Abstract
Light sensing starts with phototransduction in photoreceptor cells. The phototransduction cascade has diverged in different species, such as those mediated by transducin in vertebrate rods and cones, by Gq-type G protein in insect and molluscan rhabdomeric-type visual cells and vertebrate photosensitive retinal ganglion cells, and by Go-type G protein in scallop ciliary-type visual cells. Here, we investigated the phototransduction cascade of a prebilaterian box jellyfish, the most basal animal having eyes containing lens and ciliary-type visual cells similar to vertebrate eyes, to examine the similarity at the molecular level and to obtain an implication of the origin of the vertebrate phototransduction cascade. We showed that the opsin-based pigment functions as a green-sensitive visual pigment and triggers the Gs-type G protein-mediated phototransduction cascade in the ciliary-type visual cells of the box jellyfish lens eyes. We also demonstrated the light-dependent cAMP increase in the jellyfish visual cells and HEK293S cells expressing the jellyfish opsin. The first identified prebilaterian cascade was distinct from known phototransduction cascades but exhibited significant partial similarity with those in vertebrate and molluscan ciliary-type visual cells, because all involved cyclic nucleotide signaling. These similarities imply a monophyletic origin of ciliary phototransduction cascades distributed from prebilaterian to vertebrate.
Keywords: G protein, phototransduction, visual cell, visual pigment, rhodopsin
Many animals sense light signals for vision and nonvisual photoreceptions. Light is captured by an opsin-based photopigment in a photoreceptor cell and leads to cellular light response through a G protein-mediated phototransduction cascade. Three kinds of phototransduction cascades have been found thus far (1). In vertebrate rods and cones, the light is absorbed by visual pigment, and the information is relayed via transducin (Gt) (2), causing the decrease of intracellular cGMP concentration to close cyclic nucleotide-gated channels (3, 4). In rhabdomeric-type visual cells of higher invertebrates, such as arthropods and molluscans, Gq-type G protein passes the light information to the phosphoinositol signaling cascade (5–9), which is also found in vertebrate photosensitive retinal ganglion cells (10). In addition, we reported that the Go-type G protein-mediated phototransduction cascade (11) involving in the cGMP increase as a second messenger exists in scallop ciliary visual cells (12). Recently, the Go-mediated phototransduction cascade was also found in the ciliary photoreceptor cells of lizard parietal eyes (13). These varied phototransduction cascades are, respectively, driven by particular opsins, which belong to phylogenetically distinct opsin subfamilies (1). Because vision has evolved with phototransduction cascades and has diverged in different species, the opsin-based pigment and signaling cascade of lower invertebrates, such as prebilaterian animals, is important to understand the evolution of phototransduction, especially the origin of vertebrate vision.
The prebilaterian cnidaria is the most basal phylum having a visual system with specialized sensory organs (eyes), and, in particular, cubozoans or box jellyfish are distinguished from all other cnidarians by possessing elaborate lens eyes, which resemble those of higher animals (14, 15). In addition, visual cells in the box jellyfish eyes have ciliary morphology as do vertebrate rods and cones. Therefore, it has been of great interest for more than a century whether the box jellyfish visual system is similar to that of vertebrate at the molecular level (16). However, the underlying molecular mechanisms of the jellyfish vision, including the particular photopigment and signal transduction cascade, remain to be elucidated. Because opsin sequences were recently found in other classes of cnidarians, a sea anemone (anthozoan), hydra (17), and hydrozoan jellyfish (hydrozoan) (18), opsin is a candidate for the photoreceptive pigment in cnidarian vision.
Here, we investigated the box jellyfish visual system to elucidate the prebilaterian phototransduction cascade and to understand the phototransduction evolution throughout the animal kingdom. We showed that the opsin-based pigment functions as a green-sensitive visual pigment and triggers Gs-type G protein-mediated phototransduction cascade in the ciliary-type visual cells of the box jellyfish lens eyes. We also demonstrated that the opsin–Gs cascade causes a light-dependent cAMP increase in the jellyfish visual cells. The first identified prebilaterian cascade was distinct from any known phototransduction cascades, but it exhibited significant partial similarity to those in vertebrate and molluscan ciliary-type visual cells because all involved cyclic nucleotide signaling. Based on these similarities, we discussed evolutionary linkage among ciliary phototransduction cascades distributed from prebilaterian to vertebrate.
Results
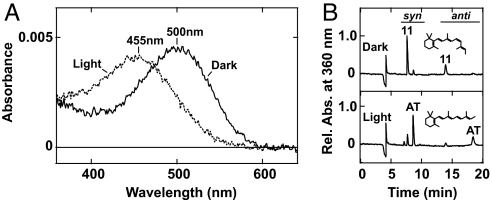
To obtain the direct evidence of the involvement of an opsin-based pigment in cnidarian vision and to characterize its molecular basis, we isolated a cDNA encoding opsin from rhopalia, which contain lens eyes, of the box jellyfish Carybdea rastonii [supporting information (SI) Fig. S1 A and B]. The amino acid sequence of the box jellyfish opsin exhibited typical features of opsins (1), such as seven putative membrane-spanning domains and a lysine residue in the seventh membrane-spanning domain that binds the retinal chromophore (Fig. S1C). In the phylogenetic tree of the opsin family, the box jellyfish opsin fell into the cnidarian opsins, clustering with the hydrozoan opsins, which was consistent with the relationship among cnidarian classes (19) (Fig. S2A). We then expressed the box jellyfish opsin in HEK293S cells, to demonstrate that the jellyfish opsin forms a photosensitive pigment, and we succeeded in obtaining the purified pigment from these cells. Fig. 1A shows the spectroscopic features of the opsin-based pigment, demonstrating that it is a green-light-sensitive pigment with an absorption maximum at ≈500 nm. The absorption spectrum agreed with the spectral sensitivity (maximum at ≈500 nm) of an electroretinogram obtained from the lens eyes of a related species of box jellyfish, Tripedalia crystophora (20). Green light irradiation of the jellyfish opsin-based pigment did not result in bleached photoproduct with an absorption maximum at 360 nm, in the UV region, but resulted in the blue-shifted photoproduct with an absorption maximum at ≈455 nm, in the visible region, unlike vertebrate visual pigments (1) (Fig. 1A). The photoproduct was stable but did not show photoregeneration by subsequent light absorption (data not shown), suggesting that the jellyfish opsin does not have a bistable nature, which bilaterian invertebrate visual pigments exhibit (10, 21). The chromophore configurations of the jellyfish opsin and its photoproduct were revealed to be 11-cis and all-trans forms, respectively, by HPLC analyses of the jellyfish opsin-based pigment expressed in HEK293S cells (Fig. 1B). These results may suggest that the jellyfish visual cells contain a chromophore retinal-replacement system from the all-trans to 11-cis form, to restore the photosensitivity of the pigment, as found in the dark regeneration of bistable pigments (22). Therefore, we concluded that the box jellyfish opsin forms a photosensitive pigment with similar characteristics in photoisomerization profile and with differences in the photoproduct property, which is involved in the G protein activation, to the visual pigments of higher animals, such as insects, molluscs, and vertebrates.
Fig. 1.
Box jellyfish opsin as a photosensitive pigment. (A) Absorption spectra of the box jellyfish opsin-based pigment in the dark state (solid curve) and after irradiation with green-light (dotted curve). (B) Chromophore configurations of the pigment in the dark (Upper) and after irradiation (Lower) analyzed with HPLC as retinal oximes (syn- and anti-forms of 11-cis- and all-trans-retinal oximes). Relative absorbances, which were normalized with absorbance of syn-form of 11-cis-retinal oxime in the dark state, are indicated.
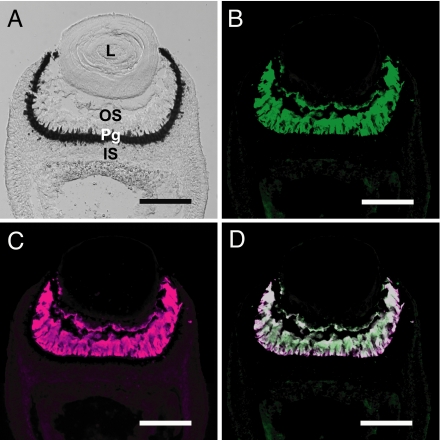
Each rhopalium of the box jellyfish contains two highly sophisticated lens eyes, upper small lens eye and lower large lens eye, in addition to two pairs of simple pit eyes (14, 16, 23) (Fig. S1B). The lens eyes contain ciliary-type visual cells composed of three parts: an outer segment derived from a modified cilium (putative photoreceptive region), a pigment granule-rich region, and an inner segment (14) (Fig. 2A and Fig. S3A). We raised a polyclonal antibody specific for the box jellyfish opsin (Fig. S1D) and conducted immunohistochemical staining of the jellyfish eyes to determine the cellular localization of the opsin. In the lens eyes, the antibody strongly labeled all of the outer segments of visual cells, demonstrating abundant expression of the opsin (Fig. 2B and Fig. S3B). In situ hybridization analysis confirmed the specific expression of the box jellyfish opsin in visual cells on the nucleotide level (Fig. S1 E and F). These localizations, together with the matching of the absorption spectrum of the box jellyfish opsin and the spectral sensitivity of the electroretinogram from lens eyes (20), demonstrate that the opsin is the visual pigment of the jellyfish.
Fig. 2.
Colocalization of the opsin and Gs in the box jellyfish visual cells. (A) Nomarski image of the large lens eye of the box jellyfish. L, lens; OS, outer segment; Pg, pigment granule-rich region; IS, inner segment. (B and C) Immunofluorescence labeling of outer segments of box jellyfish visual cells with the anti-box jellyfish opsin antibody (B, green) and the anti-Gαs antibody (C, magenta). (D) Merged image showing colocalization of the opsin and Gs in the visual cells. (Scale bars: 100 μm.)
Next, we investigated which phototransduction cascade the box jellyfish opsin triggers in vivo. We first examined the possibility that Gt, Gq, and Go, which mediate phototransduction in higher animals, exist in the jellyfish visual cells. Unexpectedly, the anti-α subunit of Gt (Gαt), anti-Gαq, and anti-Gαo antibodies did not label the visual cells (Fig. S4 A–C), raising the possibility that they contain a novel phototransduction cascade. We conducted PCR-based screening of the α subunit of G protein against the cDNAs derived from the jellyfish lens eyes and obtained cDNAs encoding Gαs and Gα12. We performed immunohistochemical analyses with antibodies against Gαs and Gα12 and observed strong fluorescent signals in outer segments of visual cells when employing the anti-Gαs antibody (Fig. 2C and Fig. S3C), whereas no signal was obtained by using the anti-Gα12 antibody (Fig. S4D). A merged image of immunofluorescent labeling with the anti-jellyfish opsin antibody and with the anti-Gαs antibody showed colocalization of the opsin and Gs in the jellyfish visual cells (Fig. 2D). These results suggest that the jellyfish opsin triggers a Gs-mediated signal transduction cascade in vivo and that the phototransduction cascade in box jellyfish ciliary visual cells differs from that in higher animal photoreceptor cells.
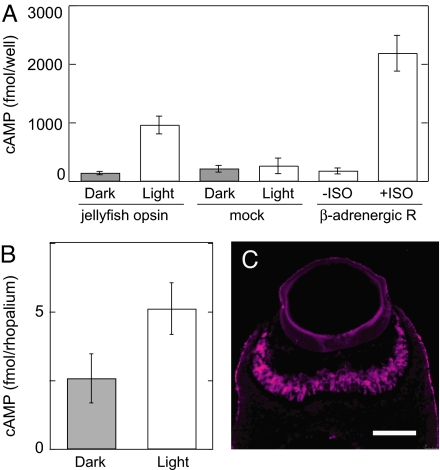
According to studies on G protein-mediated signal transduction in higher animals, Gs activates adenylyl cyclase, which elevates intracellular cAMP (24). Therefore, to obtain evidence that the jellyfish opsin activates Gs, we heterologously expressed the box jellyfish opsin in HEK293S cells and analyzed the light-dependent increase in intracellular cAMP. An enzyme-linked immunoassay showed that the cAMP concentration in irradiated cells was ≈10-fold higher than that of nonirradiated cells and was comparable with the level of agonist-induced cAMP elevation in β2-adrenergic receptor-expressing cells (Fig. 3A). Irradiation of mock-transfected cells did not increase cAMP, demonstrating that box jellyfish opsin triggers the Gs–adenylyl cyclase cascade in a light-dependent manner. Furthermore, we investigated whether the light-dependent cAMP increase also occurs in vivo. We measured the amount of cAMP in dark-adapted and irradiated rhopalia of the box jellyfish. Fig. 3B shows that the levels of cAMP in irradiated rhopalia were significantly higher than those in dark-adapted rhopalia, which provides clear evidence that a light-dependent increase of cAMP concentration occurred in the jellyfish eyes. We also confirmed the existence of an adenylyl cyclase in the box jellyfish visual cells by immunohistochemical analysis by using the anti-adenylyl cyclase antibody that recognizes most members of adenylyl cyclase family. The antibody labeled the outer segment of the jellyfish visual cells, as do the antibodies against the opsin and Gαs (Fig. 3C and Fig. S3D). Because such abundant expression of adenylyl cyclase was not observed in any parts of the rhopalia other than the visual cells (data not shown), we concluded that the light-dependent increase of cAMP shown here occurred in these cells. These results demonstrate that the jellyfish phototransduction cascade is composed of opsin, Gs, and adenylyl cyclase.
Fig. 3.
Light-dependent cAMP increase mediated by the opsin-based pigment. (A) A cAMP increase caused by light irradiation was observed in HEK293S cells transfected with the box jellyfish opsin cDNA (jellyfish opsin) but not in cells transfected with expression vector alone (mock). n = 3. As a control, the cAMP increase caused by the agonist isoprotenol (ISO) in HEK293S cells transfected with human β2-adrenergic receptor cDNA was measured (n = 3). (B) Light-dependent cAMP increase in the box jellyfish eyes. The amounts of cAMP extracted from dark-adapted (Dark) and irradiated rhopalia containing eyes (Light) were compared (n = 3). Error bars represent SEM. (C) Immunofluorescence labeling of outer segments of the box jellyfish visual cells with the anti-adenylyl cyclase antibody (magenta). (Scale bar: 100 μm.)
Discussion
We have shown here that opsin functions as a visual pigment in cnidarians, and its photoproduct property differs from that of bilaterian visual pigments. In addition, we have clearly demonstrated that the box jellyfish opsin triggers a Gs–adenylyl cyclase signal transduction cascade and consequently elicits a light-dependent increase in cAMP in the visual cells. Recent phylogenetic analyses, including genome data of cnidarian opsins, showed that most cnidarian opsins formed a single group (17), and we showed that the box jellyfish opsin belonged to the cnidarian opsin group (Fig. S2A and Fig. 4). Our findings suggest that other members of the cnidarian opsin group can also function as photopigments and activate Gs. We emphasize that the “opsin–Gs–adenylyl cyclase” cascade that we report here is direct evidence not only of phototransduction but also of G protein-mediated signal transduction in the lower animals, the prebilaterians. Furthermore, the discovery of Gs-coupled opsin is also important because it provides an elegant method to investigate G protein-coupled receptor-linked cAMP-dependent cellular or physiological responses by using light instead of chemical ligands as a trigger (Fig. 3A).
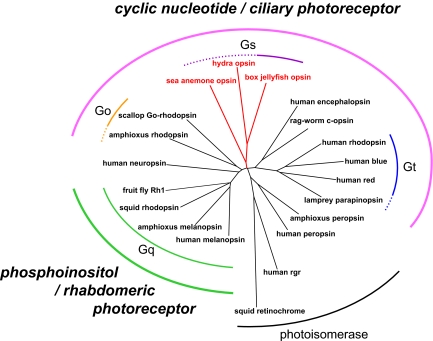
Fig. 4.
Phylogenetic relationship of opsins and animal phototransduction cascades. The unrooted tree of the opsin family was inferred based on Fig. S2A. The amino acid sequences of opsins that were revealed to function as photopigments and their apparent homologues were included. The cnidarian lineage is highlighted (red). The G protein subtypes that the opsins are coupled with and the second messengers in the phototransduction cascades, as well as the photoreceptor cell types, are indicated.
Two morphologically distinct photoreceptor cell types, ciliary-type cells with membranes of modified cilia and rhabdomeric-type cells with apical microvilli, exist in animals (25), and their relationships to the photopigment and signal transduction cascade have been analyzed (11, 26, 27). In rhabdomeric-type cells and their relatives, which are found in many higher invertebrate visual cells and vertebrate photosensitive retinal ganglion cells, Gq-coupled opsin or r-opsin functions as a photopigment (1, 28); that is, it triggers the inositol phospholipid signaling cascade via Gq (7, 9, 10). Therefore, rhabdomeric-type photoreceptor cells appear to be of monophyletic origin from both morphological and functional viewpoints (Fig. 4). By contrast, several types of opsins and phototransduction cascades are found in ciliary-type photoreceptor cells (1) (Fig. 4). In vertebrate rods and cones, visual pigment triggers transducin (referred to as Gt-coupled opsin) (3), and its related pigment encephalopsin or c-opsin is found in ciliary cells in the marine ragworm brain (26). In addition, Go-coupled opsin, which is phylogenetically distinct from Gt-coupled opsin, couples with Go in scallop ciliary visual cells (11, 12). Recently, parietopsin, which is closely related to Gt-coupled opsins, was also shown to couple with Go in the ciliary photoreceptor cells of lizard parietal eyes (13). Here, we demonstrated that jellyfish ciliary-type visual cells contain a Gs-mediated phototransduction cascade (Figs. 2 and 3). Accordingly, judging from the G protein subtype that mediates light information, the ciliary-type photoreceptor cells seem to have polyphyletic origins (Fig. 4). However, despite these differences, all of these cells employ cyclic nucleotides (cGMP or cAMP) as the second messenger in the phototransduction signaling cascade. In the Gt-mediated phototransduction of vertebrate rods and cones, it has been shown that cyclic nucleotide-gated (CNG) channels are used for generating cellular responses (4, 29). Similarly, in the Go-mediated phototransduction of molluscan photoreceptors, the functioning of CNG channels for generating cellular responses was inferred from pharmacological experiments (30, 31). Interestingly, we also isolated a cDNA encoding a channel, which fell into the CNG subfamily including vertebrate rod, cone, and olfactory CNGs, from the box jellyfish lens eyes (Fig. S4B). These observations, together with the fact that all known CNGs respond to both cAMP and cGMP (29), suggest a monophyletic group of animal phototransduction cascades characterized by employing cyclic nucleotides in phototransduction signaling, presenting an evolutionary linkage from prebilaterian phototransduction to vertebrate phototransduction. Therefore, we propose a classification of animal photoreceptor cells, including the previous reports (11, 25–27), the rhabdomeric photoreceptor cell containing phosphoinositol signaling mediated by Gq, and the ciliary photoreceptor cell containing cyclic nucleotide signaling mediated by Gt, by Go, or by Gs (Fig. 4). Surprisingly, the Gs-mediated phototransduction cascade in jellyfish visual cells exhibits overall similarities with the vertebrate olfactory signaling cascade, which is composed of Golf (a kind of Gs) and adenylyl cyclase type III, and elicits increases in cAMP and activation of CNG channels (32). Thus, it may be an intriguing hypothesis that the vertebrate olfactory sensory neuron, which also has ciliary morphology, shares an evolutionarily common origin with the ciliary photoreceptor cells.
Since submission of this paper, Kozmik et al. (33) reported the photosensitivity of the opsin-based pigment of other box jellyfish species, based on the difference spectrum, which does not provide the absolute absorption spectrum of the pigment or its photoreaction properties, basically. They also reported existence of some signal transduction-related molecules other than Gs and adenylyl cyclase in the rhopalia. It would be interesting to investigate their relation to the opsin–Gs–adenylyl cyclase cascade.
Materials and Methods
Animals.
Box jellyfish (C. rastonii) were collected in the Japanese Sea around the Oki islands, Japan. The jellyfish were kept in the dark overnight before experiments. The rhopalia, which contain the lens eyes, were dissected under dim red light.
cDNA Cloning.
The opsin, Gαs, Gα12, and CNG cDNAs of the box jellyfish were isolated from the rhopalia by RT-PCR with following degenerated primers: 5′-GCITTYYTIITIGCITGGACNCCNTA-3′ as the sense primer and 5′-GAATTCAIIGCRTADATIAINGGRTT-3′ as the antisense primer for cloning of the opsin, designed based on FLVAWTPY and NPIIYAL, respectively; 5′-GTIAARCARATGAARATHATHCA-3′ as the sense primer and 5′-TCIGAICKYTGICCICCNACRTC-3′ as the antisense primer for cloning of the G protein, designed based on VKQMKIIH and DVGGQRSE, respectively; and 5′-YTIACIYTIACNACNATHGG-3′ as the sense primer and 5′-ATRTARTCICCNGGNGARAA-3′ as the antisense primer for cloning of the CNG channel, designed based on LTLTTIG and FSPGDYI, respectively. PCR amplifications were carried out with annealing temperatures of 40 or 50°C. The 3′ and 5′ ends of the cDNAs were obtained by using the 3′-RACE and 5′-RACE systems, respectively (Invitrogen).
Expression of Opsin-Based Pigment and Spectroscopy.
The box jellyfish opsin was expressed in HEK293S cells and purified as described in ref. 34. To reconstitute the pigment, the expressed proteins were incubated with an excess of 11-cis-retinal. The absorption spectra of purified samples were recorded at 4°C with a Shimadzu UV2450 spectrophotometer. Green light was supplied by a 1-kW halogen lamp (Philips) with a 500-nm interference filter (Toshiba).
HPLC Analysis.
The chromophore configurations of irradiated and nonirradiated purified box jellyfish opsin were analyzed by HPLC as described in ref. 35.
Antibodies.
The anti-box jellyfish opsin and anti-Gα12 antibodies were generated against the C-terminal region of the box jellyfish opsin and the helical domain of Gα12, respectively, by using the pMAL protein fusion and purification system (New England Biolabs) according to the method reported (27). The anti-Gαt and anti-Gαq antibodies were a generous gift from Tatsuo Suzuki (Hyogo College of Medicine) (36, 37), and the anti-Gαo, anti-Gαs, and anti-adenylyl cyclase antibodies were commercially obtained (MBL; Santa Cruz Biotechnology).
Immunohistochemistry.
The dissected rhopalia of the box jellyfish were immersion-fixed in 4% paraformaldehyde, cryoprotected in 0.1 M phosphate buffer containing 15% sucrose, frozen with OCT medium (Sakura), and sectioned at 12 μm. The sections were incubated with 1:500 diluted antiserum followed by incubation with Alexa Fluor 488 anti-mouse IgG or 594 anti-rabbit IgG (Molecular Probes) for immunofluorescent detections.
cAMP Assay.
The cAMP content of HEK293S cells and rhopalia of the box jellyfish was measured with an enzyme-linked immunoassay system (Amersham Biosciences) according to the manufacturer's protocol. Cells transfected with the box jellyfish opsin cDNA and mock-transfected cells were incubated with 11-cis-retinal overnight in the dark followed by irradiation with white light for 30 s (as a light stimulus) before lysis. Cells transfected with human β2-adrenergic receptor cDNA were incubated with 10 nM isoprotenol for 20 min to induce cAMP formation. To prevent the degradation of cAMP by intrinsic cAMP phosphodiesterase activity, cells were treated with Hepes-buffered saline containing 1 mM 3-isobutyl-1-methylxanthine, an inhibitor of cAMP phosphodiesterases, before stimulation. To measure the light-dependent cAMP increase in the jellyfish eyes, eight rhopalia from two jellyfish were used for one experiment. Half of them were kept in the dark, and the other half were irradiated with white light for 2 min followed by immediate lysis. A 320-W halogen lamp (Cabin) was used for sample irradiation.
Phylogenetic Analyses.
Phylogenetic tree inferences were carried out as described in ref. 38. Multiple alignments of the amino acid sequences including the box jellyfish genes were calculated by using the XCED software (39). The accession numbers of amino acid sequences used for analyses are provided in the SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank M. Nishizaki for collecting the box jellyfish. This work was supported in part by grants-in-aid for Scientific Research from the Japanese Ministry of Education, Science, Sports, and Culture (to A.T. and M.K.), the Yamada Science Foundation (to A.T.), and the Naito Foundation (to M.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the DNA Data Bank of Japan (DDBJ) (accession nos. AB435549–AB435552).
This article contains supporting information online at www.pnas.org/cgi/content/full/0806215105/DCSupplemental.
References
- 1.Terakita A. The opsins. Genome Biol. 2005;6:213. doi: 10.1186/gb-2005-6-3-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhn H. Light- and GTP-regulated interaction of GTPase and other proteins with bovine photoreceptor membranes. Nature. 1980;283:587–589. doi: 10.1038/283587a0. [DOI] [PubMed] [Google Scholar]
- 3.Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- 4.Yau KW, Baylor DA. Cyclic GMP-activated conductance of retinal photoreceptor cells. Annu Rev Neurosci. 1989;12:289–327. doi: 10.1146/annurev.ne.12.030189.001445. [DOI] [PubMed] [Google Scholar]
- 5.Terakita A, Hariyama T, Tsukahara Y, Katsukura Y, Tashiro H. Interaction of GTP-binding protein Gq with photoactivated rhodopsin in the photoreceptor membranes of crayfish. FEBS Lett. 1993;330:197–200. doi: 10.1016/0014-5793(93)80272-v. [DOI] [PubMed] [Google Scholar]
- 6.Lee YJ, et al. The Drosophila dgq gene encodes a Gα protein that mediates phototransduction. Neuron. 1994;13:1143–1157. doi: 10.1016/0896-6273(94)90052-3. [DOI] [PubMed] [Google Scholar]
- 7.Yarfitz S, Hurley JB. Transduction mechanisms of vertebrate and invertebrate photoreceptors. J Biol Chem. 1994;269:14329–14332. [PubMed] [Google Scholar]
- 8.Kikkawa S, Tominaga K, Nakagawa M, Iwasa T, Tsuda M. Simple purification and functional reconstitution of octopus photoreceptor Gq, which couples rhodopsin to phospholipase C. Biochemistry. 1996;35:15857–15864. doi: 10.1021/bi961360v. [DOI] [PubMed] [Google Scholar]
- 9.Hardie RC, Raghu P. Visual transduction in Drosophila. Nature. 2001;413:186–193. doi: 10.1038/35093002. [DOI] [PubMed] [Google Scholar]
- 10.Koyanagi M, Terakita A. Gq-coupled rhodopsin subfamily composed of invertebrate visual pigment and melanopsin. Photochem Photobiol. 2008;84:1024–1030. doi: 10.1111/j.1751-1097.2008.00369.x. [DOI] [PubMed] [Google Scholar]
- 11.Kojima D, et al. A novel Go-mediated phototransduction cascade in scallop visual cells. J Biol Chem. 1997;272:22979–22982. doi: 10.1074/jbc.272.37.22979. [DOI] [PubMed] [Google Scholar]
- 12.Gomez MP, Nasi E. Light transduction in invertebrate hyperpolarizing photoreceptors: Possible involvement of a Go-regulated guanylate cyclase. J Neurosci. 2000;20:5254–5263. doi: 10.1523/JNEUROSCI.20-14-05254.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su CY, et al. Parietal-eye phototransduction components and their potential evolutionary implications. Science. 2006;311:1617–1621. doi: 10.1126/science.1123802. [DOI] [PubMed] [Google Scholar]
- 14.Martin VJ. Photoreceptors of Cnidarians. Can J Zool. 2002;80:1703–1722. [Google Scholar]
- 15.Piatigorsky J, Kozmik Z. Cubozoan jellyfish: An Evo/Devo model for eyes and other sensory systems. Int J Dev Biol. 2004;48:719–729. doi: 10.1387/ijdb.041851jp. [DOI] [PubMed] [Google Scholar]
- 16.Berger EW. The histological structure of the eyes of cubomedusae. J Comp Neurol. 1898;8:223–230. [Google Scholar]
- 17.Plachetzki DC, Degnan BM, Oakley TH. The origins of novel protein interactions during animal opsin evolution. PLoS ONE. 2007;2:e1054. doi: 10.1371/journal.pone.0001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suga H, Schmid V, Gehring WJ. Evolution and functional diversity of jellyfish opsins. Curr Biol. 2008;18:51–55. doi: 10.1016/j.cub.2007.11.059. [DOI] [PubMed] [Google Scholar]
- 19.Bridge D, Cunningham CW, Schierwater B, DeSalle R, Buss LW. Class-level relationships in the phylum Cnidaria: Evidence from mitochondrial genome structure. Proc Natl Acad Sci USA. 1992;89:8750–8753. doi: 10.1073/pnas.89.18.8750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coates MM, Garm A, Theobald JC, Thompson SH, Nilsson DE. The spectral sensitivity of the lens eyes of a box jellyfish, Tripedalia cystophora (Conant) J Exp Biol. 2006;209:3758–3765. doi: 10.1242/jeb.02431. [DOI] [PubMed] [Google Scholar]
- 21.Tsukamoto H, Terakita A, Shichida Y. A rhodopsin exhibiting binding ability to agonist all-trans-retinal. Proc Natl Acad Sci USA. 2005;102:6303–6308. doi: 10.1073/pnas.0500378102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terakita A, Hara R, Hara T. Retinal-binding protein as a shuttle for retinal in the rhodopsin-retinochrome system of the squid visual cells. Vision Res. 1989;29:639–652. doi: 10.1016/0042-6989(89)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson DE, Gislen L, Coates MM, Skogh C, Garm A. Advanced optics in a jellyfish eye. Nature. 2005;435:201–205. doi: 10.1038/nature03484. [DOI] [PubMed] [Google Scholar]
- 24.Simon MI, Strathmann MP, Gautam N. Diversity of G proteins in signal transduction. Science. 1991;252:802–808. doi: 10.1126/science.1902986. [DOI] [PubMed] [Google Scholar]
- 25.Eakin RM. Evolution of photoreceptors. Cold Spring Harbor Symp Quant Biol. 1965;30:363–370. doi: 10.1101/sqb.1965.030.01.036. [DOI] [PubMed] [Google Scholar]
- 26.Arendt D, Tessmar-Raible K, Snyman H, Dorresteijn AW, Wittbrodt J. Ciliary photoreceptors with a vertebrate-type opsin in an invertebrate brain. Science. 2004;306:869–871. doi: 10.1126/science.1099955. [DOI] [PubMed] [Google Scholar]
- 27.Koyanagi M, Kubokawa K, Tsukamoto H, Shichida Y, Terakita A. Cephalochordate melanopsin: Evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr Biol. 2005;15:1065–1069. doi: 10.1016/j.cub.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 28.Provencio I, Jiang G, De Grip WJ, Hayes WP, Rollag MD. Melanopsin: An opsin in melanophores, brain, and eye. Proc Natl Acad Sci USA. 1998;95:340–345. doi: 10.1073/pnas.95.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 30.Gotow T, Nishi T, Kijima H. Single K+ channels closed by light and opened by cyclic GMP in molluscan extraocular photoreceptor cells. Brain Res. 1994;662:268–272. doi: 10.1016/0006-8993(94)90824-9. [DOI] [PubMed] [Google Scholar]
- 31.Gomez MP, Nasi E. Antagonists of the cGMP-gated conductance of vertebrate rods block the photocurrent in scallop ciliary photoreceptors. J Physiol (London) 1997;500:367–378. doi: 10.1113/jphysiol.1997.sp022027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Firestein S. How the olfactory system makes sense of scents. Nature. 2001;413:211–218. doi: 10.1038/35093026. [DOI] [PubMed] [Google Scholar]
- 33.Kozmik Z, et al. Assembly of the cnidarian camera-type eye from vertebrate-like components. Proc Natl Acad Sci USA. 2008;105:8989–8993. doi: 10.1073/pnas.0800388105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terakita A, et al. Counterion displacement in the molecular evolution of the rhodopsin family. Nat Struct Mol Biol. 2004;11:284–289. doi: 10.1038/nsmb731. [DOI] [PubMed] [Google Scholar]
- 35.Koyanagi M, Terakita A, Kubokawa K, Shichida Y. Amphioxus homologs of Go-coupled rhodopsin and peropsin having 11-cis- and all-trans-retinals as their chromophores. FEBS Lett. 2002;531:525–528. doi: 10.1016/s0014-5793(02)03616-5. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki T, Narita K, Yoshihara K, Nagai K, Kito Y. Immunochemical detection of GTP-binding protein in cephalopod photoreceptors by anti-peptide antibodies. Zoolog Sci. 1993;10:425–430. [PubMed] [Google Scholar]
- 37.Suzuki T, Narita K, Yoshihara K, Nagai K, Kito Y. Phosphatidylinositol-phospholipase C in squid photoreceptor membrane is activated by stable metarhodopsin via GTP-binding protein, Gq. Vision Res. 1995;35:1011–1017. doi: 10.1016/0042-6989(94)00219-c. [DOI] [PubMed] [Google Scholar]
- 38.Koyanagi M, et al. Bistable UV pigment in the lamprey pineal. Proc Natl Acad Sci USA. 2004;101:6687–6691. doi: 10.1073/pnas.0400819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.