Abstract
Intercellular lipids of the stratum corneum (SC), the outer layer of the epidermis, form a barrier to water vapor diffusion through the skin. Previously, we measured cutaneous water loss (CWL) and lipid composition of the SC of adult house sparrows from two populations, one living in the deserts of Saudi Arabia and another living in mesic Ohio. Adult desert house sparrows had a lower CWL, a lower proportion of free fatty acids, and a higher proportion of ceramides and cerebrosides in the SC compared with mesic sparrows. In this study, we investigated developmental plasticity of CWL and lipid composition of the SC in desert and mesic nestling house sparrows reared in low and high humidity and compared our results with previous work on adults. We measured CWL of nestlings and analyzed the lipid composition of the SC using thin-layer chromatography. We showed that nestling house sparrows from both localities had higher CWL than adults in their natural environment, a result of major modifications of the lipid composition of the SC. The expression of plasticity in CWL seems to be a response to opposed selection pressures, thermoregulation and water conservation, at different life stages, on which regulation of CWL plays a crucial role. Desert nestlings showed a greater degree of plasticity in CWL and lipid composition of the SC than did mesic nestlings, a finding consistent with the idea that organisms exposed to more environmental stress ought to be more plastic than individuals living in more benign environments.
Keywords: ceramides, cerebrosides
A fundamental goal of evolutionary physiologists is to understand how physiological attributes of animals change relative to environmental heterogeneity, an especially relevant avenue of research in light of anthropomorphic environmental perturbations, including global warming, now occurring on our planet (1, 2). Insight into the evolution of complex physiological traits requires knowledge of evolutionary diversification relative to environment and an appreciation of molecular mechanisms that underlie adjustment of a phenotype to a given environment. Differences in physiological phenotype of organisms living in different environments are thought to be the result of natural selection across generations (3, 4) or through the expression of phenotypic plasticity within individuals, including reversible phenotypic flexibility of adults and developmental plasticity of young. Of course, phenotypic plasticity per se can also be under the influence of natural selection (5–7).
Cutaneous water loss (CWL) and its regulation by the outer layer of the skin, the stratum corneum (SC), constitutes a useful model for studying phenotypic plasticity and its consequences on fitness. Because CWL contributes at least half of total water loss in birds (4, 8, 9), reducing CWL would be an important mechanism to save water for desert birds, at least at moderate ambient temperatures (Ta). However, in deserts, Ta can become extreme during summer with the result that body temperature of some desert birds can reach 45°C during periods of activity in the hottest part of the day (J.B.W. and B. I. Tieleman, unpublished work). At such high body temperatures, desert birds rapidly increase rates of respiratory water loss, and to a small extent CWL, to maintain body temperature below lethal limits (10). Thus, the skin of desert birds serves antagonistic roles, water conservation at normothermic temperatures and, to some degree, thermoregulation at high environmental temperatures.
Variation in CWL is thought to be primarily mediated by alteration in lipid composition of SC (11–14). The SC is formed by flattened dead cells, called corneocytes, embedded in an extracellular lipid matrix consisting of cholesterol, free fatty acids (FFA), ceramides (a sphingosine molecule ester bonded to a fatty acid) and cerebrosides (a ceramide molecule attached to a hexose). Studies on the SC of larks, house sparrows, and several species of tropical birds suggest that changes in the proportions of FFA and ceramides in the extracellular matrix of the SC influence rates of CWL (11, 13, 14). Desert birds have a lower percentage of FFA and a higher percentage of ceramides in the SC than birds from mesic environments, a relationship observed across and within species (11, 13). Changes in the ratios of lipid classes in the SC might influence CWL in birds (14). Apparently, appropriate ratios of ceramides, cerebrosides, and FFAs are necessary for the formation of intracellular lipid bilayers, called lamellae, and the molecular organization of these lamellae affects CWL (14, 15).
Previously, we studied how lipids of the SC affect CWL in adult house sparrows, Passer domesticus, from two natural populations, one living in the deserts of Saudi Arabia and another living in mesic Ohio. Desert individuals had a lower CWL, a lower proportion of FFA, and a higher proportion of ceramides and cerebrosides in the SC than birds from Ohio (13). These changes could be the result of genetic differences between populations, of phenotypic flexibility of adults, of developmental plasticity of young, or a combination of these. We have previously shown that adult sparrows from Ohio reduced their CWL by 36.1% when acclimated to a dry environment compared with birds acclimated to a humid environment (16). Attendant to this physiological adjustment, dry-acclimated sparrows had a significantly lower FFA/ceramide ratio than humid-acclimated sparrows (16).
The expression of phenotypic plasticity might have adaptive value in environments, such as deserts, that are temporally heterogeneous (17). Therefore, one might predict that individuals from deserts should alter their physiological traits more when exposed to different environments than individuals from mesic habitats. However, Tieleman et al. (18) found no evidence that adult desert birds were more flexible in total evaporative water loss than mesic species when exposed to different Tas, and Tracy and Walsberg (5) showed that desert and mesic individuals of Merriam's kangaroo rat (Dipodomys merriami) reared in different humidity regimes were equally flexible in CWL.
Hence, predictions about the occurrence and the magnitude of phenotypic plasticity in different environments do not consistently align with empirical evidence. The role that phenotypic flexibility of adults and developmental plasticity of young plays in the evolutionary process is at the forefront of modern thinking in evolutionary physiology (6, 19–24). Few studies have sought to examine the relative importance of nongenetic effects and their adaptive value at different life stages (but see ref. 25). One might envision that selection pressures for plasticity might be markedly different for young birds in nests and for adults. Under these circumstances, phenotypic plasticity could be a mechanism that, through the production of phenotypic variation, allows individuals to survive different environments during their lifetime.
In this study, we investigated developmental plasticity of CWL and lipid composition of the SC in desert and mesic nestling house sparrows reared in low and high humidity and compared our results with previous work on adults. We chose to study the effect of humidity on the SC, given that the properties of the permeability barrier, at least in mammals, change in response to the level of hydration of the SC (14, 15, 26). Our study showed that, regardless of environmental origin, nestling house sparrows had a more permeable skin than that of adults exposed to their natural environment. During the maturation process, sparrows acquired a SC that is less permeable to water vapor, a result of major modifications of the lipid composition of the extracellular matrix of the SC. Nestlings from Saudi Arabia showed a greater degree of plasticity in CWL and in lipid composition of the SC than did nestlings from Ohio, a finding consistent with the idea that organisms exposed to more environmental stress ought to be more plastic than individuals living in more benign environments.
Results
Body Mass and Surface Area.
Fledglings from Saudi Arabia weighed significantly less than fledglings from Ohio (14.9 ± 2.1 g and 22.2 ± 1.3 g, respectively) (habitat: F = 173.69, P < 0.001). However, with individuals from both habitats combined, body mass was not significantly different between dry- and humid-acclimated fledglings (treatment: F = 0.01, P > 0.96). Using Meeh's equation as modified by Walsberg and King (27), we estimated skin surface area for fledglings from Saudi Arabia and Ohio as 60.3 ± 6.0 cm2 and 79.0 ± 3.1 cm2, respectively.
CWL.
Treatment.
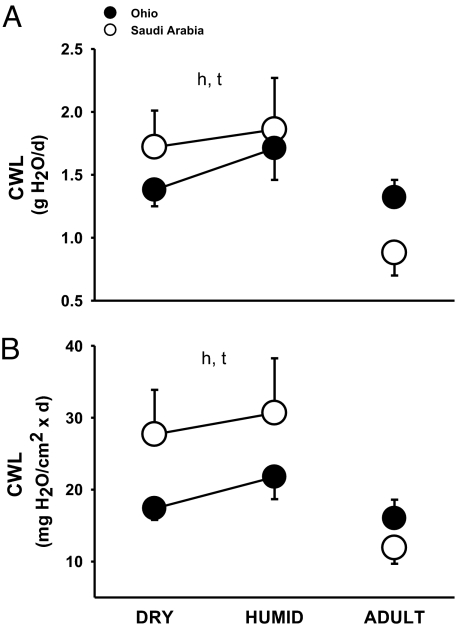
Nestling sparrows were developmentally plastic for CWL (Fig. 1). Whole-organism and surface-specific CWL of fledglings after acclimation was lower in dry- than in humid-acclimated fledglings, by 15.7% and 11.2%, respectively (F = 9.18; P = 0.004; F = 7.49, P = 0.01) (Fig. 1 A and B).
Fig. 1.
CWL in fledgling and adult house sparrows from desert (open circles) and mesic (filled circles) environments after acclimation to humidity. (A) Whole-organism CWL. (B) Surface-specific CWL. “h” and “t” indicate significance for habitat and treatment, respectively. Error bars show ±1 SD.
Habitat.
Part of the difference in CWL between desert and mesic nestlings could be attributed to a genetic component. Whole-organism and surface-specific CWL of fledglings from Ohio was significantly lower than CWL of birds from Saudi Arabia, in sharp contrast to findings for adults (F = 7.74, P < 0.01; F = 47.01; P < 0.001, respectively). The difference in CWL for nestlings exposed to a dry compared with a humid environment was 14.8% for whole-organism CWL and 52.9% for surface-specific CWL (Fig. 1).
Developmental plasticity and genetic differences between populations of fledglings contributed almost equally to variability of whole-organism CWL; however, habitat explained most of the variation in surface-specific CWL (Table 1).
Table 1.
Semipartial correlation coefficients after multiple regression on CWL, lipid amounts, lipid percentages, and lipid ratios of the SC using habitat and treatment as independent variables (see Methods for details)
| Response variable | Habitat R2 | Treatment R2 | Interaction R2 |
|---|---|---|---|
| CWL-whole | 0.16** | 0.17** | NS |
| CWL | 0.55** | 0.05* | NS |
| Lipid amounts | |||
| FFA | 0.49** | 0.10 NS | 0.12* |
| Ceramide | 0.48** | 0.01 NS | 0.06* |
| Cerebroside | 0.10* | 0.01 NS | NS |
| Lipid percentages | |||
| Cholesterol, % | 0.13* | 0.19** | 0.11* |
| FFA, % | 0.41** | 0.02 NS | 0.06* |
| Ceramide, % | 0.66** | 0.06** | NS |
| Lipid ratios | |||
| FFA/ceramide | 0.52** | 0.12** | NS |
| Ceramide/cerebroside | 0.54** | 0.00 NS | 0.03* |
When the interaction term was significant, we included it in our model. We show only variables for which at least one factor was significant. NS, factor not significant. *, P < 0.05; **, P < 0.01.
Lipid Quantities in the SC of Fledglings.
Treatment.
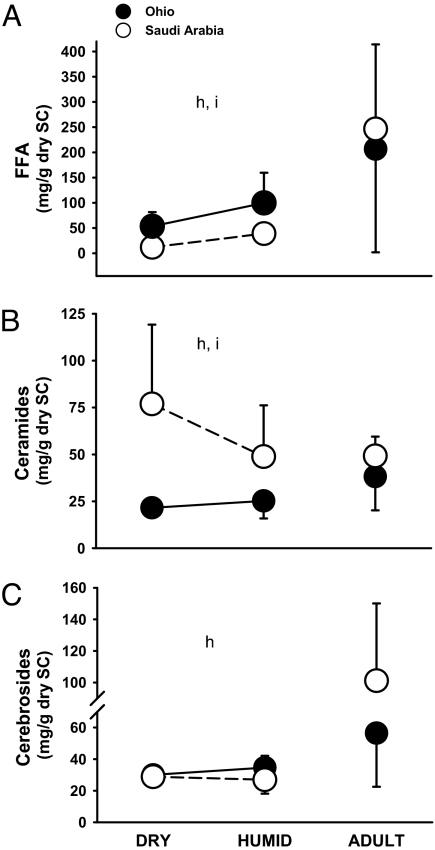
Fledglings that developed in different humidity treatments varied in the amounts of FFA and ceramides in their SC. Fledglings from desert and mesic populations had different degrees of plasticity for FFA and ceramides (interaction: F > 4.89, P < 0.04; Fig. 2). The magnitude of change in the concentration of FFA after acclimation was higher in fledglings from Ohio than in fledglings from Saudi Arabia. Fledglings from Saudi Arabia showed plasticity for the quantity of ceramides with higher concentrations of ceramides in their SC when reared in a dry environment than in a humid environment.
Fig. 2.
Lipid quantities in fledgling and adult house sparrows from desert (open circles) and mesic (filled circles) environments after acclimation to humidity. “h” and “i” indicate significance for habitat and interaction term, respectively. Error bars show ±1 SD.
Habitat.
Fledglings from Ohio had significantly higher amounts of FFA, but lower amounts of ceramides in their SC than did fledglings from Saudi Arabia (F > 4.34, P < 0.04; Fig. 2). Habitat explained a higher proportion of the variance in the amounts of FFA, ceramides, and cerebrosides in the SC of fledglings (Table 1).
Lipid Percentages in the SC of Fledglings.
Treatment.
The interaction habitat-by-treatment was significant for percentage of cholesterol (F = 5.0, P = 0.03) and FFA (F = 6.3, P < 0.02) in the SC, indicating that fledglings expressed developmental plasticity for these variables when reared in different humidity and that the degree of plasticity was different between desert and mesic populations. The magnitude of change in percentage of cholesterol in the SC after acclimation was higher in fledglings from Ohio, whereas the magnitude of change in percentage of FFA was higher in fledglings from Saudi Arabia. Fledglings also exhibited developmental plasticity for percentage of ceramides, although the degree of plasticity was the same in both populations (F = 9.0, P = 0.005).
Habitat.
Fledglings from Saudi Arabia had a lower percentage of cholesterol and FFA in their SC, but a higher percentage of ceramides than fledglings from Ohio (F > 4.9, P < 0.04).
Developmental plasticity explained a higher proportion of the variance in percentage of cholesterol in SC than habitat; on the other hand, variation in percentage of FFA and ceramides in the SC of fledglings was explained by habitat (Table 1).
Ratios of Lipids in the SC of Fledglings.
Treatment.
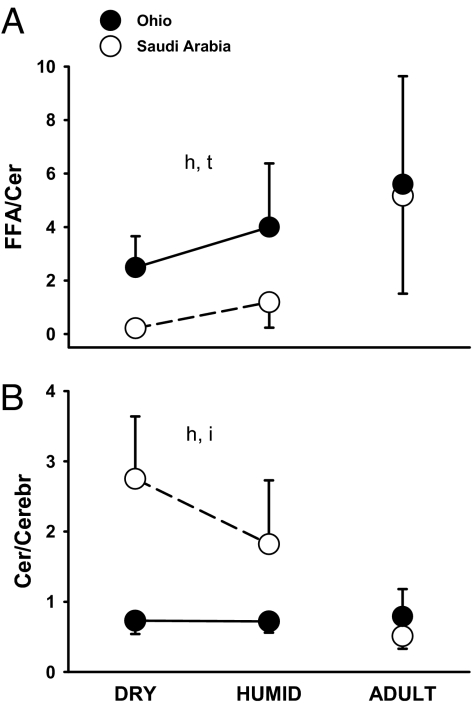
We found that fledglings exhibited developmental plasticity for the ratio of FFA to ceramides (F = 7.39, P = 0.01; Fig. 3A). The ceramide/cerebroside ratio was also plastic but only in fledglings from Saudi Arabia (interaction: F = 6.0, P < 0.02) (Fig. 3B).
Fig. 3.
FFA/ceramide ratio (A) and ceramide/cerebroside ratio (B) in fledgling and adult house sparrows from desert (open circles) and mesic (filled circles) habitats after humidity acclimation. “h,” “t,” and “i” indicate significance for habitat, treatment, and interaction term, respectively. Error bars show ±1 SD.
Habitat.
The FFA/ceramide ratio was higher in mesic fledglings than in those from the desert (F = 28.75, P < 0.001; Fig. 3A), whereas the ceramide/cerebroside ratio was higher in fledglings from Saudi Arabia (F = 64.7, P < 0.001; Fig. 3B). Habitat accounted for most of the variance in both lipid ratios in the SC of fledglings; developmental plasticity explained a significant part of the variance only for the FFA/ceramide ratio (Table 1).
Comparisons of Pairs of Siblings.
When we evaluated differences between pairs of siblings reared in different humidity, results showed that fledglings raised in the dry environment had lower CWL, significantly less FFA, a higher percentage of FFA, a lower percentage of ceramides, and a higher FFA/ceramide ratio than their humid-acclimated siblings (t > 2.55, P < 0.03). These results suggest that fledglings showed developmental plasticity for CWL and the concentration of FFA and ceramides, but not for the concentrations of cholesterol, triglycerides, and cerebrosides, in agreement with results from the comparison between acclimation groups.
CWL and Lipids.
We investigated the relationship between quantities of lipid classes in the SC and CWL of fledglings using stepwise multiple regression. With all fledglings combined, we found a significant association between CWL and amounts of ceramides and cholesterol in the SC (R2 = 0.46, P < 0.001). When we restricted analyses to dry-acclimated fledglings, we found that CWL was correlated with amounts of ceramides and cerebrosides (R2 = 0.62, P < 0.001). In humid-acclimated fledglings, CWL was negatively associated with quantity of FFA (R2 = 0.21, P < 0.05).
Again, with data for all fledglings combined, multiple regression analysis showed that CWL was significantly related to percentage of ceramides and percentage of cholesterol (R2 = 0.44, P < 0.001). When we considered dry-acclimated and humid-acclimated fledglings separately, we found that CWL was significantly correlated with percentage of ceramides in both cases (R2 = 0.61, P < 0.001; R2 = 0.39, P < 0.01, respectively).
Regressions of FFA/ceramide and ceramide/cerebroside ratios on CWL were significant when we combined all fledglings and when we separated dry- from humid-acclimated sparrows (R2 > 0.25, P < 0.05).
Transition of CWL and Lipids of the SC from Nestlings to Adults.
To understand changes that occur in CWL and lipids of the skin between nestlings and adults, we compared adults from natural environments with fledglings reared in a similar humidity environment to what they experience as adults; therefore, we compared nestlings from Saudi Arabia reared in a dry environment with adults from Saudi Arabia, and we compared nestlings from Ohio raised in a humid environment with adults from Ohio.
Fledglings from Saudi Arabia and Ohio reduced CWL when adults. Adult sparrows from Saudi Arabia reduced their CWL by 57.0% compared with desert fledglings reared in the dry environment. Adults from Ohio decreased their CWL by 25.8% compared with mesic fledglings raised in the humid environment (Fig. 1).
Nestlings altered lipid composition of their SC during ontogeny. Adult house sparrows from natural populations in Saudi Arabia had more FFA and more cerebrosides, but less ceramides in the SC than desert fledglings acclimated to a dry environment. Adult sparrows from natural habitats in Ohio had more FFA, more ceramides, and more cerebrosides in their SC than did fledglings raised in a humid environment (Fig. 2).
Compared with desert fledglings reared in the dry environment, adults from Saudi Arabia had a higher percentage of FFA and cerebrosides in the SC and a lower percentage of ceramides. Percentage of FFA was higher in adults from Ohio than in mesic fledglings acclimated to the humid environment. However, percentages of ceramides and cerebrosides did not vary with ontogeny in mesic birds.
The FFA/ceramide ratio was higher in adults from both desert and mesic populations (Fig. 3A). For Saudi Arabia sparrows, the ceramide/cerebroside ratio of adults was lower than that of fledglings acclimated to the dry environment, but that ratio remained constant in mesic birds (Fig. 3B).
Discussion
We have found that developmental plasticity of house sparrow fledglings in environments that differ in water vapor pressure and, thus, the environment–skin humidity gradient, is an important process modifying CWL through changes of the lipid composition of the SC. Dry-acclimated fledglings from both Saudi Arabia and Ohio had a lower proportion of FFA in the SC when compared with humid-acclimated birds. Fledglings from Saudi Arabia decreased the proportion of ceramides in the SC in response to high humidity, whereas fledglings from Ohio kept a constant proportion of ceramides. Comparisons between siblings also indicated that ceramides and FFA were the more flexible lipid classes in the SC. These results suggest that desert and mesic fledglings modified the lipid composition of the SC in different ways in response to acclimation. In both cases, however, the functional significance of these changes was the same: a reduction in CWL in dry-acclimated fledglings.
Adult house sparrows had lower CWL rates than fledglings in both Saudi Arabia and Ohio (13, 28). To achieve a more efficient skin permeability barrier, adult sparrows from Saudi Arabia increased the ratio of FFA to ceramides and decreased the ratio of ceramides to cerebrosides in the SC compared with nestlings. Adult house sparrows from Ohio, on the other hand, had a higher FFA/ceramide ratio than fledglings from Ohio, but they had the same ceramide/cerebroside ratio as fledglings, suggesting that this ratio is not a plastic trait in house sparrows from Ohio. Hence, adjustment of CWL rates in environments that differ in humidity seems to depend on two key lipid molecules, sphingolipids (ceramides and cerebrosides) and FFA in the SC; desert sparrows regulate mainly the former, whereas mesic sparrows modify the latter.
Comparisons of adults with fledglings from both Saudi Arabia and Ohio suggest that birds at different life stages face different selection pressures with regard to water conservation and thermoregulation. As one might expect, CWL in desert adult sparrows was lower than in mesic adult birds (13). Most likely, natural selection has acted on adult sparrows to minimize CWL in desert environments, where a frugal water economy is crucial for survival and reproduction. Adults can avoid heat gain using behavioral strategies, such as finding shade or restricting foraging time to cooler parts of the day. However, the intensity of selection for thermoregulation in desert and mesic habitats would be higher in nestlings because, confined to a nest, chicks will need to rely on physiological mechanisms to regulate their body temperatures. Rates of CWL were higher in desert than in mesic fledglings in both dry and humid acclimation environments. In the light of differential selection pressures at different life stages, we suggest that nestlings in Saudi Arabia have higher rates of water loss through their skin because they are subjected to episodes of heat stress more often than mesic nestlings.
Desert and mesic fledglings regulated rates of CWL in response to environmental conditions through modifications of lipid composition of the SC. However, adjustments of lipid concentrations of the SC were different between populations of fledglings. Both desert and mesic fledglings changed concentrations of FFA in their SC, but only desert fledglings altered concentrations of ceramides. In an evolutionary context, the regulation of the sphingolipid content of the SC is more important for desert birds than for mesic ones. Ceramides of the SC form the structural backbone of intercellular lipid lamellae, thus playing a major role in preventing high water vapor diffusion rates through the skin (29). The role of cerebrosides in the SC is not well understood, but they might interact with water molecules in the SC and promote water evaporation. Therefore, the proper combination of ceramides and cerebrosides in the SC of desert birds appears to be crucial for the dual function of the SC of water conservation and thermoregulation in this environment (13, 14). Lipids can also change their phase behavior in response to humidity (15). High levels of hydration of the SC lead to increased fluidity of intercellular lipids. Because permeability in fluid phases is higher than in solid phases (30), an increase in fluidity will be associated with higher CWL without the need to modify the lipid composition of the SC. We cannot exclude the possibility that both mechanisms, changes in lipid composition of the SC and changes in phase behavior of lipids in response to humidity, act together to influence CWL through the skin in house sparrows.
In conclusion, the picture emerges that variation in CWL between desert and mesic populations of house sparrows in response to humidity is attributable to developmental plasticity of nestlings and natural selection on both nestlings and adults. The expression of plasticity in CWL may be a response to opposite selection pressures, for attributes that influence their ability to thermoregulate and for those that influence water conservation. These selective forces occur with different intensities during the lifetime of house sparrows in different environments. House sparrow nestlings from deserts show larger developmental plasticity because the intensity of selection is larger in xeric environments. This result supports the idea that in stressful environments, such as deserts, organisms will show a higher degree of plasticity than those inhabiting more benign habitats (17, 31). Sparrows from different habitats modify different classes of lipids in the SC to regulate CWL. Desert house sparrows seemed to control more closely the concentration of sphingolipids, whereas mesic birds prioritized the regulation of the concentration of FFA. Modification of sphingolipids, FFA, or both led to changes in interactions of different lipid classes in the SC, hence altering CWL.
Materials and Methods
Study Species.
Native to Saudi Arabia, Passer domesticus indicus diverged from sparrows in Europe at least 5,000 years ago (32). House sparrows in Ohio (P.d. domesticus) were introduced from England in the 19th Century (32). Because house sparrows in Saudi Arabia live in human settlements, they have daily access to water.
Collection of House Sparrow Nestlings.
In Saudi Arabia, we placed nest boxes around the National Wildlife Research Center, near Taif (22°15′ N, 41°50′ E), between March and April, whereas we set them up in Columbus, OH (40°00′ N, 83°10′ W), between mid April and late August. Average maximum Ta during the breeding season in Taif, Saudi Arabia, was 32–35°C, and it was 23.4°C in Columbus (33, http://www.worldclimate.com). After pairs built nests and laid eggs, we checked boxes regularly until eggs hatched, designated as day 0. We collected two 3- to 4-day-old chicks from each nest, which we assumed were full-siblings. We fed nestlings Friskies cat food (78% water, 11% protein, 2.5% fat), crickets, and mealworms. Experiments were approved by the Institutional Laboratory Animal Care and Use Committee of Ohio State University (Protocol 2006-A0085) and the National Commission for Wildlife Conservation and Development, Riyadh, Saudi Arabia.
Humidity Acclimation.
In our experimental design, we had two variables, “habitat” and “treatment,” with two levels each, dry or humid. For habitat, we classified nestlings as desert or mesic. For treatment, we randomly assigned one of the siblings from each pair to a dry treatment and the other to a humid treatment. Therefore we had four groups of nestlings in our experiment; dry-acclimated and humid-acclimated individuals from Saudi Arabia (n = 9 and n = 7, respectively) and from Ohio (n = 12 for each group).
We placed nestlings in separate environmental chambers at a constant Ta of 35°C (±1°C) and a photoperiod of 12 light/12 dark. A vacuum pump brought atmospheric air into the chambers at ≈75 ml/min. Humidity and Ta were monitored continuously by using HOBO ProSeries data loggers. To maintain low humidity for the dry treatment, we routed inlet air through two columns of Drierite before entry into the chambers. To maintain a high humidity in the humid treatment, we bubbled air through water in a sealed stainless steel chamber before entry into the chamber and placed pans filled with water on the bottom of the chamber. Average relative humidity was 15–20% (mean absolute humidity of 6.5 g of H2O/m3) in the dry chambers, and 90–95% (mean absolute humidity of 31 g of H2O/m3) in the humid chambers. Nestlings remained in the dry or humid environments until they fledged (day 16–17).
Measurement of CWL.
We measured CWL using standard flow-through respirometry methods (10, 34) on postabsortive fledglings during their inactive phase. We placed fledglings in a sealed stainless-steel metabolic chamber (0.44 liters) at 35°C, on a wire mesh platform over a layer of mineral oil to trap feces.
We measured CWL and respiratory water loss using a mask system to separate these two avenues of water loss (10). Atmospheric air was drawn through a column of Drierite to remove water, and then into the metabolism chamber. Under negative pressure, air exited the chamber through two ports. From the first port, air was drawn through a mask and then routed to a dew-point hygrometer (2001-C1-S3; EdgeTech or M4; General Eastern). Water was removed from the air exiting the dew-point hygrometer with a column of Drierite and then routed to a mass-flow controller (model 5850; Brooks) set at 500–600 ml/min, calibrated with a 1-liter bubble meter (35) and then through a vacuum pump. Air exited through a second port, through a separate dew-point hygrometer, a column of Drierite, a calibrated mass-flow controller (model 5850; Brooks) set at 300–400 ml/min., and finally through a second vacuum pump. Air from the mask contained all respiratory water plus some water vapor from the skin, whereas air drawn from the chamber at a lower flow rate contained water from the skin only.
After 1 h of equilibration, when readings were constant for at least 10 min, we recorded averages of dew-point temperatures, Ta at the dew-point hygrometers, and Ta inside the chamber. CWL was calculated by using the equation CWL = (ρchamber − ρin) (V′e1 + V′e2), where ρchamber is absolute humidity of air in the chamber (g/m3, STP), ρin is absolute humidity of air entering the chamber (STP), V′e1 is flow rate at the dew-point hygrometer of the first port, and V′e2 is flow rate at the dew-point hygrometer of the second port; flow rates were calculated as in ref. 10.
Separation and Identification of Skin Lipids.
After measuring CWL, we weighed fledglings and killed them by cervical dislocation. Then, after plucking feathers, we removed the skin, and pinned it to a thin sheet of Teflon. After immersing the skin in distilled water at 65°C for 3 min, we peeled the epidermis from the dermis (11, 36) and placed it in a solution of 0.5% trypsin at 4°C overnight. The following day, we reimmersed the tissue in fresh 0.5% trypsin solution for 3 h at 38°C, effectively isolating the SC (37). After rinsing it with distilled water, we freeze-dried the SC for 12 h and stored it at −20°C in an atmosphere of nitrogen or argon.
Immediately before lipid extraction, we determined dry mass of the SC (± 0.01 mg). To extract lipids, we submerged the SC in a series of chloroform/methanol mixtures containing 50 mg/liter butylated hydroxytoluene (BHT), 2:1, 1:1, and 1:2 vol/vol for 2 h for each step (38). We dried mixtures under a stream of nitrogen gas by placing them in a water bath at 60°C (N-EVAP 11155-O; Organomation Associates).
We redissolved extracted lipids in chloroform/methanol 2:1 containing BHT and separated lipid classes of the SC of fledglings by using analytical TLC on 20 × 20-cm glass plates (0.25 mm thick; Adsorbosil-Plus 1; Altech). Each plate received a series of lipid standards of different concentrations. For plates used to quantify more polar lipids, the standard mixture contained nonhydroxy fatty acid ceramides, galactocerebrosides, and cholesterol, whereas for plates on which we determined nonpolar lipids, it consisted of a mixture of FFA and cholesterol. We dissolved known amounts of lipid standards in chloroform/methanol (2:1) with BHT and made a serial dilution to obtain five standard concentrations (13). Plates were developed with chloroform/methanol (2:1) to the top to remove contaminants and then activated in an oven at 110°C for 30 min. We pipetted 5 μl of each lipid extract and each standard solution in duplicate in the preadsorbent area of the plates. To separate ceramides and cerebrosides, we developed plates with chloroform/methanol/water (60:40:5) run 5 cm from the bottom, followed by two developments with a mixture of chloroform/methanol/acetic acid (190:9:1) to the top, and a final development with hexane/ethyl ether/acetic acid (70:30:1) run to 15 cm from the bottom. To separate cholesterol and FFAs, plates were developed with hexane to the top, toluene to the top, and with hexane/ethyl ether/acetic acid (70:30:1) run to 15 cm from the bottom. After development, we sprayed plates with a solution of 3% cupric acetate in 8% phosphoric acid and then heated them on an aluminum hotplate at 160°C for 1 h to visualize lipid bands.
To quantify concentrations of lipid classes, we scanned plates and measured the absorbance of lipid bands using TN-Image (39). After calculating standard curves, we measured the amount of each lipid class in our samples by comparing their absorbance with that of standards.
Statistics.
The quantity of some classes of lipids as determined by TLC were not normally distributed, so we log-transformed them (40). Percentages were logit transformed [Ln (Y/1 − Y)] before analyses (40). To test for significance of effects of habitat and humidity acclimation on CWL and the lipid composition of the SC, we used a two-way ANOVA, with habitat and treatment as fixed factors. If the interaction was not significant, we dropped it from the analysis and tested for significance of factors.
We interpreted results from our ANOVA following the rationale of reaction norms (41, 42). A trait lacked developmental plasticity when neither the interaction term nor treatment was significant. If habitat was significant, we inferred the existence of genetic differences between fledglings from Saudi Arabia and Ohio in that trait. If we found an insignificant interaction term and significant differences in treatment, the trait was developmentally plastic to the same degree for fledglings from both habitats. When habitat was significant, we inferred the existence of genetic differences between fledglings from both populations in addition to an acclimation effect. A significant interaction term suggested occurrence of developmental plasticity in one habitat or existence of developmental plasticity in both habitats to different degrees.
We used multiple regression to simultaneously investigate the relationship of habitat and treatment, included in the regression model as dummy independent variables, with our response variables. To assess the contribution of habitat and treatment to the variability of our response variables, we calculated semipartial correlation coefficients, which evaluate the contribution of one of the independent variables to the variance of the dependent variable, not explained by the other independent variables (43).
To explore functional consequences of variation in lipid composition of the SC of fledglings, we performed stepwise multiple regression with CWL as the dependent variable and lipid amounts and lipid percentages as independent variables (14). We also regressed CWL against total lipid and ratios of lipid classes in the SC of fledglings.
We conducted comparisons between CWL and the lipid composition of the SC of siblings assigned to different acclimation regimes using a paired t test. All statistical tests were conducted with SPSS 16.0. Averages are reported ±1 SD. We rejected the null hypothesis at P > 0.05.
Acknowledgments.
We express our appreciation to the National Commission for Wildlife Conservation and Development, Riyadh, Saudi Arabia, for support during our research. We thank Patrick Paillat, Stephane Ostrowski, Abdulrahman Khoja, and the staff of the National Wildlife Research Center (NWRC), Taif, Saudi Arabia, and Mark Schmittgen and Reagan Bluel of Waterman Farm at Ohio State University for their help with our experiments. Estela Quintero provided invaluable help in the laboratory and the field. We also thank Maria Gonzalez, Susana Rodriguez, and Catherine Tsagarakis for their assistance in the field. Wildlife research programs at the NWRC have been made possible through the support of His Royal Highness Prince Saud Al Faisal and under the guidance of Dr. Abdulaziz H. Abuzinada. This work was supported by the NWRC and National Science Foundation Grant IBN-0212092 (to J.B.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Feder ME. Ecological, evolutionary, and comparative physiology. Annu Rev Physiol. 2005;67:175–176. [Google Scholar]
- 2.Helmuth B, Kingsolver JG, Carrington E. Biophysics, physiological ecology, and climate change: Does mechanism matter? Annu Rev Physiol. 2005;67:177–201. doi: 10.1146/annurev.physiol.67.040403.105027. [DOI] [PubMed] [Google Scholar]
- 3.Bennet AF, Lenski RE. Evolutionary adaptation to temperature. 6. Phenotypic acclimation and its evolution in Escherichia coli. Evolution (Lawrence, Kans) 1997;51:36–44. doi: 10.1111/j.1558-5646.1997.tb02386.x. [DOI] [PubMed] [Google Scholar]
- 4.Williams JB, Tieleman BI. Physiological adaptation in desert birds. Bioscience. 2005;55:416–426. [Google Scholar]
- 5.Tracy RL, Walsberg GE. Development and acclimatory contributions to water loss in a desert rodent: Investigating the time course of adaptive change. J Comp Physiol B. 2001;171:669–679. doi: 10.1007/s003600100218. [DOI] [PubMed] [Google Scholar]
- 6.West-Eberhard MJ. Developmental Plasticity and Evolution. New York: Oxford Univ Press; 2003. [Google Scholar]
- 7.Piersma T, Drent J. Phenotypic flexibility and the evolution of organismal design. Trends Ecol Evol. 2003;18:228–233. [Google Scholar]
- 8.Webster MD, Campbell GS, King JR. Cutaneous resistance to water-vapor diffusion in pigeons and the role of the plumage. Physiol Zool. 1985;58:58–70. [Google Scholar]
- 9.Wolf BO, Walsberg GE. Respiratory and cutaneous evaporative water loss at high environmental temperatures in a small bird. J Exp Biol. 1996;199:451–457. doi: 10.1242/jeb.199.2.451. [DOI] [PubMed] [Google Scholar]
- 10.Tieleman BI, Williams JB. Cutaneous and respiratory water loss in larks from arid and mesic environments. Physiol Biochem Zool. 2002;75:590–599. doi: 10.1086/344491. [DOI] [PubMed] [Google Scholar]
- 11.Haugen M, Williams JB, Wertz PW, Tieleman BI. Lipids of the stratum corneum vary with cutaneous water loss among larks along a temperature-moisture gradient. Physiol Biochem Zool. 2003;76:907–917. doi: 10.1086/380213. [DOI] [PubMed] [Google Scholar]
- 12.Haugen M, Tieleman BI, Williams JB. Phenotypic flexibility in cutaneous water loss and lipids of the stratum corneum. J Exp Biol. 2003;206:3581–3588. doi: 10.1242/jeb.00596. [DOI] [PubMed] [Google Scholar]
- 13.Muñoz-Garcia A, Williams JB. Cutaneous water loss and lipids of the stratum corneum in house sparrows, Passer domesticus, from arid and mesic environments. J Exp Biol. 2005;208:3689–3700. doi: 10.1242/jeb.01811. [DOI] [PubMed] [Google Scholar]
- 14.Muñoz-Garcia A, Ro J, Brown JC, Williams JB. Cutaneous water loss and sphingolipids in the stratum corneum of house sparrows, Passer domesticus L, from desert and mesic environments as determined by reversed phase high-performance liquid chromatography coupled with atmospheric pressure photospray ionization mass spectrometry. J Exp Biol. 2008;211:447–458. doi: 10.1242/jeb.013649. [DOI] [PubMed] [Google Scholar]
- 15.Silva CL, et al. Stratum corneum hydration: phase transformations and mobility in stratum corneum, extracted lipids and isolated corneocytes. Biochim Biophys Acta. 2007;1768:2647–2659. doi: 10.1016/j.bbamem.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 16.Muñoz-Garcia A, Cox RL, Williams JB. Phenotypic flexibility in cutaneous water loss and lipids of the stratum corneum in house sparrows (Passer domesticus) following acclimation to high and low humidity. Physiol Biochem Zool. 2008;81:87–96. doi: 10.1086/522651. [DOI] [PubMed] [Google Scholar]
- 17.Schlichting CD, Smith H. Phenotypic plasticity: Linking molecular mechanisms with evolutionary outcomes. Evol Ecol. 2002;16:189–211. [Google Scholar]
- 18.Tieleman BI, Williams JB, Buschur ME, Brown CR. Phenotypic variation among and within larks along an aridity gradient: Are desert birds more flexible? Ecology. 2003;84:1800–1815. [Google Scholar]
- 19.Terblanche JS, Chown SL. The relative contributions of developmental plasticity and adult acclimation to physiological variation in the tsetse fly, Glossina pallidipes (Diptera, Glossinidae) J Exp Biol. 2006;209:1064–1073. doi: 10.1242/jeb.02129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pigliucci M. Evolution of phenotypic plasticity: Where are we going now? Trends Ecol Evol. 2005;20:481–486. doi: 10.1016/j.tree.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 21.Price TD, Qvarnstrom A, Irwin DE. The role of phenotypic plasticity in driving genetic evolution. Proc R Soc London Ser B. 2003;270:1433–1440. doi: 10.1098/rspb.2003.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffmann AA, Shirriffs J, Scott M. Relative importance of plastic vs genetic factors in adaptive differentiation: Geographical variation for stress resistance in Drosophila melanogaster from eastern Australia. Funct Ecol. 2005;19:222–227. [Google Scholar]
- 23.Gibert P, Huey RB, Gilchrist GW. Locomotor performance of Drosophila melanogaster: Interactions among developmental and adult temperatures, age, and geography. Evolution (Lawrence, Kans) 2001;55:205–209. doi: 10.1111/j.0014-3820.2001.tb01286.x. [DOI] [PubMed] [Google Scholar]
- 24.Wilson RS, Franklin CE. Testing the benefitial acclimation hypothesis. Trens Ecol Evol. 2002;17:66–70. [Google Scholar]
- 25.Fischer K, Eenhoorn E, Bot ANM, Brakefield PM, Zwaan BJ. Cooler butterflies lay larger eggs: Developmental plasticity versus acclimation. Proc R Soc London Ser B. 2003;270:2051–2056. doi: 10.1098/rspb.2003.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elias PM. The epidermal permeability barrier: from the early days at Harvard to emerging concepts. J Invest Dermatol. 2004;122:36–39. doi: 10.1046/j.0022-202X.2004.22233.x. [DOI] [PubMed] [Google Scholar]
- 27.Walsberg GE, King JR. The relationship of the external surface area of birds to skin surface and body mass. J Exp Biol. 1978;76:185–189. [Google Scholar]
- 28.Groff B, Muñoz-Garcia A, Yamaguchi M, Williams JB. Development of skin structure and cutaneous water loss in nestling desert house sparrows from Saudi Arabia. Comp Biochem Physiol A. 2007;147:493–501. doi: 10.1016/j.cbpa.2007.01.680. [DOI] [PubMed] [Google Scholar]
- 29.Bouwstra JA, Honeywell-Nguyen PL, Gooris GS, Ponec M. Structure of the skin barrier and its modulation by vesicular formulations. Prog Lipid Res. 2003;42:1–36. doi: 10.1016/s0163-7827(02)00028-0. [DOI] [PubMed] [Google Scholar]
- 30.Schaefer H, Rodelmeier TE. Principles of Percutaneous Absorption. Basel: Karger; 1996. Skin barrier; pp. 43–86. [Google Scholar]
- 31.Parsons PA. Evolutionary rates under environmental stress. Evol Biol. 1987;21:311–347. [Google Scholar]
- 32.Summers-Smith JD. The House Sparrow. London: Collins; 1988. [Google Scholar]
- 33.Fisher M, Membery DA. In: Vegetation of the Arabian Peninsula. Ghazanfar SA, Fisher M, editors. Boston: Kluwer Academic Publishers; 1998. pp. 5–38. [Google Scholar]
- 34.Gessaman JA. In: Raptor Management Techniques Manual. Pendleton BA, Millsop BA, Cline KW, Bird DM, editors. New Haven, CT: Yale Univ Press; 1987. pp. 289–320. [Google Scholar]
- 35.Levy A. The accuracy of the bubble meter for gas flow measurements. J Sci Instr. 1964;41:449–453. [Google Scholar]
- 36.Wertz PW, Stover PM, Abraham W, Downing DT. Lipids of chicken epidermis. J Lipid Res. 1986;27:427–435. [PubMed] [Google Scholar]
- 37.Wertz PW, Downing DT. Covalently bound ω-hydroxyacylsphingosine in the stratum corneum. Biochim Biophys Acta. 1987;917:108–111. doi: 10.1016/0005-2760(87)90290-6. [DOI] [PubMed] [Google Scholar]
- 38.Law S, Wertz PW, Swartzendruber DC, Squier CA. Regional variation in content, composition and organization of porcine epithelial barrier lipids revealed by thin layer chromatography and transmission electron microscopy. Arch Oral Biol. 1995;40:1085–1091. doi: 10.1016/0003-9969(95)00091-7. [DOI] [PubMed] [Google Scholar]
- 39.Nelson TJ. TN-Image. 2003 Available at http://brneurosci.org/tnimage.html.
- 40.Zar JH. Biostatistical Analysis. Engelwood Cliffs, NJ: Prentice Hall; 1996. [Google Scholar]
- 41.Via S. Adaptive phenotypic plasticity: Target or by-product of selection in a variable environment? Am Nat. 1993;142:352–365. doi: 10.1086/285542. [DOI] [PubMed] [Google Scholar]
- 42.Pigliucci M. Beyond nature and nurture. Baltimore: The Johns Hopkins Univ Press; 2001. Phenotypic plasticity. [Google Scholar]
- 43.Field A. London: Sage Publications; 2005. Discovering statistics using SPSS. [Google Scholar]