al., 1995). With the exception of specialized microgametocytes, which only develop in cat intestinal epithelial cells, the crescent-shaped zoites of Toxoplasma are not flagellated and do not express cilia. The remaining life-cycle stages, including tachyzoites, bradyzoites, and sporozoites, are all invasive and likely rely on a common means of locomotion. Motility in Toxoplasma tachyzoites has been previously described as gliding on solid substrates while leaving trails of the surface membrane protein SAG1 that are readily visualized by staining with antibodies (Dobrowolski and Sibley, 1996; Dobrowolski et al., 1997a). Similar trails have been described for the related parasites Cryptosporidium (Arrowood et al., 1991), Plasmodium sporozoites (Vanderberg, 1974), and Eimeria (Russell and Sinden, 1981). Formation of these trails is disrupted by cytochalasin, indicating that motility depends on the actin cytoskeleton of the parasite (Russell and Sinden, 1981; Russell, 1983; Dobrowolski and Sibley, 1996).
Thus far, the study of motility by Toxoplasma and related parasites has relied on this static assessment of trail formation; however, these studies have revealed little information about the dynamics of this process. Herein, we take advantage of the fact that Toxoplasma is relatively easily cultured, can be purified in large quantities, and maintains excellent viability in vitro. Time-lapse video microscopy was used to analyze the kinetics of gliding motility, thereby revealing that the parasite uses a combination of three different modes of movement to achieve locomotion across the substrate and during cell penetration.
MATERIALS AND METHODS
Cell Culture and Chemicals
Tachyzoites of the RH strain T. gondii were maintained by serial passage in human fibroblast as described previously (Morisaki et al., 1995). Bone marrow macrophages were harvested and cultured as described previously (Morisaki et al., 1995). Parasites were harvested by passage though 3.0-μm filters and washed in HBBS containing 0.1 mM EGTA and 10 mM HEPES (referred to as HHE) (Morisaki et al., 1995). Cytochalasin D (Sigma, Saint Louis, MO) was dissolved in DMSO at 1 mM and stored at 4°C. Butanedione monoxime was obtained from Sigma (Saint Louis, MO) and made fresh in HHE at 40 mg/ml. DiIC18 was obtained from Molecular Probes (Eugene, OR) and dissolved in DMSO at 1 mg/ml and stored at −20°C.
Gliding Assays
Glass chamber slides (Lab-Tek, Nalge Nunc, Milwaukee, WI) or coverslips were coated by incubation in 50% fetal bovine serum diluted in PBS or with 100 μg/ml bovine serum albumin (BSA) for 1 h at 37°C followed by rinsing in PBS. Freshly harvested tachyzoites were resuspended in HHE at ∼107 cells/ml and added to precoated Lab-Tek four-chamber glass slides or glass coverslips and incubated at 37°C for 15 min.
Immunofluoresence Detection of Trails
Slides were briefly rinsed and fixed in 2.5% formalin–PBS for 10 min. Staining of surface proteins in trails was performed by indirect immunofluorescence using the following antibodies diluted 1:500. SAG1 was detected with mAb DG52 (Burg et al., 1988) (kindly provided by John Boothroyd, Stanford University), SAG2 was detected with mAb 3G11 (kindly provided by Jean-Francois Dubremetz, INSERM U42, Lille, France); p35 was detected with mAb 3F12 (Jean-Francois Dubremetz), SAG3 was detected with mAb 1F12 (Jean-Francois Dubremetz) (Couvreur et al., 1988). After incubation in primary antibodies, slides were rinsed and stained with goat anti-mouse IgG conjugated to FITC at 1:500 (Jackson ImmunoResearch Labs, West Grove, PA). To detect lipids in the trails, slides were rinsed in PBS and incubated at room temperature for 15 min in DiIC18 diluted to 1 μg/ml in 300 mM sorbitol–10 mM HEPES, pH 7.2. Slides were rinsed and mounted in 20% glycerol–PBS or in PBS alone and examined using a Zeiss Axioscope equipped for phase-contrast and epifluorescence microscopy.
Electron Microscopy
Freshly isolated tachyzoites were allowed to glide on serum- or carbon-coated glass coverslips as described above. After short incubation at 37°C, coverslips were flash-frozen in liquid nitrogen, and platinum-coated replicas were prepared and examined using a JOEL (Tokyo, Japan) CX100 electron microscope.
Video Microscopy
Time-lapse video microscopy was conducted using a Zeiss Axiovert equipped with phase-contrast and epifluorescence microscopy and a temperature-controlled stage (Medical Systems Corp., Greenvale, NY) to maintain 37°C incubation. Freshly harvested parasites were resuspended in HHE and allowed to glide on glass-bottom microwells (MatTek Corp., Ashland, MA) precoated with BSA (Sigma). Images were collected under low-light illumination using an intensified CCD C2400 camera (Hamamatsu Photonics K.K., Hamamatsu City, Japan) at 63× magnification. The video signal was digitally processed using a video-frame capture board (Perceptics Corp., Knoxville, TN) controlled by the Biological Detection Systems image analysis software (BDS Image V 1.4.1) running on a Macintosh Quadra 900 computer. The analog output was recorded to S-VHS tape using a JVC model SR-S360U videocassette recorder. Video images from S-VHS tape were transferred onto optical disk using a Panasonic Disk Recorder TQ-3038F and digitized using a Targa 2000 image capture board controlled by Adobe Premier 4.0 software to generate Quick-Time 3.0 movies at 2× real-time with frame speeds of 30 frames per second. The movies were produced at 240 × 240, 300 × 200, or 320 × 209 pixels in size.
RESULTS
Circular Gliding and Twirling Revealed by Video Microscopy
To characterize the mechanics of gliding motility in vitro, we used time-lapse video microscopy to examine the pattern of movement by live extracellular parasites. Freshly harvested parasites in suspension were added to BSA-coated glass microwells in HHE medium, and motile cells were recorded by phase-contrast video microscopy using standard NTSC format (30 frames per second at 512 × 480 lines). By analyzing the video recordings at slower than normal rates, we were able to distinguish three different modes of parasite motility. Two of these modes, referred to as circular and helical gliding, result in net forward movement, whereas the third mode, denoted twirling, is stationary with respect to the substratum. These different forms of motility are not mutually exclusive because the parasite frequently changes between these modalities in an apparently random manner (our unpublished results).
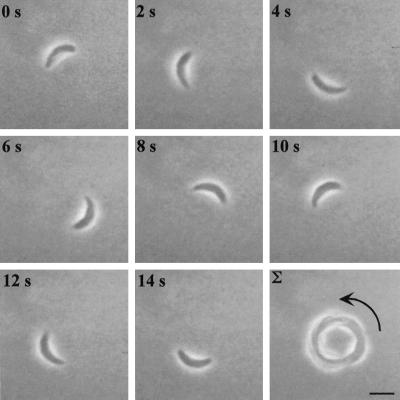
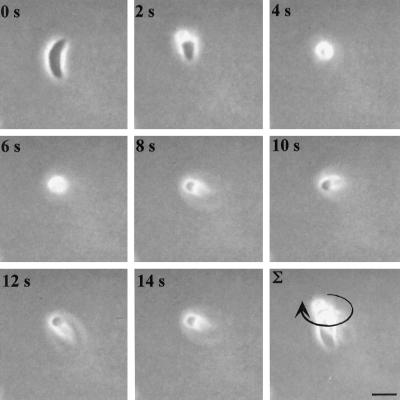
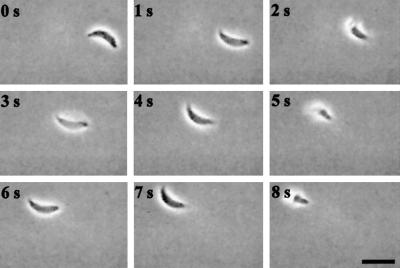
Circular gliding is characterized by forward movement while the parasite is attached to the substratum on its right side (the convex surface of the parasite was defined as the dorsal side) (Figure 1, accompanied by video 1). This form of motility results in a circular pattern as the tachyzoite moves forward in a counterclockwise direction that appears to be dictated by the crescent shape of the cell (Figure 1). The speed of circular gliding is not constant because the parasites both pause and move with variable velocity; however, the average net forward speed of gliding motility was measured to be 1.5 μm/s under these assay conditions (Table 1). During twirling, the parasite is oriented vertically while remaining attached to the substrate at its posterior end. Twirling is characterized by clockwise spinning (as viewed from above) of the parasite around a fixed point of attachment at the posterior end (Figure 2, accompanied by video 2). The average time required to complete a 360° rotation was measured to be 2.0 s (Table 1).
Figure 1.
Time-lapse video microscopy of a Toxoplasma cell engaged in circular gliding. Freshly harvested parasites in HHE medium were put on BSA-coated glass-bottom microwell plates, and their motility was documented with phase-contrast video microscopy using a 63× lens. The temperature was maintained at 37°C throughout the experiment using a temperature-controlled stage. The time elapsed between each frame is indicated in seconds. The final panel shows a frame-summation of the video, with the arrow indicating the net direction of movement. Bar, 6 μm. The video is shown at 2× real time and is representative of four independent experiments.
Table 1.
Effects of inhibitors on T. gondii motility as determined by time-lapse video microscopy
| Circular glidea | Twirlingb | Helical glidea | |
|---|---|---|---|
| Control | 1.5 | 2 | 1.1 |
| Cytochalasin D (1 μM) | − | − | − |
| Butanedione monoxime (40 mM) | − | − | − |
Estimates of rates of motility were based on at least four documented individual parasites by measuring the traveled distance or the number of 360° revolutions and the time required to do so. (−) indicates no motility observed in at least two independent experiments.
Distance traveled in microns per second.
Number of seconds per 360° revolution.
Figure 2.
Time-lapse video microscopy of a twirling Toxoplasma cell. Freshly harvested parasites in HHE medium were put on BSA-coated glass-bottom microwell plates, and their motility was documented with phase-contrast video microscopy using a 63× lens. The temperature was maintained at 37°C throughout the experiment using a temperature-controlled stage. The time elapsed between each frame is indicated in seconds. The final panel shows a frame-summation of the video, with the arrow indicating the net direction of movement. Bar, 6 μm. The video is shown at 2× real time and is representative of four independent experiments.
Gliding Motility Deposits Trails Rich in Surface Proteins and Membrane
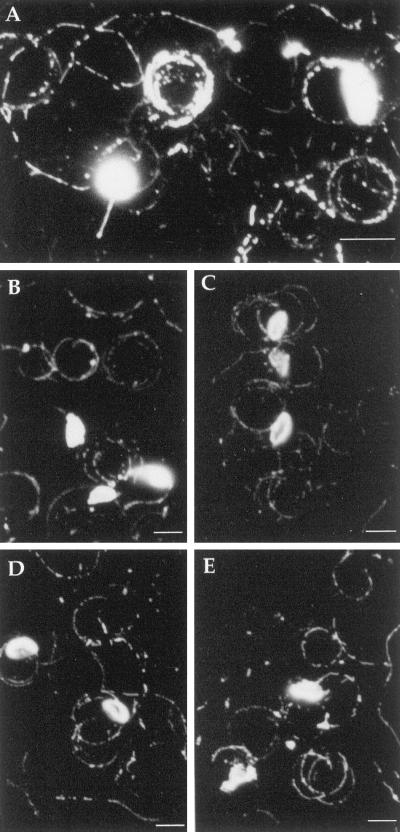
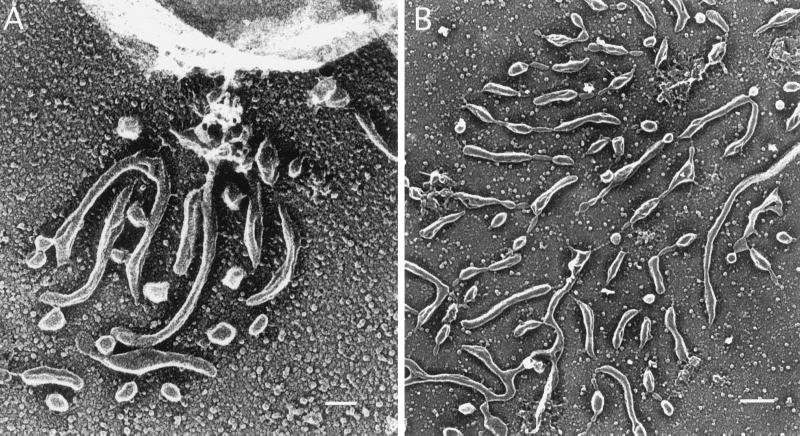
We have shown previously that the major surface protein SAG1 is found in the trails left by Toxoplasma (Dobrowolski and Sibley, 1996). SAG1 is anchored in the parasite membrane by a glycosylphosphoinositol lipid addition (Nagel and Boothroyd, 1989), hence it is not clear whether its presence in the trail is caused by release of the protein or shedding of the surface membrane. To further investigate the nature of these trails, we examined the trails left by gliding Toxoplasma for additional surface antigens. Indirect immunofluorescence labeling of surface antigens of Toxoplasma showed strong staining of both cells and gliding trails for SAG1, SAG2, SAG3, and p35 (Figure 3). In contrast, no staining was detected with GRA2, a secretory protein released from dense granules, or ROP1, a secretory protein released form rhoptries (our unpublished results). On staining with DiIC18, a fluorescent divalent cation that specifically intercalates into lipid bilayers (Struck and Pagano, 1980), strong labeling was detected on both the cell surface and in trails produced by gliding Toxoplasma (Figure 3A). Together, these results are indicative of shedding of plasma membrane during gliding motility. In agreement with this observation, scanning electron micrographs of the gliding trails show that these are made up of a multitude of small droplets, which suggests a hydrophobic nature of this material (Figure 4). As exemplified in Figure 4A, electron microscopy of gliding Toxoplasma cells shows material being deposited close to the posterior of the cell that appears to be shed from the plasma membrane.
Figure 3.
Gliding produces trails that are rich in surface proteins and lipids. (A) DiIC18-labeled Toxoplasma cells and gliding trails. (B) Indirect immunofluorescence of SAG1-stained trails with mAb DG52. (C) Trail deposits of SAG2 stained with mAb 3G11. (D) Trail deposits of p35 stained with mAb 3F12. (E) Trail deposits of SAG3 stained with mAb 1F12. Bar, 5 μm.
Figure 4.
Gliding trails are formed by membrane shedding. Electron microscopy analysis of Toxoplasma parasites gliding on coated glass reveals that the surface membrane is shed from the posterior end of the cell during gliding (A). Bar, 10 nm. The resulting trail has a feathered appearance, being composed of numerous small droplets of membrane (B). Bar, 20 nm.
Video Microscopy Analysis of Helical Gliding
Helical gliding of Toxoplasma is characterized by a forward movement with concomitant clockwise 360° rotation of the parasite along its longitudinal axis (as viewed from the apical end). Three successive helical cycles are depicted in Figure 5, which shows a parasite moving from right to left across the field. Interestingly, this motion is initiated when the parasite rest on its left side, opposite to that used for circular gliding (Figure 5, accompanied by video 3). As the parasite moves forward along the convex surface, the rotation around the long axis traces out a helical pattern as the point of contact between the cell and the substrate moves backward. The natural curvature of the cell results in the apical end lifting off the substrate, and at the halfway point it is suspended in midair, having moved forward one body length (Figure 5). The parasite then reorients itself to complete the turn and rest on its left side before commencing another step of helical gliding (Figure 5).
Figure 5.
Time-lapse video microscopy of a Toxoplasma cell engaged in helical gliding. Freshly harvested parasites in HHE medium were put on BSA-coated glass-bottom microwell plates, and their motility was documented with phase-contrast video microscopy using a 63× lens. The temperature was maintained at 37°C throughout the experiment using a temperature-controlled stage. The time elapsed between each frame is indicated in seconds. Bar, 6 μm. The video is shown at 2× real time and is representative of four independent experiments.
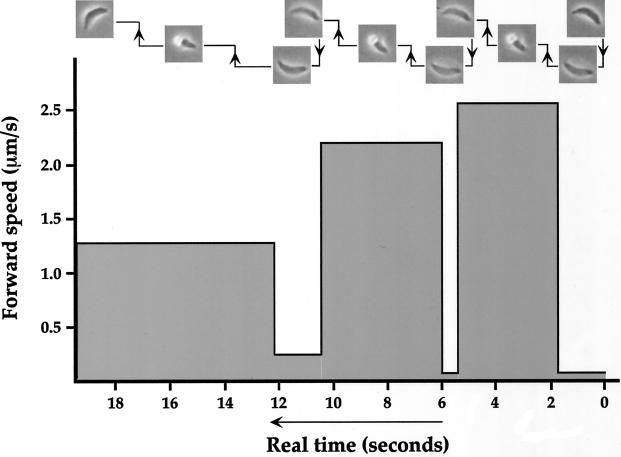
Helical Gliding Is Biphasic
Examination of the time-lapse sequences at slower than real time revealed that the forward movement during helical gliding is not continuous. After moving forward one body length and rotating 180°, the cell ends up in a vertically angled position with its right side facing downward. For the parasite to continue its helical gliding pattern, it reorients itself by touching its apical end onto the substratum and then flipping over its dorsal (convex) side and rotating another 180° (Figure 5). This flipping motion results in the cell resting on its left side, ready for a new cycle of helical gliding. During the second step of right to left reorientation, which ranges in time between 0.5 and 2 s, there is little or no forward movement, whereas the first step propels the cell forward at a speed of 1–3 μm/s (Figure 6). The forward heading of helical gliding can be altered by changing the position, as viewed from above, at which the apical end of the parasite comes into contact with the substratum before the flip over to the left side (see end of Figure 5 video). Consequently, the parasite can navigate in a straight line or, by turning inward at each step, in a large and counterclockwise circular pattern as it moves across the substrate.
Figure 6.
Helical gliding is biphasic. On the basis of the video shown in Figure 5, the speed and duration of the forward body-length movement and the right to left reorientation (flip) were determined. As shown in the top of the figure, the cell was moving from right to left. The measurements of speeds and durations of each phase are shown in real time.
We have shown previously that the trails formed by gliding Toxoplasma are disrupted by cytochalasin (Dobrowolski and Sibley, 1996) and the myosin–ATPase inhibitor butanedione monoxime (Dobrowolski et al., 1997a). To investigate whether these inhibitors block all three forms of motility, we examined the behavior of parasites in the presence of inhibitors by time-lapse video microscopy. We observed that all three modes of extracellular motility were inhibited by actin filament (cytochalasin D) and myosin inhibitors (butanedione monoxime). Inhibition was evident in a reduction of the number of cells that were motile from ∼10% in the control to nearly zero in the treated cells and a significant decrease in the average trail length (Table 1). Collectively, these results demonstrate that all of these processes are powered by a parasite actinomyosin motor.
Gliding Motility Leads to Host Cell Invasion
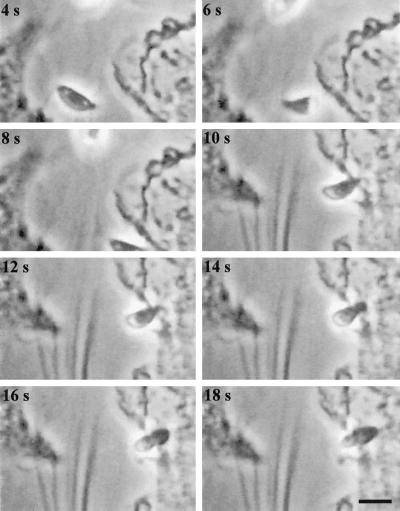
To investigate whether gliding motility can promote migration to and invasion of new host cells, we monitored the fate of parasites lysing out from an infected cell in the proximity of noninfected host cells. We observed numerous instances of parasites using gliding motility to propel themselves toward another host cell and subsequently invade it (as exemplified in Figure 7, accompanied by video 4). This sequence was filmed using an upright microscope that resulted in an inversion in the optical path. Consequently, although the parasite appears to be rotating in an opposite direction relative to the earlier images (below), it actually undergoes the typical clockwise helical twist that is characteristic of gliding motility seen in Figures 5 and 6.
Figure 7.
Time-lapse video microscopy of a Toxoplasma tachyzoite lysing out from one host cell, move by helical gliding to a neighboring cell and invade. The time elapsed between each frame is indicated in seconds. The video was produced by capturing one frame per second and is shown at 2× real time. The invading parasite was centered by moving the microscope stage at the time of cell contact, which leads to a slight lag in the sequence. Because this sequence was filmed on an upright microscope, it is inverted with respect to those shown in the earlier figures. Bar, 6 μm.
DISCUSSION
We demonstrate here that during gliding motility, Toxoplasma releases trails composed of surface membrane proteins and lipids that are deposited on the substrate. Time-lapse video microscopy of gliding reveals a complexity of movements that is not fully appreciated by merely examining the static trails. Gliding is composed of three major modes of locomotion: circular gliding, twirling, and helical gliding. Moreover, the parasite undergoes several intricate reorientations in performing these motions, the net result of which is forward movement. Gliding not only propels the parasite across the substrate but is used in cell invasion. Given the highly conserved nature of gliding motility among apicomplexan parasites, which infect various mammalian cells, our studies on the mechanism of gliding in Toxoplasma are relevant to a large group of medically and economically important parasites.
Time-lapse video microscopy revealed that Toxoplasma motility is composed of three distinct forms of locomotion. The first of these is simple circular gliding, which occurs in a counterclockwise direction leading to circular patterns of varying diameters that appear to be related primarily to the arc of the parasite body. Circular gliding corresponds closely to the deposit of trails that are detected using antibodies to major surface proteins (Figure 3); however, it is not the primary means of movement across the substrate or during cell entry. The second form of motility, referred to as twirling, has recently been the subject of a video kinetic analysis that concluded that the parasite moves by a combination of clockwise spinning and longitudinal contractions (Frixione et al., 1996). Our recordings of twirling are in complete agreement with this previous analysis; however, this form of motility does not result in net forward motion. As such, its significance remains unclear, although it is possible that this form of behavior represents a form of random chemotaxis in the absence of a clear gradient or signal to trigger forward motion. Parasites often spontaneously enter this phase of upright twirling and remain there for variable periods before returning to the helical gliding pattern that results in net forward motion.
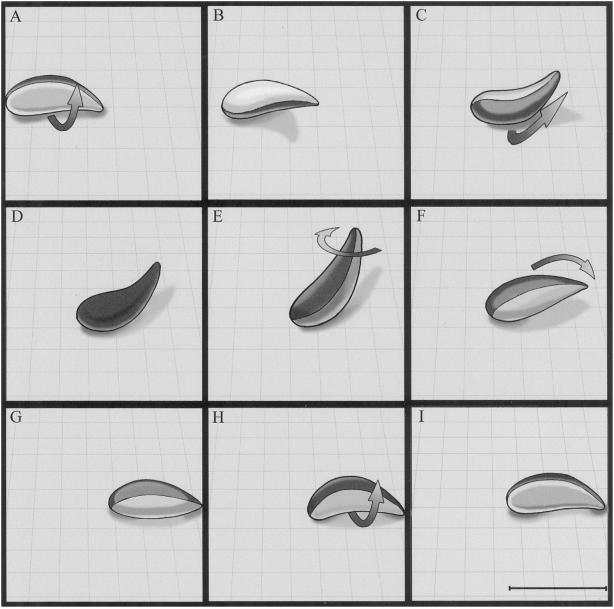
Helical gliding shares many of the same features as twirling, the key difference being that the parasite begins the motion while lying horizontal with respect to the substrate. From this position it undergoes a clockwise revolution along the long axis while keeping a portion of the cell body attached to the substrate. A similar helical form of substrate-dependent motility was previously described for Eimeria as occurring in distinct, body-length units of forward motion (Russell and Sinden, 1981). We demonstrate here that helical gliding occurs by a two-step process during which the parasite moves forward one body length during the first 180o and then flips from the right side to the left to complete the revolution before repeating the process (Figure 8). The relative rates of motion in these two steps are quite different: the time elapsed during the first half is considerably longer as the parasite moves forward, whereas in the second phase, which happens very rapidly, there is little net forward motion. Using this form of helical gliding motility, the parasite is able to traverse distances of 10–200 μm in a period of several minutes. The pattern created by helical gliding varies from a nearly straight line to a wide circle with a left-handed arc, reflecting the fact that the forward steps are often shortened by an inward turning (relative to the arc of the parasite). Helical gliding is the most common form of motility associated with cell invasion by Toxoplasma.
Figure 8.
Model of a single complete cycle in the helical gliding pattern exhibited by Toxoplasma. During helical gliding, the organism maintains only a limited contact with the substrate along its convex face. This contact zone progresses backward along the organism as it moves forward and turns as the organism rotates along its long axis (A–E); thus, the contact zone can be described as progressing along a helical path from one end of the organism to the other. The parasite’s crescent shape prevents this helix from being completed because the organism cannot achieve contact with the substrate along its concave face. Toxoplasma has “solved” this problem by rotating another 180o along its long axis while maintaining contact with the substrate at the two poles but without progressing forward (F–G), thus completing the cycle of helical motility. This biphasic motion assures that the parasite will move forward a single body length (achieved during the first 180° revolution) and reorient (accomplished during the flip) to begin the cycle again. During twirling, the parasite remains upright on its posterior end (E) and pirouettes around its long axis. Bar, 7 μm.
Gliding motility is exhibited by sporozoites of various apicomplexan parasites and is likely a defining feature of this phylum because even more distantly related members like the Gregarines display substrate-dependent gliding (King, 1981, 1988). The sporozoite stages of other apicomplexans, such as Plasmodium (Stewart and Vanderberg, 1988), Eimeria (Russell and Sinden, 1981), and Cryptosporidum (Arrowood et al., 1991), also display gliding motility in vitro. The rates of gliding in these organisms vary from 1 to 10 μm/s (King, 1988). Like Toxoplasma, these stages also lack obvious organelles for locomotion, and thus it is likely that gliding propels them during cell invasion. Although gliding is only capable of sustaining movement of several millimeters per hour, it is still relatively fast compared with the amoeboid movement of vertebrate cells (see below). Studies on Toxoplasma indicate that a similar actinomyosin motor propels the parasite during gliding and cell invasion (Dobrowolski and Sibley 1996); thus it may be that gliding is largely an adaptation for active cell penetration. During in vivo infection, the parasite infects various nucleated host cells, which leads to rapid lysis and invasion of nearby host cells. Toxoplasma also readily crosses the intestinal barrier and gains entry into the CNS and across the placenta, thereby causing the most serious forms of toxoplasmosis (Dubey, 1994). Thus, gliding may also participate in tissue migration, as the parasite penetrates through epithelial barriers or basement membranes, for example.
Several prokaryotic organisms, such as cyanobacteria, flavobacteria, and myxobacteria, exhibit forms of gliding motility (Youderian, 1998). Gliding by Myxococcus xanthus depends on the depositing of trails made up of polysaccharides and fibrillin protein (Behmlander and Dworkin, 1994a,b; Chang and Dworkin, 1994). In the case of M. xanthus, type IV pili have been shown to be required but are not sufficient for motility (Wu and Kaiser, 1995; Wu et al., 1997). The implication of pili in the motility of M. xanthus indicates that in spite of superficial similarities, bacterial and apicomplexan gliding are based on different mechanisms because the latter organisms do not contain pili or other locomotory organelles.
In higher eukaryotes, actin-based motility occurs by several different mechanisms, including filament elongation (an actin-based motor), filament sliding (myosin II-based motor), and filament translocation (unconventional myosin-based motors) (Mitchison and Cramer, 1996). Filament translocation participates in crawling motility and phagocytosis events that occur at rates of 1–10 μm/min (Mitchison and Cramer, 1996). Because gliding motility is capable of moving the parasite at a rate of 1 μm/sec, it may be that its chief advantage lies in propelling efficient invasion by the parasite before the host is able to respond by phagocytosis. We have shown previously that active invasion by Toxoplasma is approximately five times faster than phagocytosis by macrophages (Morisaki et al., 1995). The net result of active invasion is formation of a nonfusigenic vacuole that supports the survival and growth of the parasite, whereas engulfment by phagocytosis results in lysosome fusion and destruction of the parasite (Mordue and Sibley, 1997).
Myosins also participate in cell motility in vertebrate cells as shown by inhibitors that block myosin function, including KT5926, a myosin light-chain kinase inhibitor, and butanedione monoxime, an inhibitor of myosin ATPases (Cramer and Mitchison, 1995, 1997). Such inhibitors of myosin function also block all three forms of Toxoplasma motility (Table 1) and inhibit cell invasion (Dobrowolski et al., 1997a), indicating that these processes are likely driven by a common actinomyosin motor in the parasite. Indeed actin and myosin colocalize in Toxoplasma beneath the plasma membrane where actin filaments likely serve as a scaffold for a myosin motor (Dobrowolski et al., 1997a,b; Heintzleman and Schwartzman 1997).
Gliding motility by apicomplexan parasites is a unique biological process that occurs in the absence of other known appendages involved in ciliary or flagellar motility. The force generated by the actinomyosin motor during gliding motility may be linked to the outside world through interactions with a transmembrane protein that serves as an adhesin for the substrate. One candidate for this function is the secretory protein known as TRAP that mediates cell attachment by malarial sporozoites; in the absence of this protein the parasites are rendered nonmotile and noninfectious (Sultan et al., 1997). Most apicomlexans contain close homologues of this protein, including Toxoplasma (Wan et al., 1996), again suggesting a common theme of motility that may be characteristic of the phylum. Given the relative flexibility of Toxoplasma for genetic (Roos et al., 1994) and cell biological studies (Sibley et al., 1998), this system offers an excellent experimental model for deciphering the mechanism of gliding motility by this primitive yet medically important group of parasites.
Supplementary Material
ACKNOWLEDGMENTS
We thank John Boothroyd and Jean-François Dubremetz for the generous gift of antibodies used here. We also acknowledge Robin Roth and Olivia Giddings for expert technical assistance and David Russell for helpful comments and critical review of the manuscript. This work was supported in part by a grant from National Institutes of Health (AI-34036) to L.D.S. S.H. was supported by the Wenner-Gren Foundation, Sweden.
Footnotes
REFERENCES
- Arrowood MJ, Sterling CR, Healey MC. Immunofluorescent microscopical visualization of trails left by gliding Cryptosporidium parvum sporozoites. J Parasitol. 1991;77:315–317. [PubMed] [Google Scholar]
- Behmlander RM, Dworkin M. Biochemical and structural analyses of the extracellular matrix fibrils of Myxococcus xanthus. J Bacteriol. 1994a;176:6295–6303. doi: 10.1128/jb.176.20.6295-6303.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behmlander RM, Dworkin M. Integral proteins of the extracellular matrix fibrils of Myxococcus xanthus. J Bacteriol. 1994b;176:6304–6311. doi: 10.1128/jb.176.20.6304-6311.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg JL, Perlman D, Kasper LH, Ware PL, Boothroyd JC. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J Immunol. 1988;141:3584–3591. [PubMed] [Google Scholar]
- Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- Chang BY, Dworkin M. Isolated fibrils rescue cohesion and development in the Dsp mutant of Myxococcus xanthus. J Bacteriol. 1994;176:7190–7196. doi: 10.1128/jb.176.23.7190-7196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couvreur G, Sadak A, Fortier B, Dubremetz JF. Surface antigens of Toxoplasma gondii. Parasitology. 1988;97:1–10. doi: 10.1017/s0031182000066695. [DOI] [PubMed] [Google Scholar]
- Cramer LP, Mitchison TJ. Myosin is involved in postmitotic cell spreading. J Cell Biol. 1995;131:179–189. doi: 10.1083/jcb.131.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer LP, Mitchison TJ. Investigation of the mechanism of retraction of the cell margin and rearward flow of nodules during mitotic cell rounding. Mol Biol Cell. 1997;8:109–119. doi: 10.1091/mbc.8.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowolski JM, Carruthers VB, Sibley LD. Participation of myosin in gliding motility and host cell invasion by Toxoplasma gondii. Mol Microbiol. 1997a;26:163–173. doi: 10.1046/j.1365-2958.1997.5671913.x. [DOI] [PubMed] [Google Scholar]
- Dobrowolski JM, Niesman IR, Sibley LD. Actin in Toxoplasma gondii is encoded by a single-copy gene, ACT1, and exists primarily in a globular form. Cell Motil Cytoskeleton. 1997b;37:253–262. doi: 10.1002/(SICI)1097-0169(1997)37:3<253::AID-CM7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Dobrowolski JM, Sibley LD. Toxoplasma invasion of mammalian cells is powered by the actin cytoskeleton of the parasite. Cell. 1996;84:933–939. doi: 10.1016/s0092-8674(00)81071-5. [DOI] [PubMed] [Google Scholar]
- Dubey JP. Toxoplasmosis. J Am Vet Med Assoc. 1994;205:1593–1598. [PubMed] [Google Scholar]
- Dubremetz JF, Achbarou A, Bermudes D, Joiner KA. Kinetics and pattern of organelle exocytosis during Toxoplasma gondii/host cell interaction. Parasitol Res. 1993;79:402–408. doi: 10.1007/BF00931830. [DOI] [PubMed] [Google Scholar]
- Frixione E, Mondragon R, Meza I. Kinematic analysis of Toxoplasma gondii motility. Cell Motil Cytoskeleton. 1996;34:152–163. doi: 10.1002/(SICI)1097-0169(1996)34:2<152::AID-CM6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Gajadhar AA, Marquardt WC, Hall R, Gunderson J, Ariztia-Carmona EV, Sogin ML. rRNA sequences of Sarcocystis muris, Theileria annulata and Crypthecodinium cohnii reveal evolutionary relationships among apicomplexans, dinoflagelates, and ciliates. Mol Biochem Parasitol. 1991;45:147–154. doi: 10.1016/0166-6851(91)90036-6. [DOI] [PubMed] [Google Scholar]
- Heintzleman MB, Schwartzman JD. A novel class of unconventional myosins from Toxoplasma gondii. J Mol Biol. 1997;271:139–146. doi: 10.1006/jmbi.1997.1167. [DOI] [PubMed] [Google Scholar]
- King CA. Cell surface interaction of the protozoan Gregarina with Concanavalin A beads: implications for models of gregarine gliding. Cell Biol Int Rep. 1981;5:297–305. doi: 10.1016/0309-1651(81)90228-9. [DOI] [PubMed] [Google Scholar]
- King CA. Cell motility of sporozoan protozoa. Parasitol Today. 1988;11:315–318. doi: 10.1016/0169-4758(88)90113-5. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- Mordue DG, Sibley LD. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J Immunol. 1997;159:4452–4459. [PubMed] [Google Scholar]
- Morisaki JH, Heuser JE, Sibley LD. Invasion of Toxoplasma gondii occurs by active penetration of the host cell. J Cell Sci. 1995;108:2457–2464. doi: 10.1242/jcs.108.6.2457. [DOI] [PubMed] [Google Scholar]
- Morrissette NS, Murray JM, Roos DS. Subpellicular microtubules associate with an intermembranous particle lattice in the protozoan parasite Toxoplasma gondii. J Cell Sci. 1997;110:35–42. doi: 10.1242/jcs.110.1.35. [DOI] [PubMed] [Google Scholar]
- Nagel SD, Boothroyd JC. The major surface antigen, p30, of Toxoplasma gondii is anchored by a glycolipid. J Biol Chem. 1989;264:5569–5574. [PubMed] [Google Scholar]
- Nichols B, Chiappino M. Cytoskeleton of Toxoplasma gondii. J Protozool. 1987;34:217–226. doi: 10.1111/j.1550-7408.1987.tb03162.x. [DOI] [PubMed] [Google Scholar]
- Roos DS, Donald RGK, Morrissette NS, Moulton AL. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. Methods Cell Biol. 1994;45:28–61. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- Russell DG. Host cell invasion by Apicomplexa: an expression of the parasite’s contractile system? Parasitology. 1983;87:199–209. doi: 10.1017/s0031182000052562. [DOI] [PubMed] [Google Scholar]
- Russell DG, Burns RG. The polar ring of coccidian sporozoites: a unique microtubule-organizing center. J Cell Sci. 1984;65:193–207. doi: 10.1242/jcs.65.1.193. [DOI] [PubMed] [Google Scholar]
- Russell DG, Sinden RE. The role of the cytoskeleton in the motility of coccidian sporozoites. J Cell Sci. 1981;50:345–359. doi: 10.1242/jcs.50.1.345. [DOI] [PubMed] [Google Scholar]
- Sibley LD, Håkansson S, Carruthers VB. Gliding motility: an efficient mechanism for cell penetration. Curr Biol. 1998;8:R12–14. doi: 10.1016/s0960-9822(98)70008-9. [DOI] [PubMed] [Google Scholar]
- Stewart MJ, Vanderberg JP. Malaria sporozoites leave behind gliding trails of circumsporozoite protein during gliding motility. J Protozool. 1988;35:389–393. doi: 10.1111/j.1550-7408.1988.tb04115.x. [DOI] [PubMed] [Google Scholar]
- Struck DK, Pagano RE. Insertion of fluorescent phospholipids into the plasma membrane of a mammalian cell. J Biol Chem. 1980;255:5404–5410. [PubMed] [Google Scholar]
- Sultan AA, Thathy V, Frevert U, Robson KJH, Crisanti A, Nussenzweig V, Nussenzweig RS, Menard R. TRAP is necessary for gliding motility and infectivity of Plasmodium sporozoites. Cell. 1997;90:511–522. doi: 10.1016/s0092-8674(00)80511-5. [DOI] [PubMed] [Google Scholar]
- Suss-Toby E, Zimmerberg J, Ward GE. Toxoplasma invasion: the parasitophorous vacuole is formed from host cell plasma membrane and pinches off via a fusion pore. Proc Natl Acad Sci USA. 1996;93:8413–8418. doi: 10.1073/pnas.93.16.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderberg JP. Studies on the motility of Plasmodium sporozoites. J Protozool. 1974;21:527–537. doi: 10.1111/j.1550-7408.1974.tb03693.x. [DOI] [PubMed] [Google Scholar]
- Wan KL, Carruthers VB, Sibley LD, Ajioka JW. Molecular characterization of an expressed sequence tag locus of Toxoplasma gondii encoding the micronemal protein MIC2. Mol Biochem Parasitol. 1996;84:203–214. doi: 10.1016/s0166-6851(96)02796-x. [DOI] [PubMed] [Google Scholar]
- Wu SS, Kaiser D. Genetic and functional evidence that Type IV pili are required for social gliding motility in Myxococcus xanthus. Mol Microbiol. 1995;18:547–558. doi: 10.1111/j.1365-2958.1995.mmi_18030547.x. [DOI] [PubMed] [Google Scholar]
- Wu SS, Wu J, Kaiser D. The Myxococcus xanthus pilT locus is required for social gliding motility although pili are still produced. Mol Microbiol. 1997;23:109–121. doi: 10.1046/j.1365-2958.1997.1791550.x. [DOI] [PubMed] [Google Scholar]
- Youderian P. Bacterial motility: secretory secrets of gliding bacteria. Curr Biol. 1998;8:408–411. doi: 10.1016/s0960-9822(98)70264-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.