Abstract
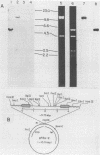
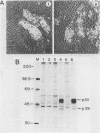
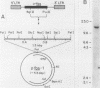
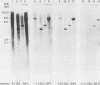
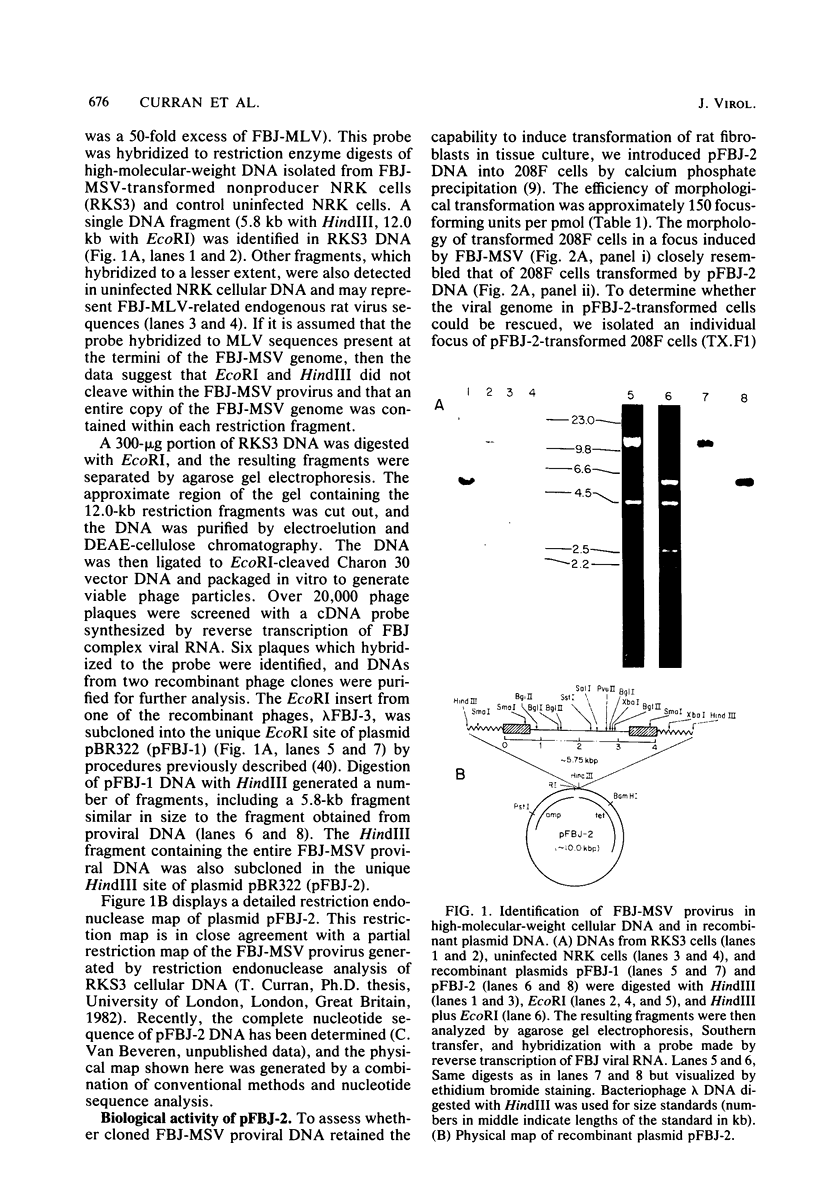
A 12.0-kilobase EcoRI restriction fragment containing FBJ murine osteosarcoma virus (FBJ-MSV) proviral DNA was identified in FBJ-MSV-transformed nonproducer rat cells and molecularly cloned in bacteriophage Charon 30 (lambda FBJ-1). A 5.8-kb HindIII fragment containing the entire FBJ-MSV proviral DNA was isolated from lambda FBJ-1 and subsequently subcloned in plasmid pBR322 (pFBJ-2). The DNA from recombinant plasmid pFBJ-2 was able to induce morphological transformation of rat fibroblasts in tissue culture. Transfected cells contained the p55 and p39 antigens specific for cells transformed by FBJ-MSV (T. Curran and N. M. Teich, J. Virol. 42:114-122, 1982). The organization of the FBJ-MSV provirus was analyzed by restriction endonuclease mapping, and a region of nonhomology with the helper virus was delineated. Sequences specific for this region (presumably the viral fos gene) were subcloned and used as a probe to identify related sequences present in the normal genomes of cells from a variety of mammalian species (cellular fos). A single-size (3.4 kilobases long) class of RNA hybridizing to the viral fos probe was identified in FBJ-MSV-transformed cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen P. R., Devare S. G., Tronick S. R., Ellis R. W., Aaronson S. A., Scolnick E. M. Generation of BALB-MuSV and Ha-MuSC by type C virus transduction of homologous transforming genes from different species. Cell. 1981 Oct;26(1 Pt 1):129–134. doi: 10.1016/0092-8674(81)90041-6. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Enemies within: the genesis of retrovirus oncogenes. Cell. 1981 Jan;23(1):5–6. doi: 10.1016/0092-8674(81)90263-4. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Maryak J. M., Wei C. M., Shih T. Y., Shober R., Cheung H. L., Ellis R. W., Hager G. L., Scolnick E. M., Lowy D. R. Functional organization of the Harvey murine sarcoma virus genome. J Virol. 1980 Jul;35(1):76–92. doi: 10.1128/jvi.35.1.76-92.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Curran T., Teich N. M. Candidate product of the FBJ murine osteosarcoma virus oncogene: characterization of a 55,000-dalton phosphoprotein. J Virol. 1982 Apr;42(1):114–122. doi: 10.1128/jvi.42.1.114-122.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Teich N. M. Identification of a 39,000-dalton protein in cells transformed by the FBJ murine osteosarcoma virus. Virology. 1982 Jan 15;116(1):221–235. doi: 10.1016/0042-6822(82)90415-9. [DOI] [PubMed] [Google Scholar]
- DeFeo D., Gonda M. A., Young H. A., Chang E. H., Lowy D. R., Scolnick E. M., Ellis R. W. Analysis of two divergent rat genomic clones homologous to the transforming gene of Harvey murine sarcoma virus. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3328–3332. doi: 10.1073/pnas.78.6.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner L., Fedele L. A., Garon C. F., Anderson S. J., Sherr C. J. McDonough feline sarcoma virus: characterization of the molecularly cloned provirus and its feline oncogene (v-fms). J Virol. 1982 Feb;41(2):489–500. doi: 10.1128/jvi.41.2.489-500.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eva A., Robbins K. C., Andersen P. R., Srinivasan A., Tronick S. R., Reddy E. P., Ellmore N. W., Galen A. T., Lautenberger J. A., Papas T. S. Cellular genes analogous to retroviral onc genes are transcribed in human tumour cells. Nature. 1982 Jan 14;295(5845):116–119. doi: 10.1038/295116a0. [DOI] [PubMed] [Google Scholar]
- Finkel M. P., Biskis B. O., Jinkins P. B. Virus induction of osteosarcomas in mice. Science. 1966 Feb 11;151(3711):698–701. doi: 10.1126/science.151.3711.698. [DOI] [PubMed] [Google Scholar]
- Franchini G., Even J., Sherr C. J., Wong-Staal F. onc sequences (v-fes) of Snyder-Theilen feline sarcoma virus are derived from noncontiguous regions of a cat cellular gene (c-fes). Nature. 1981 Mar 12;290(5802):154–157. doi: 10.1038/290154a0. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Gilboa E., Witte O. N., Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980 Dec;22(3):777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Production of altered cell foci in tissue culture by defective Moloney sarcoma virus particles. Proc Natl Acad Sci U S A. 1966 Apr;55(4):780–786. doi: 10.1073/pnas.55.4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huu Duc-Nguyen, Rosenblum E. N., Zeigel R. F. Persistent infection of a rat kidney cell line with Rauscher murine leukemia virus. J Bacteriol. 1966 Oct;92(4):1133–1140. doi: 10.1128/jb.92.4.1133-1140.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M., Bosselman R. A., van der Hoorn F. A., Berns A., Fan H., Verma I. M. Identification and molecular cloning of Moloney mouse sarcoma virus-specific sequences from uninfected mouse cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2651–2655. doi: 10.1073/pnas.77.5.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Hartley J. W., Rowe W. P., Huebner R. J. Studies of FBJ osteosarcoma virus in tissue culture. I. Biologic characteristics of the "C"-type viruses. J Natl Cancer Inst. 1973 Aug;51(2):525–539. [PubMed] [Google Scholar]
- Levy J. A., Kazan P. L., Reilly C. A., Finkel M. P. FBJ osteosarcoma virus in tissue culture. III. Isolation and characterization of non-virus-producing FBJ-transformed cells. J Virol. 1978 Apr;26(1):11–15. doi: 10.1128/jvi.26.1.11-15.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra N. K., Ryan W. L. Effect of 3-methylcholanthrene and dimethylnitrosamine on anchorage dependence of rat fibroblasts chronically infected with Rauscher leukemia virus. Int J Cancer. 1973 Jan 15;11(1):123–130. doi: 10.1002/ijc.2910110114. [DOI] [PubMed] [Google Scholar]
- Oskarsson M., McClements W. L., Blair D. G., Maizel J. V., Vande Woude G. F. Properties of a normal mouse cell DNA sequence (sarc) homologous to the src sequence of Moloney sarcoma virus. Science. 1980 Mar 14;207(4436):1222–1224. doi: 10.1126/science.6243788. [DOI] [PubMed] [Google Scholar]
- Parker R. C., Varmus H. E., Bishop J. M. Cellular homologue (c-src) of the transforming gene of Rous sarcoma virus: isolation, mapping, and transcriptional analysis of c-src and flanking regions. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5842–5846. doi: 10.1073/pnas.78.9.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B., Baluda M. A. Avian myeloblastosis virus transforming gene is related to unique chicken DNA regions separated by at least one intervening sequence. J Virol. 1982 Jan;41(1):250–257. doi: 10.1128/jvi.41.1.250-257.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quade K. Transformation of mammalian cells by avian myelocytomatosis virus and avian erythroblastosis virus. Virology. 1979 Oct 30;98(2):461–465. doi: 10.1016/0042-6822(79)90569-5. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robins T., Bister K., Garon C., Papas T., Duesberg P. Structural relationship between a normal chicken DNA locus and the transforming gene of the avian acute leukemia virus MC29. J Virol. 1982 Feb;41(2):635–642. doi: 10.1128/jvi.41.2.635-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Rubtsov P. M., Musakhanov M. M., Zakharyev V. M., Krayev A. S., Skryabin K. G., Bayev A. A. The structure of the yeast ribosomal RNA genes. I. The complete nucleotide sequence of the 18S ribosomal RNA gene from Saccharomyces cerevisiae. Nucleic Acids Res. 1980 Dec 11;8(23):5779–5794. doi: 10.1093/nar/8.23.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalloway D., Zelenetz A. D., Cooper G. M. Molecular cloning and characterization of the chicken gene homologous to the transforming gene of Rous sarcoma virus. Cell. 1981 May;24(2):531–541. doi: 10.1016/0092-8674(81)90344-5. [DOI] [PubMed] [Google Scholar]
- Shibuya M., Hanafusa H., Balduzzi P. C. Cellular sequences related to three new onc genes of avian sarcoma virus (fps, yes, and ros) and their expression in normal and transformed cells. J Virol. 1982 Apr;42(1):143–152. doi: 10.1128/jvi.42.1.143-152.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehner R. L., Dmochowski L. Induction of bone tumours in rats and hamsters with murine sarcoma virus and their cell-free transmission. Nature. 1969 Oct 11;224(5215):191–192. doi: 10.1038/224191a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Taylor J. M., Illmensee R., Summers J. Efficeint transcription of RNA into DNA by avian sarcoma virus polymerase. Biochim Biophys Acta. 1976 Sep 6;442(3):324–330. doi: 10.1016/0005-2787(76)90307-5. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., Rands E., Chattopadhyay S. K., Lowy D. R., Verma I. M. Long terminal repeat of murine retroviral DNAs: sequence analysis, host-proviral junctions, and preintegration site. J Virol. 1982 Feb;41(2):542–556. doi: 10.1128/jvi.41.2.542-556.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Lai M. H., Bosselman R. A., McKennett M. A., Fan H., Berns A. Molecular cloning of unintegrated Moloney mouse sarcoma virus DNA in bacteriophage lambda. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1773–1777. doi: 10.1073/pnas.77.4.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward J. M., Young D. M. Histogenesis and Morphology of periosteal sarcomas induced by FBJ virus in NIH Swiss mice. Cancer Res. 1976 Nov;36(11 Pt 1):3985–3992. [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. Methods in Enzymology volume 68 recombinant DNA. Methods Enzymol. 1979;68:1–555. doi: 10.1016/0076-6879(79)68001-1. [DOI] [PubMed] [Google Scholar]