Abstract
The splicing factor SF2/ASF is an oncoprotein that is up-regulated in many cancers and can transform immortal rodent fibroblasts when slightly overexpressed. The mTOR signaling pathway is activated in many cancers, and pharmacological blockers of this pathway are in clinical trials as anticancer drugs. We examined the activity of the mTOR pathway in cells transformed by SF2/ASF and found that this splicing factor activates the mTORC1 branch of the pathway, as measured by S6K and eIF4EBP1 phosphorylation. This activation is specific to mTORC1 because no activation of Akt, an mTORC2 substrate, was detected. mTORC1 activation by SF2/ASF bypasses upstream PI3K/Akt signaling and is essential for SF2/ASF-mediated transformation, as inhibition of mTOR by rapamycin blocked transformation by SF2/ASF in vitro and in vivo. Moreover, shRNA-mediated knockdown of mTOR, or of the specific mTORC1 and mTORC2 components Raptor and Rictor, abolished the tumorigenic potential of cells overexpressing SF2/ASF. These results suggest that clinical tumors with SF2/ASF up-regulation could be especially sensitive to mTOR inhibitors.
Keywords: alternative splicing, mTOR, Raptor, transformation, rapamycin
The PI3K-mTOR pathway is an important contributor to the transformed phenotype and is activated in many cancers (1, 2). Many components of this signaling pathway are mutated in tumors, generating activated oncogenes that promote the pathway (PI3K, AKT) or inactivated tumor suppressors that normally inhibit the pathway (PTEN, LKB1, TSC1, TSC2) (1, 3). The importance of this pathway is also underscored by the fact that specific inhibitors of mTOR can reverse transformation alone, or more efficiently in combination with cytotoxic drugs; these inhibitors are currently in clinical trials as anticancer drugs (4, 5).
The PI3K-mTOR pathway is a major contributor to tumor growth and survival (1, 2). The mTOR kinase is part of two different protein complexes that result in phosphorylation of distinct substrates. The mTORC1 complex phosphorylates and inactivates the translation inhibitors 4E-BP1 and 4E-BP2 and activates S6K1 to enhance translation (1–3). Enhancement of translation contributes to tumorigenesis by elevating the levels of transcription factors with high turnover, such as HIF1-α—which induces metabolic changes leading to aerobic glycolysis, and enhances angiogenesis (6, 7)—the oncoprotein c-myc (8), β-catenin, and others (9). mTORC2 phosphorylates the oncoprotein Akt, which contributes to transformation by phosphorylating many substrates, leading to inhibition of apoptosis and enhancement of cell proliferation (10, 11).
We reported recently that the splicing factor SF2/ASF is a potent oncoprotein that is up-regulated in lung and colon cancers, and whose gene (SFRS1) is amplified in some breast tumors; moreover, SF2/ASF can transform immortal rodent cells when slightly overexpressed, and these cells form high-grade sarcomas in nude mice (12). We also found that elevated SF2/ASF expression did not activate the Ras-MAPK pathway, but changed the alternative splicing of Mnk2 kinase pre-mRNA, correlating with increased eIF4E phosphorylation downstream of the MAPK pathway (12). Because activation of the PI3K-mTOR pathway is seen in many cancers (2, 5), we sought here to measure the status of this pathway in cells transformed by SF2/ASF.
Akt, a major effector of the PI3K pathway, is both an upstream activator of mTOR and a substrate of mTORC2 (2, 3, 13). We examined the activity of this pathway in cells transformed by SF2/ASF overexpression. We observed no activation of Akt, but phosphorylation of substrates of the mTORC1 complex, S6K and 4E-BP1, was elevated in these cells and was reduced upon SF2/ASF knockdown. To examine whether the activation of this pathway is important for SF2/ASF-mediated transformation, we blocked mTOR pharmacologically using rapamycin and genetically using shRNAs to mTOR, Raptor, or Rictor and found that all these treatments can block SF2/ASF-mediated transformation.
These results demonstrate the essential role of mTOR activation in SF2/ASF-mediated transformation and indicate that mTOR inhibitors may be useful for treatment of tumors with SF2/ASF up-regulation.
Results
The Splicing Factor SF2/ASF Activates the mTORC1 Pathway, Bypassing Akt Activation.
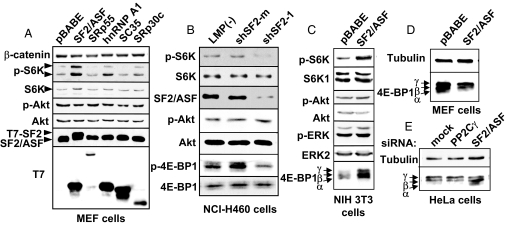
To examine the activity of the PI3K-Akt pathway in cells with elevated or reduced SF2/ASF, we measured the phosphorylation state of Akt on S473 by Western blotting. We did not detect any activation of Akt in any of the cell lines we examined, including immortal (Rat1, NIH 3T3) and primary (MEF, IMR90) cells stably transduced with human SF2/ASF cDNA [Fig. 1 A and C, supporting information (SI) Fig. S1, and data not shown]. Moreover, knockdown of SF2/ASF in the lung carcinoma cell line NCI-H460, which overexpresses SF2/ASF (12), or in NIH 3T3 cells transduced with SF2/ASF did not inhibit Akt phosphorylation. (Fig. 1B and Fig. S2). These results indicate that changes in SF2/ASF levels have no effect on phosphorylation of Akt on S473.
Fig. 1.
SF2/ASF activates mTOR, bypassing Akt activation. (A) MEF cells were infected with transducing retroviruses encoding the cDNAs of four splicing factors from the SR protein family (SF2/ASF, SRp55, SC35, and SRp30c) and the hnRNP A/B protein hnRNP A1, fused to a T7 tag at the N terminus, or with the pBABE vector alone. After selection, the cells were plated (106 cells per 10-cm plate) and lysed in SDS sample buffer after 24 h. Western blots were carried out by using the indicated primary antibodies. The antibody to phospho-S6K1 recognizes both p70 and p85, which differ by their translation initiation site (27). (B) NCI-H460 lung cancer cells, which express high levels of SF2/ASF, were infected with empty retroviral vector (LMP) or LMP encoding SF2/ASF-specific shRNA (shSF2–1) or a control shRNA with two mismatches (shSF2-m). After selection, cells were lysed as above. Western blots were carried out by using the indicated primary antibodies. (C and D) NIH 3T3 and MEF cells were transduced as described in A, and lysed after selection. Western blots were probed with the indicated antibodies. (E) HeLa cells were transfected with the indicated siRNAs and lysed after 72 h. Western blotting was done as in D. The 4E-BP1 phosphorylation in C–E is detected by its mobility shift toward the γ form (28).
We next measured the phosphorylation state of the mTOR substrates S6K1 and 4E-BP1 (1). MEF and NIH 3T3 cells overexpressing SF2/ASF had increased phosphorylation of both S6K1 and 4E-BP1, and this activation was specific for SF2/ASF, as cells overexpressing other related splicing factors did not show comparable activation (Fig. 1 A and D and data not shown). NIH 3T3 cells transformed by SF2/ASF showed increased S6K and 4E-BP1 phosphorylation (Fig. 1C), and tumors derived from these cells also showed elevated S6K and 4E-BP1 phosphorylation, compared with tumor-derived cells overexpressing hnRNPA1 (data not shown). Conversely, transformed cells (NCI-H460, HeLa) with knockdown of SF2/ASF showed reduced S6K and 4E-BP1 phosphorylation (Fig. 1 B and E).
mTOR Activity Is Required for SF2/ASF-Mediated Transformation.
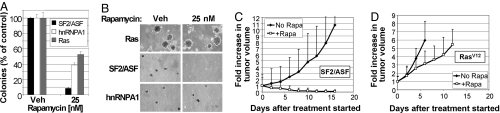
To assess the importance of mTORC1 and mTORC2 activation for SF2/ASF-mediated transformation, we took two approaches: First, we used the mTOR inhibitor rapamycin to block mTOR catalytic activity as part of mTORC1, and second, we used shRNAs to knock down either mTOR itself or Raptor or Rictor, the distinctive components of mTORC1 and mTORC2, respectively (3, 14, 15). Colony formation in soft agar by SF2/ASF transductants was completely blocked by rapamycin, whereas colony formation by cells transduced with activated Ras or hnRNPA1—another splicing factor with oncogenic activity (12)—was only partially inhibited (Fig. 2 A and B). Moreover, rapamycin treatment of NCI-H460 [a lung carcinoma cell line with high levels of SF2/ASF (12)] also blocked colony formation in soft agar (Fig. S3).
Fig. 2.
mTOR activity is required for SF2/ASF-mediated transformation in vitro and in vivo. (A) Quantitation of colony formation in soft agar of NIH 3T3 cells transduced with SF2/ASF, hnRNP A1, or oncogenic Ras, in the presence of rapamycin or vehicle control, as indicated. Mean values in the absence of rapamycin were set at 100%; error bars indicate standard deviations (n = 3). (B) Representative fields of soft-agar colonies described in A. (C) NIH 3T3 cells transduced with SF2/ASF were injected into nude mice. Tumors were allowed to reach a size of ≈200 mm3, and then mice were injected i.p. with 4 mg/kg rapamycin or vehicle control every day. (D) NIH 3T3 cells transduced with RasV12 were injected, and mice were treated as in C. Tumor volumes were measured during the treatment. Animals were killed when tumors reached a size of 1,500 mm3. Error bars in C and D indicate the top halves of the standard deviations (n = 10).
To examine the effect of mTOR inhibition on tumorigenesis in vivo, we injected the cells overexpressing SF2/ASF into nude mice, and after small tumors developed (≈200 mm3), we treated the mice daily with rapamycin (4 mg/kg, i.p.) and measured the change in tumor volume (Fig. 2 C and D). Rapamycin treatment caused shrinkage of the tumors overexpressing SF2/ASF, and complete remission for most of the tumors (Fig. 2C). The growth rate of tumors expressing activated Ras also decreased, but no tumor shrinkage was observed (Fig. 2D). This experiment shows that cells overexpressing SF2/ASF are highly sensitive to rapamycin in vivo.
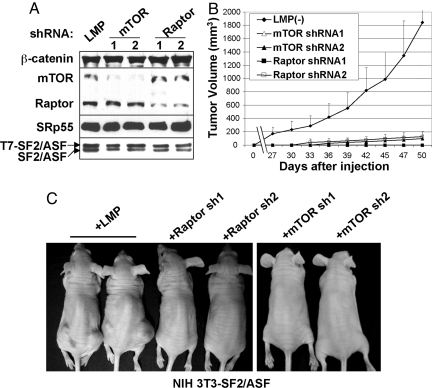
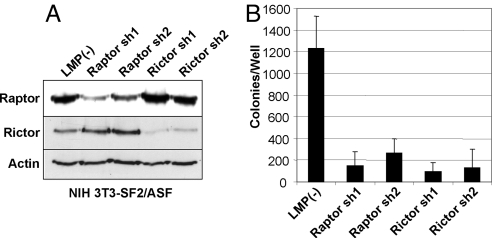
To examine the contribution of mTORC1 and mTORC2 to SF2/ASF-mediated transformation, we designed specific shRNAs to stably knock down mTOR, Raptor, or Rictor in the cells overexpressing SF2/ASF (Figs. 3A and 4A). Knockdown of any one of these three factors completely abrogated the tumorigenicity of cells overexpressing SF2/ASF (Figs. 3 B and C and 4B). We conclude that mTOR activation is essential for SF2/ASF-mediated tumorigenesis.
Fig. 3.
mTORC1 is required for SF2/ASF-induced tumorigenesis. (A) NIH 3T3 cells transduced with pWZL-Hygro-SF2/ASF were supertransduced with retroviruses encoding mTOR- or Raptor-specific shRNAs. After puromycin selection, cells were analyzed by Western blotting for mTOR and Raptor protein expression; antibodies to SF2/ASF (which detects endogenous and tagged proteins), SRp55, and β-catenin were used as controls. (B) Cells described in A were injected into nude mice, and tumor volume was measured every 3 days, as described in Fig. 2C. Error bars indicate standard deviations (n = 10). (C) Representative mice described in B are shown.
Fig. 4.
Both Raptor and Rictor are required for SF2/ASF-mediated transformation. (A) NIH 3T3 cells transduced with pWZL-Hygro-SF2/ASF were supertransduced with retroviruses encoding Raptor- or Rictor-specific shRNAs or with the empty LMP vector. After puromycin selection, cells were analyzed by Western blotting for Rictor and Raptor protein expression; antibodies to actin were used as controls. (B) Cells described in A were plated in soft agar in duplicate experiments. Colonies were counted 14 days later. Error bars indicate standard deviations (n = 2).
Discussion
mTOR activity is regulated by a complex signaling network that senses nutrient and energy levels, growth factors, and stress signals like hypoxia (2, 3). One major pathway that regulates mTOR activity and is activated in many tumors is the PI3K-Akt pathway, which inhibits the GTPase activity of the TSC1/2 complex, leading to the activation of the mTOR activator RheB (2, 3). The PI3K-Akt pathway also inhibits the mTOR inhibitor PRAS40 (16). We found recently that the splicing factor SF2/ASF is a protooncogene that is up-regulated in many tumors and modulates alternative splicing of the mTOR substrate S6K1 (12). This alternative splicing effect is downstream of mTOR and is not affected by the mTOR pathway (data not shown).
Here, we examined the activity of the mTOR pathway in cells that were transduced with human SF2/ASF cDNA to achieve modest overexpression of this splicing factor [Fig. 1A; (12)]. We found that increased SF2/ASF promotes phosphorylation of the mTORC1 substrates S6K1 and 4E-BP1, whereas SF2/ASF knockdown inhibits these phosphorylation events (Fig. 1). Interestingly, we observed no enhanced Akt phosphorylation in mouse, rat, or human cells overexpressing SF2/ASF (Fig. 1 and Fig. S1). Thus, we conclude that SF2/ASF activates mTOR downstream of Akt. In addition, because Akt is also a substrate of the mTORC2 complex in a feedback loop (17), we infer that mTORC2 is not activated by SF2/ASF.
We do not know at present the exact mechanism(s) by which SF2/ASF activates mTORC1. The effect could be indirect, involving alternative splicing of an upstream activator or inhibitor of mTOR, for example, or it could be the consequence of a direct interaction between SF2/ASF and mTOR. Relevant to the first scenario, we found recently that SF2/ASF induces the expression of a new S6K1 splicing isoform (“isoform-2”) lacking the C-terminal region phosphorylated by mTOR and that this isoform is oncogenic (12). An intriguing possibility is that isoform-2, in a positive feedback loop, might somehow activate mTORC1. Regarding the second scenario, recent studies showed that SF2/ASF can activate translation initiation in a cell-free system, probably independently of its splicing activity (18, 19). In addition, SF2/ASF was shown to be present in a complex with mTOR and to enhance 4E-BP1 phosphorylation in translation extracts (20). Moreover, the translational-stimulation effects of SF2/ASF depend on the presence of 4E-BP1 and are sensitive to rapamycin, indicating that activation of mTORC1 is responsible for these effects (20) Our results in cells are consistent with these recent findings in extracts (Figs. 1–3).
To examine the importance of mTOR activation for SF2/ASF-mediated transformation, we blocked mTOR activity either pharmacologically, using the mTOR-specific inhibitor rapamycin, or by RNA interference against mTOR itself, against Raptor, the distinctive component of mTORC1 (Figs. 2, 3), or against Rictor, the distinctive component of mTORC2 (Fig. 4). We used Ras-transformed cells as a control; Ras activates both the PI3K-Akt and the Raf-MAPK pathways, both of which contribute to transformation (21, 22). Thus, blocking the mTOR pathway should only partially inhibit Ras-induced transformation. Indeed, we found that rapamycin partially inhibited colony formation in soft agar, as well as tumor growth in nude mice, of Ras-transformed cells (Fig. 2 A, B, and D). In contrast, cells transformed by SF2/ASF were extremely sensitive to rapamycin, both in vitro and in nude mice (Fig. 2 A–C). These results indicate that mTOR activation is essential for SF2/ASF-mediated transformation.
Our results further show that mTORC1, but not mTORC2, is activated by SF2/ASF, as determined by measuring the extent of phosphorylation of their unique substrates (Fig. 1 and Fig. S1). Consistent with SF2/ASF's activation of mTORC1, we found that knockdown of Raptor or the mTOR kinase itself caused complete inhibition of tumorigenesis of cells transformed by SF2/ASF, indicating that mTORC1 activation is an essential step in SF2/ASF-mediated transformation.
Interestingly, knockdown of Rictor, the distinctive component of mTORC2, also led to inhibition of colony formation in soft agar (Fig. 4), indicating that although mTORC2 is not activated by SF2/ASF, its basal activity is still required for SF2/ASF-mediated transformation. mTORC2 phosphorylates Akt on serine 473, and Akt activity regulates many survival pathways (11). Thus, inhibiting Akt phosphorylation through Rictor down-regulation is expected to inhibit the oncogenic potential of many transformed cells.
Many studies have shown activation of mTORC1 in tumors (17). However, it was not clear whether the components of mTORC1, mTOR, and Raptor, have direct roles in transformation. Rapamycin, which has antitumor activity, blocks mainly mTORC1 activity, but recent data showed that it affects the stability of mTORC2 as well (23). Our results, based on rapamycin sensitivity and mTOR or Raptor knockdown, suggest that mTORC1 activation is essential for SF2/ASF-mediated transformation. SF2/ASF-transformed cells proved to be extremely sensitive to mTOR inhibition, raising the possibility that clinical tumors with SF2/ASF up-regulation will be especially sensitive to mTOR inhibitors. If this proves to be true, it might facilitate the diagnosis and treatment of cancers with SF2/ASF overexpression.
Materials and Methods
Cells.
HeLa and MEF cells were grown in DMEM, and NCI-H460 cells were grown in RPMI 1640 medium, supplemented with 10% FCS, penicillin, and streptomycin. NIH 3T3 cells were grown in DMEM supplemented with 10% calf serum (CS), penicillin, and streptomycin. To generate stable transductant pools, NIH 3T3 and MEF cells were infected with pBABE-puro or pWZL-hygro retroviral vectors (24) expressing T7-tagged human splicing factor cDNAs. At 24 h after infection, the medium was replaced, and 24 h later, infected cells were selected with puromycin (2 μg/ml) or hygromycin (200 μg/ml) for 72 h. In the case of double infection with LMP-puro-shRNAs vectors, NIH 3T3 cells transduced with pWZL-hygro-SF2/ASF virus were selected with hygromycin for 96 h, followed by infection with the indicated LMP-puro-shRNAs viruses and selection with puromycin for 72 h.
RNA Interference.
For inhibition of SF2/ASF and PP2Cγ expression, HeLa cells were seeded (7 × 104 cells per well) in six-well plates in antibiotic-free medium. At 24 h, cells were transfected with 200 pmol of siRNA per well (Dharmacon), by using Oligofectamine (Invitrogen). At 72 h, cells were lysed, and protein and RNA were extracted as described below. siRNA target sequences: TTGACCACTGAAGAAGTCA (PP2Cγ) and ACGATTGCCGCATCTACGT (SF2/ASF); both siRNA strands had 3′ dTdT tails. shRNAs against mouse and human SF2/ASF were as described (12). NCI-H460 and NIH 3T3 cells were transduced and selected as described above.
shRNA Sequences.
Raptor sh1: TGCTGTTGACAGTGAGCGATCGTGGCAAGTTTGTTTAGAATAGTG AAGCCACAGATGTATTCTAAACAAACTTGCCACGAGTGCCTACTGCCTCGGA. Raptor sh2: TGCTGTTGACAGTGAGCGAGGCGTTCCTTCTGTGGTCAAATAGTG AAGCCACAGATGTATTTGACCACAGAAGGAACGCCGTGCCTACTGCCTCGGA. mTOR sh1: TGCTGTTGACAGTGAGCGAAGCAGGGACTCAGAACATAAATAGT GAAGCCACAGATGTATTTATGTTCTGAGTCCCTGCTGTGCCTACTGCCTCGGA. mTOR sh2: TGCTGTTGACAGTGAGCGAACCACGTTGTATCTGAGTAAATAGTG AAGCCACAGATGTATTTACTCAGATACAACGTGGTGTGCCTACTGCCTCGGA. Rictor sh1: TGCTGTTGACAGTGAGCGCTAGGTGTTTAAGACTATTAAATAGTG AAGCCACAGATGTATTTAATAGTCTTAAACACCTATTGCCTACTGCCTCGGA. Rictor sh2: TGCTGTTGACAGTGAGCGACTCCAGCAAACTTGTAAAGAATAGTG AAGCCACAGATGTATTCTTTACAAGTTTGCTGGAGCTGCCTACTGCCTCGGA.
Immunoblotting.
Cells were lysed in SDS and analyzed for total protein concentration as described (25). Thirty or 50 μg of total protein from each cell lysate was separated by SDS/PAGE and transferred onto a nitrocellulose membrane. The membranes were blocked and probed with antibodies by using enhanced chemiluminescence detection. Primary antibodies: β-catenin (1:5,000; Transduction Laboratories, or 1:2,000; Sigma); SF2/ASF [AK96 culture supernatant 1:100 (26)]; T7 tag (1:5,000; Novagen); S6K1 (N terminus) (1:200; PharMingen); and Raptor, Rictor, mTOR, phospho-4E-BP1 T70, 4E-BP1, phospho-S6K T389, phospho-ERK (T202/Y204), and ERK1/2 (1:1,000; Cell Signaling Technology). Secondary antibodies: HRP-conjugated goat anti-mouse or anti-rabbit IgG (H+L) (1:10,000; Pierce).
Anchorage-Independent Growth.
Colony formation in soft agar was assayed as described (12). Rapamycin was added once to the top medium at the indicated concentrations. Plates were incubated at 37°C and 6% CO2. After 10–18 days, colonies from 10 different fields in each of two wells were counted for each treatment, and the average number of colonies per well was calculated. The colonies were stained as described (24) and photographed under a light microscope at magnification ×100.
Tumorigenesis Assays in Nude Mice and Rapamycin Treatment.
NIH 3T3 stable pools expressing SF2/ASF or H-RasV12 were injected (2 × 106 cells per site in 200 μl of PBS) s.c. into each rear flank of (NIH nu/nu) nude mice by using a 26-gauge needle. After small tumors appeared (100–200 mm3), mice were injected daily i.p. with 4 mg/kg rapamycin in 2% Tween 20, or just with vehicle. Tumor growth was monitored as described (12). For shRNAs experiments, NIH 3T3 cells expressing both pWZL-hygro-SF2/ASF and shRNAs to mTOR or Raptor (see sequences above) were injected, and tumor volume was monitored as described (12).
Statistical Analysis.
Where appropriate, the data are presented as the means ± SD. Data points were compared by an unpaired two-tailed Student's t test, and the calculated P values are indicated in the figure legends. For soft-agar colony assays, means ± SD and P values were calculated for 10 fields per well, in duplicate wells for each transductant pool.
Supplementary Material
Acknowledgments.
This work was supported by National Cancer Institute Grant CA13106 and by the Starr Cancer Consortium.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0801376105/DCSupplemental.
References
- 1.Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416–6422. doi: 10.1038/sj.onc.1209888. [DOI] [PubMed] [Google Scholar]
- 2.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 3.Sabatini DM. mTOR and cancer: Insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 4.Wendel HG, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 5.Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov. 2006;5:671–688. doi: 10.1038/nrd2062. [DOI] [PubMed] [Google Scholar]
- 6.Karni R, Dor Y, Keshet E, Meyuhas O, Levitzki A. Activated pp60c-Src leads to elevated hypoxia-inducible factor (HIF)-1alpha expression under normoxia. J Biol Chem. 2002;277:42919–42925. doi: 10.1074/jbc.M206141200. [DOI] [PubMed] [Google Scholar]
- 7.Lum JJ, et al. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21:1037–1049. doi: 10.1101/gad.1529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Benedetti A, Harris AL. eIF4E expression in tumors: Its possible role in progression of malignancies. Int J Biochem Cell Biol. 1999;31:59–72. doi: 10.1016/s1357-2725(98)00132-0. [DOI] [PubMed] [Google Scholar]
- 9.Holland EC, Sonenberg N, Pandolfi PP, Thomas G. Signaling control of mRNA translation in cancer pathogenesis. Oncogene. 2004;23:3138–3144. doi: 10.1038/sj.onc.1207590. [DOI] [PubMed] [Google Scholar]
- 10.Rajasekhar VK, et al. Oncogenic Ras and Akt signaling contribute to glioblastoma formation by differential recruitment of existing mRNAs to polysomes. Mol Cell. 2003;12:889–901. doi: 10.1016/s1097-2765(03)00395-2. [DOI] [PubMed] [Google Scholar]
- 11.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karni R, et al. The gene encoding the splicing factor SF2/ASF is a proto-oncogene. Nat Struct Mol Biol. 2007:14185–93. doi: 10.1038/nsmb1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aoki M, Blazek E, Vogt PK. A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. Proc Natl Acad Sci USA. 2001;98:136–141. doi: 10.1073/pnas.011528498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hara K, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 15.Kim DH, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 16.Sancak Y, et al. PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell. 2007;25:903–915. doi: 10.1016/j.molcel.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Sanford JR, Gray NK, Beckmann K, Cáceres JF. A novel role for shuttling SR proteins in mRNA translation. Genes Dev. 2004;18:755–768. doi: 10.1101/gad.286404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanford JR, Ellis JD, Cazalla D, Cáceres JF. Reversible phosphorylation differentially affects nuclear and cytoplasmic functions of splicing factor 2/alternative splicing factor. Proc Natl Acad Sci USA. 2005;102:15042–15047. doi: 10.1073/pnas.0507827102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michlewski G, Sanford JR, Cáceres JF. The splicing factor SF2/ASF regulates translation initiation by enhancing phosphorylation of 4E-BP1. Mol Cell. 2008;30:179–189. doi: 10.1016/j.molcel.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Tognon C, et al. The chimeric protein tyrosine kinase ETV6-NTRK3 requires both Ras-Erk1/2 and PI3-kinase-Akt signaling for fibroblast transformation. Cancer Res. 2001;61:8909–8916. [PubMed] [Google Scholar]
- 22.Penuel E, Martin GS. Transformation by v-Src: Ras-MAPK and PI3K-mTOR mediate parallel pathways. Mol Biol Cell. 1999;10:1693–1703. doi: 10.1091/mbc.10.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarbassov DD, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 24.McCurrach ME, Lowe SW. Methods for studying pro- and antiapoptotic genes in nonimmortal cells. Methods Cell Biol. 2001;66:197–227. doi: 10.1016/s0091-679x(01)66010-2. [DOI] [PubMed] [Google Scholar]
- 25.Karni R, Gus Y, Dor Y, Meyuhas O, Levitzki A. Active Src elevates the expression of beta-catenin by enhancement of cap-dependent translation. Mol Cell Biol. 2005;25:5031–5039. doi: 10.1128/MCB.25.12.5031-5039.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanamura A, Cáceres JF, Mayeda A, Franza BR, Jr, Krainer AR. Regulated tissue-specific expression of antagonistic pre-mRNA splicing factors. RNA. 1998;4:430–444. [PMC free article] [PubMed] [Google Scholar]
- 27.Holz MK, Blenis J. Identification of S6 kinase 1 as a novel mammalian target of rapamycin (mTOR)-phosphorylating kinase. J Biol Chem. 2005;280:26089–26093. doi: 10.1074/jbc.M504045200. [DOI] [PubMed] [Google Scholar]
- 28.von Manteuffel SR, et al. The insulin-induced signalling pathway leading to S6 and initiation factor 4E binding protein 1 phosphorylation bifurcates at a rapamycin-sensitive point immediately upstream of p70s6k. Mol Cell Biol. 1997;17:5426–5436. doi: 10.1128/mcb.17.9.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.