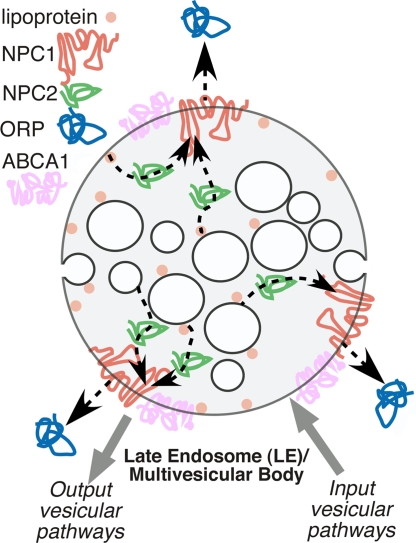
Cholesterol homeostasis is essential for the functional integrity of the cell (1). Both the cellular content and distribution of cholesterol within the cell is highly dynamic and tightly regulated through de novo synthesis of cholesterol by the endoplasmic reticulum (ER) (2), and by uptake of cholesterol ester-rich lipoprotein particles circulating in the serum by the low-density lipoprotein (LDL) receptor pathway (3). The main sorting station for cholesterol within the cell is the late endosome (LE), an intermediate stage in the endosomal-lysosomal trafficking pathway (Fig. 1). LEs are also referred to as “multivesicular” compartments in that they have numerous internalized membrane-enveloped structures generated by the ESCRT pathway (4) (Fig. 1). Within the lumen of the LE, cholesterol esters are hydrolyzed to free cholesterol by acid lipase. Released cholesterol joins the endogenous cholesterol pool for recycling to the cell surface, or transferred to the ER where they are re-esterified and, as necessary, placed in storage particles for later use (1).
Fig. 1.
Cartoon illustrating potential NPC1/2 TTD trafficking patterns of cholesterol in the LE/multivesicular body. NPC2 is then central shuttle in a unidirectional transfer pathway that mobilizes cholesterol from the LE limiting membrane, lipoprotein particles, or internal vesicles to NPC1 for transfer to ORP or vesicular trafficking pathways.
A nagging question in human physiology is how cholesterol imported to LEs is redistributed to maintain homeostasis? Current evidence suggests that sorting utilizes both vesicular and nonvesicular pathways. However, two LE proteins, Niemann-Pick C1 (NPC1) and NPC2, appear to be key players that initiate the sorting process (1, 5–7). Mutations in either one of these two proteins is the cause of the devastating childhood lysosomal storage disease, Niemann-Pick type C (NPC). NPC is characterized by the accumulation of cholesterol and glycosphingolipids in the LE. NPC1 is a multispanning protein that is localized to the limiting membrane of LEs. It contains three lumenally oriented domains including an N-terminal domain (NTD). Whether NPC1 functions as a transporter remains controversial. In contrast, NPC2 is a soluble, glycosylated protein that is present in the lumen of the LE (5, 8) (Fig. 1). Infante et al. (9, 10) earlier this year made the unexpected discovery that a major oxysterol binding protein found in liver was NPC1. Oxysterols are cholesterol analogs that are modified with 24-, 25- or 27-hydroxyl groups and regulate cholesterol pathways (11). Careful molecular dissection of NPC1 revealed that the soluble NTD of NPC1 [NPC1(NTD)] contained all of the sterol binding capacity of NPC1 and bound oxysterol stronger than cholesterol (9). This finding was in contrast to the soluble NPC2 protein that clearly showed a strong preference for cholesterol and did not bind oxysterols (8–10). Mutation of either NPC1 or NPC2 yields an identical Niemann-Pick disease etiology (1, 5, 6, 12), suggesting that they work together in an unknown fashion to export cholesterol.
In this issue of PNAS, Infante et al. (13) have now moved our understanding of the significance of sterol binding to NPC1 and NPC2 to the next level, namely, their separate and likely sequential roles in cholesterol mobilization from the LE. They first showed that NPC1(NTD) bound cholesterol, albeit slowly and had a slow dissociation rate. In contrast, NPC2 bound and released cholesterol rapidly. Next, they asked whether a functional interaction exists between NPC1(NTD) and NPC2. In a series of clever experiments in which NPC1(NTD) or NPC2 were separately preloaded with the [3H]cholesterol ([3H]Chol) substrate (the donors), they asked whether transfer could occur to unlabeled NPC1(NTD) or NPC2 (the acceptors). The [3H]Chol-NPC1(NTD) donor showed very slow transfer of [3H]Chol to either NPC1(NTD) or NPC2 acceptors. In contrast, [3H]Chol-NPC2 donor rapidly transferred [3H]Chol to either NPC1(NTD) or NPC2 acceptors. To begin to assess the relevance of this observation to lipid transfer between LE bilayers (Fig. 1), liposomes were used as either the donor or acceptor for [3H]Chol. Transfer from donor [3H]Chol-loaded liposomes to acceptor NPC2 was very fast and was not influenced by the presence of NPC1(NTD). In contrast, transfer of [3H]Chol was very slow between NPC1(NTD) and liposomes. Strikingly, addition of NPC2 accelerated [3H]Chol transfer nearly 100-fold between liposomes and NPC1. The transfer of cholesterol occurred whether NPC1(NTD) was the [3H]Chol acceptor or the liposomes were the acceptor. Moreover, transfer was inhibited by a missense mutation in NPC2 that prevented cholesterol binding. These data demonstrate for the first time that NPC1 and NPC2 function as the cellular “tag team duo” (TTD) to catalyze mobilization of cholesterol within the multivesicular environment of the LE to effect egress through the limiting bilayer of the LE (Fig. 1).
What do these observations tell us about cholesterol trafficking in the cell? One possibility is that cholesterol found in LEs is bound by the NTD of NPC1 as the first member of a tag team for either direct export or transfer to NPC2 for delivery to an cholesterol efflux transporter such as ABCA1 (1, 5). Alternatively, cholesterol may be bound by NPC2 as the first member of the tag team and delivered to the NTD of NPC1 for direct efflux or transfer to ABCA1 from the LE. Which order is correct? The first model could account for efflux of cholesterol found on the limiting membrane of the LE, particularly cholesterol derived from acid lipase hydrolysis of cholesterol esters found in lipoprotein particles that are associated with the limiting LE membrane. However, it does not account for the substantial pool of cholesterol found in the internal membranes of the LE lacking NPC1. In contrast, by having NPC2 as the first carrier of the tag team (Fig. 1), it allows mobilization from any source and could be considered the preferred model. Of course, in either model, what is needed outside of the LE is a cytosolic sink for cholesterol transferred to NPC1 in the limiting membrane of the LE. Here, key suspects include vesicle trafficking pathways and members of the oxysterol-binding protein-related proteins (ORPs) family (Fig. 1, blue) that are thought to be cytosolic cholesterol transfer proteins (5). Thus, the study by Infante et al. (13) has shown us for the first time a cholesterol transfer pathway that provides a mechanism for unidirectional efflux from the LE.
What does the NPC1/2 TTD tell us about NPC disease and the optimal path to treatment? First and foremost, we now appreciate why loss-of-function mutations in both proteins trigger NPC disease. As with any tag team effort, missing one partner renders playing the game moot. In the case of mutations affecting NPC2 function, the current results suggests that to achieve correction we need to focus on approaches that improve “donor” function, e.g., improved cholesterol mobilization within the LE. One class of drugs could include the cyclodextrin chemical scaffold. These are nontoxic small molecules, such as 2-hydroxypropyl-β-cyclodextrin (14), that are commonly used to modify the cholesterol content of cellular membranes. In principle, if optimized through structure activity relationship studies, these could provide a sustained cholesterol shuttling mechanism within the endosomal–lysosomal system to achieve unidirectional flow via the linked activities of NPC1 and ORP, or vesicle traffic.
In contrast, in the case of NPC1 deficiencies, we need to focus on compounds that improve “acceptor” function. In the complete absence of NPC1, one possibly is that correction may require mobilization of cholesterol through up-regulation of vesicular trafficking pathway(s) (15, 16) (Fig. 1). On the other hand, most missense mutations in NPC1 may actually lead to improper trafficking of the protein from its site of synthesis in the ER to the limiting membrane of the LE (17, 18). Such mutants may be more responsive to the alteration of the protein homeostasis or “proteostasis” system that is used by the cell to generate and maintain the protein fold (19). Here, both pharmacological chaperones (PCs) (i.e., small molecules such as cholesterol analogs that could bind and stabilize the fold) or a new class of compounds we refer to as proteostasis regulators (PRs) (19) that alter the cellular folding environment to “repair” the variant fold may favor repopulation of the LE with functional NPC1 and restoration of acceptor function. Strikingly, as proof-of-principle of the key insights provided by Infante et al. (13), cyclodextrins have recently been reported to correct NPC1 null mouse models of disease (20, 21). Here, cholesterol binding activity of either internalized cyclodextrin or, possibly, the presence of an extracellular sink for cholesterol could supplement the shuttle activity of endogenous NPC2 to transfer cholesterol from internal membranes to favor efflux via ABCA1 or vesicular recycling pathways.
Cyclodextrins have recently been reported to correct NPC1 null mouse models of disease.
The work of the Infante et al. tag team (13) may have found the Achilles heel of Niemann-Pick disease: the need for a series of linked kinetic equilibria driven by the NPC1/2 TTD to direct cholesterol efflux through the limiting membrane of the LE. Given that fact that NPC1(NTD) binds oxysterol with higher affinity than cholesterol (9), oxysterols may provide an unanticipated regulatory mechanism to control cholesterol efflux from the limiting membrane of the LE. Of course, there remains a large number of additional questions that need to be addressed regarding TTD function in cholesterol mobilization, particularly given the close link between cholesterol homeostasis (1, 5, 6), glycosphingolipid metabolism (6, 22), and Rab recycling pathways (15, 16).
In summary, our new understanding of cholesterol mobilization from the LE should allow us to modulate different steps in the TTD transfer pathway in a systematic fashion to repair the dysfunctional cellular pathophysiology associated with disease and provide substantial benefit to NPC children. Indeed, combination therapy of cholesterol mobilization with cyclodextrin-like cholesterol binding scaffolds (20, 21) and a PC/PR (18) may prove to be synergistic to create a substantially improved trafficking and function environment as Kelly and colleagues (23) have demonstrated recently for Gaucher's disease.
Footnotes
The authors declare no conflict of interest.
See companion article on page 15287.
References
- 1.Maxfield FR, Menon AK. Intracellular sterol transport and distribution. Curr Opin Cell Biol. 2006;18:379–385. doi: 10.1016/j.ceb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 3.Jeon H, Blacklow SC. Structure and physiologic function of the low-density lipoprotein receptor. Annu Rev Biochem. 2005;74:535–562. doi: 10.1146/annurev.biochem.74.082803.133354. [DOI] [PubMed] [Google Scholar]
- 4.Hurley JH. ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol. 2008;20:4–11. doi: 10.1016/j.ceb.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol. 2006;22:129–157. doi: 10.1146/annurev.cellbio.22.010305.104656. [DOI] [PubMed] [Google Scholar]
- 6.Vance JE. Lipid imbalance in the neurological disorder, Niemann-Pick C disease. FEBS Lett. 2006;580:5518–5524. doi: 10.1016/j.febslet.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Yu L. The structure and function of Niemann-Pick C1-like 1 protein. Curr Opin Lipidol. 2008;19:263–269. doi: 10.1097/MOL.0b013e3282f9b563. [DOI] [PubMed] [Google Scholar]
- 8.Xu S, Benoff B, Liou HL, Lobel P, Stock AM. Structural basis of sterol binding by NPC2, a lysosomal protein deficient in Niemann-Pick type C2 disease. J Biol Chem. 2007;282:23525–23531. doi: 10.1074/jbc.M703848200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Infante RE, et al. Purified NPC1 protein: II. Localization of sterol binding to a 240-amino acid soluble luminal loop. J Biol Chem. 2008;283:1064–1075. doi: 10.1074/jbc.M707944200. [DOI] [PubMed] [Google Scholar]
- 10.Infante RE, et al. Purified NPC1 protein I. Binding of cholesterol and oxysterols to a 1,278-amino acid membrane protein. J Biol Chem. 2008;283:1052–1063. doi: 10.1074/jbc.M707943200. [DOI] [PubMed] [Google Scholar]
- 11.Vaya J, Schipper HM. Oxysterols, cholesterol homeostasis, and Alzheimer disease. J Neurochem. 2007;102:1727–1737. doi: 10.1111/j.1471-4159.2007.04689.x. [DOI] [PubMed] [Google Scholar]
- 12.Cheruku SR, Xu Z, Dutia R, Lobel P, Storch J. Mechanism of cholesterol transfer from the Niemann-Pick type C2 protein to model membranes supports a role in lysosomal cholesterol transport. J Biol Chem. 2006;281:31594–31604. doi: 10.1074/jbc.M602765200. [DOI] [PubMed] [Google Scholar]
- 13.Infante RE, et al. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc Natl Acad Sci USA. 2008;105:15287–15292. doi: 10.1073/pnas.0807328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim CH, et al. Cholesterol depletion and genistein as tools to promote 508delCFTR, retention at the plasma membrane. 2007;20:473–482. doi: 10.1159/000107531. [DOI] [PubMed] [Google Scholar]
- 15.Linder MD, et al. Rab8-dependent recycling promotes endosomal cholesterol removal in normal and sphingolipidosis cells. Mol Biol Cell. 2007;18:47–56. doi: 10.1091/mbc.E06-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narita K, et al. Protein transduction of Rab9 in Niemann-Pick C cells reduces cholesterol storage. FASEB J. 2005;19:1558–1560. doi: 10.1096/fj.04-2714fje. [DOI] [PubMed] [Google Scholar]
- 17.Runz H, Dolle D, Schlitter AM, Zschocke J. NPC-db, a Niemann-Pick type C disease gene variation database. Hum Mutat. 2008;29:345–350. doi: 10.1002/humu.20636. [DOI] [PubMed] [Google Scholar]
- 18.Gelsthorpe ME, et al. Niemann-Pick type C1 I1061T mutant encodes a functional protein that is selected for endoplasmic reticulum-associated degradation due to protein misfolding. J Biol Chem. 2008;283:8229–8236. doi: 10.1074/jbc.M708735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 20.Liu B, Li H, Repa JJ, Turley SD, Dietschy JM. Genetic variations and treatments that affect the lifespan of the NPC1 mouse. J Lipid Res. 2008;49:663–669. doi: 10.1194/jlr.M700525-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Lope-Piedrafita S, Totenhagen JW, Hicks CH, Erickson RP, Trouard TP. MRI detects therapeutic effects in weanling Niemann-Pick type C mice. J Neurosci Res. 2008;86:2802–2807. doi: 10.1002/jnr.21707. [DOI] [PubMed] [Google Scholar]
- 22.Walkley SU, Suzuki K. Consequences of NPC1 and NPC2 loss of function in mammalian neurons. Biochim Biophys Acta. 2004;1685:48–62. doi: 10.1016/j.bbalip.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 23.Mu T-W, et al. Chemical and biological approaches synergize to ameliorate protein-folding diseases. Cell. 2008;134:769–781. doi: 10.1016/j.cell.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]