Abstract
Perfluorooctanoic acid (PFOA) and perfluorodecanoic acid (PFDA) have been detected globally in wildlife and humans. Data from a gene array indicate that PFOA decreases organic anion transporting polypeptides (Oatps) in liver. Na+-taurocholate cotransporting polypeptide (Ntcp) and Oatp1a1, 1a4, and 1b2 are major transporters responsible for uptake of bile acids (BAs) and other organic compounds into liver. The purpose of the present study was to determine the effects of two perfluorinated fatty acids, PFOA and PFDA, on mRNA and protein expression of hepatic uptake transporters Oatps and Ntcp, and to determine the underlying regulatory mechanisms by using peroxisome proliferator-activated receptor alpha (PPAR-α), constitutive androstane receptor, pregnane-X receptor, NF-E2–related factor 2, and farnesoid X receptor-null mouse models. After 2 days following a single i.p. administration, PFOA did not alter serum BA concentrations, but PFDA increased serum BA concentrations 300%. Furthermore, PFOA decreased mRNA and protein expression of Oatp1a1, 1a4, and 1b2, but not Ntcp in mouse liver. In contrast, PFDA decreased mRNA and protein expression of all four transporters, and decreased the mRNA expression in a dose-dependent manner, with the decrease of Oatp1a4 occurring at lower doses than the other three transporters. Multiple mechanisms are likely involved in the down-regulation of mouse Oatps and Ntcp by PFDA. By using the various transcription factor-null mice, PPAR-α was shown to play a central role in the down-regulation of Oatp1a1, 1a4, 1b2, and Ntcp by PFDA. The current studies provide important insight into understanding the mechanisms by which PFDA regulate the expression of hepatic uptake transporters. In conclusion, PFOA and PFDA decrease mouse liver uptake transporters primarily via activation of PPAR-α.
Keywords: Na+-taurocholate cotransporting polypeptide (Ntcp), Organic anion transporting polypeptide (Oatp), perfluorodecanoic acid (PFDA), perfluorooctanoic acid (PFOA), peroxisome proliferator-activated receptor alpha (PPAR-α)
Certain perfluorocarboxylic acids (PFCAs or perfluorinated fatty acids), such as perfluooctanoic acid (PFOA), perfluorononanoic acid, and perfluorodecanoic acid (PFDA) have been used for decades to make products that resist heat, oil, stains, grease, and water, such as in stain-resistant carpets and fabrics, in the manufacture of non-stick cookware, components of fire-fighting foam, and other industrial uses (the information available through Environmental Protection Agency web site: http://www.epa.gov/oppt/pfoa/pubs/pfoainfo.htm). These perfluorinated compounds have been detected globally in wildlife (Falandysz et al., 2006; Gannon et al., 2006; Houde et al., 2006; Kim and Kannan, 2007; Sinclair et al., 2006) and humans (Calafat et al., 2007a, b; Fei et al., 2007; Olsen et al., 2004, 2007; Tao et al. 2008). Perfluorooctanoic acid (PFOA) and certain perfluorochemicals have become a public health concern in recent years as studies have revealed their environmental persistence (De Silva and Mabury, 2004; Oono et al., 2008; Skutlarek et al., 2006; Trudel et al., 2008), and as data have emerged about their toxicity in laboratory animals (George and Andersen, 1986; Harris et al., 1989; Jensen and Leffers, 2008; Langley, 1990; Lau et al., 2004; Son et al., 2008; Starkov and Wallace, 2002; Van Rafelghem et al., 1987; Wei et al., 2008; Wolf et al., 2008). Liver is thought to be a prime target organ of PFOA and related perfluorochemicals, due to efficient hepatic uptake and persistence in liver (George and Andersen, 1986; Van Rafelghem et al., 1987; Vanden Heuvel et al., 1991a, b). In livers of mice and rats, PFOA and/or PFDA caused hepatomegaly, peroxisome proliferation, and alteration in hepatic lipid content (Brewster and Birnbaum, 1989; Harris et al., 1989; Kudo and Kawashima, 1997; Son et al., 2008; Van Rafelghem et al., 1987; Wolf et al., 2008; Yamamoto and Kawashima, 1997). PFOA has been shown to promote hepatocarcinogenesis in rats, but PFDA has not (Abdellatif et al., 1991; Borges et al., 1993). Both PFOA and PFDA are peroxisome proliferator-activated receptor alpha (PPAR-α) activators and capable of triggering oxidative stress (Cai et al., 1995; Langley, 1990; Takagi et al., 1991; Vanden Heuvel et al., 2006). In addition, PFDA, but not PFOA, increased tumor necrosis factor-α concentrations in rat sera (Adinehzadeh and Reo, 1998).
The Na+-taurocholate cotransporting polypeptide (Ntcp) and organic anion transporting polypeptides (Oatps) are major transporters responsible for uptake of bile acids (BAs) and other organic compounds into liver. Ntcp is a sodium-dependent hepatic BA uptake transporter (Kullak-Ublick et al., 2000; Meier et al., 1997; Meier and Stieger, 2002). Organic anion transporting polypeptides (rodents: Oatps; human: OATPs) represent a gene superfamily that mediates sodium-independent transport of various amphipathic organic compounds such as BAs, drugs (e.g., rifampicin, digoxin), toxins (e.g., microcystin), steroid conjugates, eicosanoids, and thyroid hormones into cells. In rats and mice, Oatp1a1, 1a4, and 1b2 are highly expressed in liver (Cheng et al., 2005a; Hagenbuch and Meier, 2003; Li et al., 2002; Ogura et al., 2000; Ogura et al., 2001). The effects of some xenobiotics on the regulation of Oatps have been reported previously (Cheng et al., 2005b, 2006; Guo et al., 2002a; Staudinger et al., 2001a). For example, the PXR ligand pregnenolone-16α-carbonitrile (PCN) markedly induces mouse and rat Oatp1a4 expression (Cheng and Klaassen, 2006; Cheng et al., 2005b; Guo et al., 2002b).
Drugs and other xenobiotics can alter target gene expression through activation of nuclear receptors and other transcription factors including aryl hyrdrocarbon receptor, constitutive androstane receptors (CAR), pregnane-X receptor (PXR), PPAR-α, and NF-E2–related factor (Nrf2). For example, PCN increases Oatp1a4 expression through activation of PXR in rats and mice (Guo et al., 2002b; Staudinger et al., 2001a), whereas oltipraz and butylated hydroxyanisole activate Nrf2, and induce Mrp2, 3, and 4 expression (Maher et al., 2007).
In a recent gene array of rat liver, it was reported that PFOA down-regulates Oatp1a1, 1a4, and 1b2 mRNA expression (Guruge et al., 2006). Therefore, the purpose of the present study was to determine in mice the effects of two perfluorinated fatty acids, namely PFOA and PFDA, on the gene expression of hepatic uptake transporters, and to determine the underlying regulatory mechanisms by using PPAR-α, CAR-, PXR-, Nrf2-, and FXR-null mouse models.
MATERIALS AND METHODS
Materials.
Sodium chloride, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) sodium salt, HEPES free acid, lithium lauryl sulfate, ethylenediaminetetraacetic acid, and d-(+)-glucose were purchased from Sigma-Aldrich (St Louis, MO). Micro-O-protect was purchased from Roche Diagnostics (Indianapolis, IN) for the bDNA assay. Formaldehyde, 4-morpholinepropanesulfonic acid, sodium citrate, and NaHCO3 were purchased from Fischer Chemicals (Fairlawn, NJ). Chloroform, agarose, and ethidium bromide were purchased from AMRESCO, Inc. (Solon, OH). PFOA (free acid form; 96% purity; M.W. 413 g/mol) and PFDA (free acid form; 98% purity; M.W. 513 g/mol) were both purchased from Sigma-Aldrich Co. (St Louis, MO). All other chemicals, unless otherwise indicated, were purchased from Sigma-Aldrich Co. Total BA (Colorimetric assay) kit was purchased from Bio-Quant, Inc. (San Diego, CA).
Rabbit anti-rat Ntcp antibody, which has cross-reactivity with mouse Ntcp (Aleksunes et al., 2006; Wolters et al., 2002), was generously provided by Dr Bruno Stieger (Department of Medicine, University Hospital, Zurich, Switzerland). Polyclonal antibodies of mouse Oatp1a1, 1a4, and 1b2 were developed against mouse Oatp-specific peptide (Oatp1a1-specific peptide, NH2-CRKFHFPGDIHSPDTE-COOH; Oatp1a4-specific peptide, NH2-CFPGDIDSSDTDP-COOH; Oatp1b2-specific peptide, NH2-CNPEPVNNNGYSCVPSDEKNSETPL-COOH), and were raised in rabbits by Covance Research Products, Inc. (Berkeley, CA). β-Actin antibody (ab8227) was purchased from Abcam, Inc. (Cambridge, MA). Goat anti-rabbit IgG horseradish peroxidase-linked secondary antibody was purchased from Sigma-Aldrich Co.
Animals and treatment.
Eight-week-old adult male C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME), and housed according to the American Animal Association Laboratory Animal Care (AAALAC) guidance. For the dose-response study of PFDA, adult C57BL/6 male mice (n = 4 per treatment) were administered a single i.p. dose of PFDA (0.5, 1.0, 10, 20, 40, or 80 mg/kg of body weight), whereas control mice were treated i.p. with the vehicle, propylene glycol:water (1:1, vol/vol). Two days later, livers were collected and snap-frozen in liquid nitrogen and stored at −80°C.
Breeding pairs of FXR-null mice (Sinal et al., 2000) and PXR-null mice (Staudinger et al., 2001b) in the C57BL/6 background were kindly provided by Dr Frank J. Gonzalez (National Institutes of Health/National Cancer Institute, Bethesda, MD). Breeding pairs of CAR-null mice in the C57BL/6 background were obtained from Dr Ivan Rusyn (University of North Carolina, Chapel Hill, NC), which were engineered by Tularik, Inc. (South San Francisco, CA) as described previously (Ueda et al., 2002). Nrf2-null mice were kindly provided by Dr Jefferson Y. Chan at University of California, Irvine (Leung et al., 2003), and bred to a second generation of C57BL/6 background in our facility. Each mouse genotype (n = 5) was administered a single i.p. dose of PFOA (40 mg/kg; or 90 μmol/kg) or PFDA (80 mg/kg; or 156 μmol/kg), or the vehicle propylene glycol:water (1:1, vol/vol). Two days later, livers were removed and immediately frozen in liquid nitrogen, and stored at −80°C.
The PPAR-α-null mice originally engineered by the laboratory of Dr Frank J. Gonzalez (Lee et al., 1995), were back-crossed to a C57BL/6 background (Akiyama et al., 2001), and bred and housed at an AAALAC-accredited facility at Pennsylvania State University (University Park, PA). The PPAR-α-null mice and their WT controls were treated in the laboratory of Dr. Jeffrey M. Peters (Pennsylvania State University); the dose of PFDA was decreased to 40 mg/kg (or 78 μmol/kg) due to increased lethality at 80 mg/kg (or 156 μmol/kg). Two days later, livers were collected, frozen, and shipped to the University of Kansas Medical Center (Kansas City, KS) for further studies.
Total BA quantification in mouse serum.
Adult C57BL/6 male mice (n = 5 per treatment) were administered a single i.p. dose of PFOA (40 mg/kg; or 90 μmol/kg) or PFDA (80 mg/kg; or 156 μmol/kg), and control mice were injected i.p. with the vehicle, propylene glycol:water (1:1, vol/vol). Two days later, mice were euthanized with an overdose of pentobarbital. Blood was collected by cardiac puncture, allowed to coagulate, and centrifuged at 10,000 × g for 15 min. The resulting supernatant (serum) was collected for analysis. Serum BA concentrations were quantified according to the manufacturer's protocol (Total BA [Colorimetric assay] kit, Bio-Quant, Inc.).
Total RNA isolation.
Total RNA was isolated using RNA Bee reagent (Tel-Test, Inc., Friendswood, TX) per the manufacturer's protocol. The concentration of total RNA in each sample was quantified spectrophotometrically at 260 nm.
Branched DNA signal amplification assay.
The mRNA levels for mouse Oatps and Ntcp were quantified using the branched DNA (bDNA) assay (Quantigene bDNA signal amplification kit; Panomics, Inc., Fremont, CA). The strategy of multiple oligonucleotide probe set design and the probe set of mouse Ntcp and Oatps have been described previously (Cheng and Klaassen, 2006; Cheng et al., 2007; Hartley and Klaassen, 2000). The luminescence for each gene is reported as relative light units (RLUs) per 10 μg of total RNA.
Membrane protein preparation.
Crude plasma membrane samples were prepared from mouse livers as described previously (Johnson et al., 2002). Briefly, 200–300 mg liver was minced in 10 ml of ice-cold homogenizing buffer (0.25M sucrose, 10mM Tris-HCl at pH 7.5, containing 25 μg/ml leupeptin, 50 μg/ml aprotinin, 40 μg/ml phenylmethanesulphonylfluoride, 0.5 μg/ml pepstatin, and 50 μg/ml antipain). The minced tissue was poured into a Dounce homogenizer (Kontes, Vineland, NJ) and homogenized on ice for 10 strokes. The homogenate was filtered through one layer of gauze sponges (Tyco Healthcare Group LP, Mansfield, MA), and then centrifuged at 100,000 × g for 1 h at 4°C. The resulting pellet was mixed with resuspension buffer (0.25M sucrose, 10mM HEPES, and 40 μg/ml phenylmethanesulphonylfluoride). Protein concentration of each sample was determined with a Bradford protein assay kit from Sigma.
Western blots.
Membrane protein samples mixed with sample loading buffer (75 μg protein/lane) were loaded after heating onto a 10% sodium dodecyl sulfate-polyacrylamide gel. Following electrophoresis, proteins in the gel were electrotransferred to a nitrocellulose membrane for 4.5 h at 34 V at 4°C. Membranes were blocked for 2 h at room temperature with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween-20 (TBS-T). Blots were then incubated overnight with each polyclonal antibody of mouse Oatps and rat Ntcp at 4°C. β-Actin antibody was used as a loading control. After thorough washing (three 20-min washes with excess TBS-T), blots were incubated with goat anti-rabbit IgG horseradish peroxidase-linked secondary antibody (1:5000 dilution with 5% non-fat milk in TBS-T) for 1 h. Blots were washed again. Immunoreactive bands were detected with an enhanced chemical luminescence kit (Pierce Biotechnology Inc., Rockford, IL). Oatp1a1, 1a4, 1b2, and Ntcp protein bands were visualized by exposure to Fuji Medical X-Ray film. The protein band intensity on the films was quantified with Gel-Pro 3.1 image analysis software (MediaCybernetics, Silver Spring, MD).
Statistical analysis.
Data are represented as mean ± SEM. Data were analyzed by one-way ANOVA, followed by Duncan's post hoc test. Statistical significance was set at p < 0.05. When only the difference between control and PFDA treatment was determined, data were analyzed by student's t-test, and statistical significance was considered at p < 0.05.
RESULTS
Effect of PFOA and PFDA on Total BA Concentrations in Adult Male C57BL/6 WT Mouse Serum
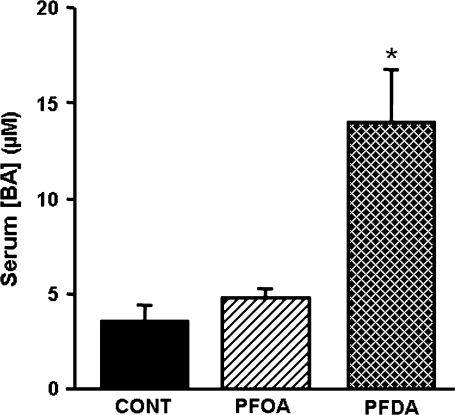
As shown in Figure 1, 2 days after treatment, PFOA did not increase serum BA concentrations. In contrast, PFDA increased serum BA concentrations 300%.
FIG. 1.
Effects of PFOA and PFDA on total BA concentrations in mouse serum. BA concentration in serum from adult male C57BL/6 WT mice 2 days after a single i.p. administration of control (propylene glycol:water; vol/vol, 1:1), PFOA (40 mg/kg), or PFDA (80 mg/kg). The data are presented as mean ± SEM (n = 5 mice). Asterisks (*) represent a statistical difference (p < 0.05) between control and treatment group.
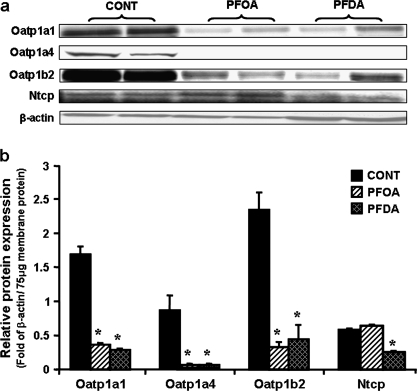
FIG. 2.
Effects of PFOA and PFDA on Oatp1a1, 1a4, and 1b2, as well as Ntcp protein expression in mouse livers. Adult male C57BL/6 WT mice (n = 5 per treatment) were given a single i.p. administration of control (propylene glycol:water; vol/vol, 1:1), PFOA (40 mg/kg), or PFDA (80 mg/kg). Two days after, mouse livers were collected. Total membrane protein was isolated from mouse livers. Two of five protein samples from each treatment were analyzed by Western blots. (a) Protein levels of Oatp1a1, 1a4, and 1b2, as well as Ntcp in mouse liver membranes were analyzed by Western blotting. (b) Protein levels of Oatp1a1, 1a4, and 1b2, as well as Ntcp in mouse liver membranes are expressed as ratio of each transporter to β-actin protein levels per 75 μg total membrane protein. Data are presented as mean ± SEM. Asterisks indicate statistically significant differences between control and PFOA or PFDA-treated male mice (p < 0.05).
Effects of PFOA and PFDA on Oatp1a1, 1a4, and 1b2, as well as Ntcp Protein Expression in Adult Male C57BL/6 WT Mouse Liver
The effects of PFOA and PFDA on the four hepatic uptake transporters in mouse livers are shown in Figure 2. Two days after treatment, PFOA decreased protein levels of Oatp1a1, 1a4, and 1b2 about 79, 97, and 87%, respectively. However, PFOA did not alter Ntcp protein expression. Similar to PFOA, 2 days of PFDA treatment decreased Oatp1a1, 1a4, and 1b2 by 84, 99, and 82%, respectively. In contrast to PFOA, PFDA decreased Ntcp protein levels approximately 65%.
Effects of PFOA and PFDA on Oatp1a1, 1a4, and 1b2, as well as Ntcp mRNA Expression in Adult Male C57BL/6 WT Mouse Liver
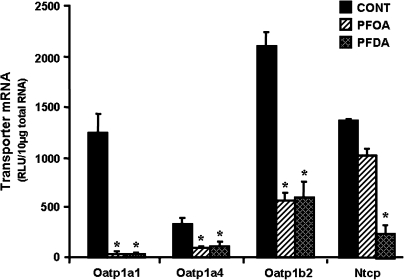
To determine whether the decrease in mouse Oatp and Ntcp protein expression by PFOA and PFDA corresponds to a decrease in mRNA, mRNA levels of each transporter were quantified. As depicted in Figure 3, similar to the alterations at the protein level, PFOA and PFDA markedly decreased mRNA levels of Oatp1a1, 1a4, and 1b2. PFOA tended to decrease mRNA expression of Ntcp (25%), but this was not statistically significant, whereas PFDA decreased Ntcp mRNA levels 82%. Therefore, the downregulation of Oatp protein expression by both PFOA and PFDA, and Ntcp by PFDA correlates with the decrease in the mRNA.
FIG. 3.
Effects of PFOA and PFDA on mRNA expression of Oatp1a1, 1a4, and 1b2, as well as Ntcp in mouse livers. Adult male C57BL/6 WT mice (n = 5 per treatment) were given a single i.p. administration of control (propylene glycol:water; vol/vol, 1:1), PFOA (40 mg/kg), or PFDA (80 mg/kg). Two days later, mouse livers were collected. Total RNA from mouse livers (n = 5 per treatment) was analyzed by the bDNA assay for the mRNA expression of each transporter. The data are reported as RLUs per 10 μg of total RNA, and are presented as mean ± SEM. Asterisks indicate statistically significant differences between control and PFOA or PFDA-treated male mice (p < 0.05).
Due to the apparent similarity in regulation of mouse Oatps by PFOA and PFDA and the fact that Ntcp was only altered by PFDA, further mechanistic studies focused mainly on PFDA.
Effects of Various Doses of PFDA on Oatp1a1, 1a4, and 1b2, as well as Ntcp mRNA Expression in Adult Male C57BL/6 WT Mouse Liver
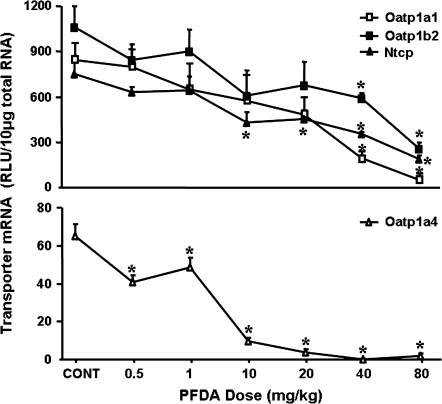
As shown in Figure 4, the lowest doses of PFDA used that decreased Oatp1a1 (75%), 1a4 (50%), 1b2 (45%), and Ntcp (43%) mRNA expression are 40, 0.5, 40, and 10 mg/kg, respectively. When doses increased, the mRNA expression of each transporter decreased further. With 80 mg/kg of PFDA, the mRNA of Oatp1a1, 1a4, 1b2, and Ntcp decreased approximately 94, 97, 75, and 75%, respectively.
FIG. 4.
Effects of various doses of PFDA on Oatp1a1, 1a4, and 1b2, as well as Ntcp mRNA expression in mouse livers. Adult male C57BL/6 WT mice were administered PFDA at a single i.p. dose (0.5, 1.0, 10, 20, 40, or 80 mg/kg of body weight), whereas control mice were treated i.p. with the vehicle, propylene glycerol:water (1:1, vol/vol). After 2 days, livers were collected. Total RNA samples from treated male mouse livers (n = 4) were analyzed by the bDNA assay. The data are reported as RLUs per 10 μg of total RNA, and are presented as mean ± SEM. Asterisks indicate statistically significant differences between control and PFDA-treated male mice (p < 0.05).
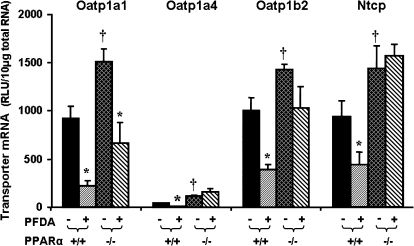
Effects of PFDA on Oatp1a1, 1a4, and 1b2, as well as Ntcp mRNA Expression in WT and PPAR-α-Null Mice
PFDA is a well-known peroxisome proliferator and a potent PPAR-α agonist (Cai et al., 1995; Cimini et al., 2000; Johnson and Klaassen, 2002). Therefore, the wild-type and PPAR-α-null mice were first used to determine whether the PFDA-induced down-regulation of Oatp1a1, 1a4, 1b2, and Ntcp is via PPAR-α. As shown in Figure 5, disruption of PPAR-α increased the constitutive expression of Oatp1a1 mRNA 65%. PFDA decreased Oatp1a1 mRNA 76% in wild-type mice, and 56% in PPAR-α-null mice. Constitutive mRNA levels of Oatp1a4 increased 170% in PPAR-α-null mice. PFDA decreased mRNA levels of Oatp1a4 in wild-type mice 75%, but not in PPAR-α-null mice, suggesting a PPAR-α–dependent mechanism. Removal of functional PPAR-α protein, as illustrated in the PPAR-α-null mice, increased constitutive mRNA levels of Oatp1b2 (40%). PFDA decreased Oatp1b2 mRNA expression 73% in wild-type mice and only 27% in PPAR-α-null mice, which is not statistically significant, indicating downregulation of Oatp1b2 mRNA expression by PFDA is PPAR-α dependent. Disruption of PPAR-α increased constitutive Ntcp mRNA 54%. PFDA decreased Ntcp mRNA 54% in wild-type mice, but not in PPAR-α-null mice, indicating that downregulation of Ntcp by PFDA is also PPAR-α dependent.
FIG. 5.
Effects of PFDA on mouse Oatp1a1, 1a4, and 1b2, as well as Ntcp mRNA expression in wild-type and PPAR-α-null mice. The mouse treatments are separated into 4 groups: PPAR(+/+) + CONT, wild-type mice treated with the vehicle, propylene glycerol:water (1:1, vol/vol); PPAR(+/+) + PFDA, wild-type mice treated with PFDA at dose 40 mg/kg of body weight; PPAR(−/−) + CONT, PPAR-α-null mice treated with the vehicle; PPAR(−/−) + PFDA, PPAR-α-null mice treated with PFDA at dose 40 mg/kg of body weight. The mice were given a single i.p. administration. After 2 days, livers were removed. Total RNA from treated mouse livers was analyzed by the bDNA assay. All data are expressed as mean ± SEM of four to five mice for each treatment. Asterisk indicates statistically significant difference between treated and control mice (p < 0.05). Single dagger (†) represents statistically significant differences (p < 0.05) between the vehicle-treated wild-type male mice and the vehicle-treated PPAR-α-null male mice.
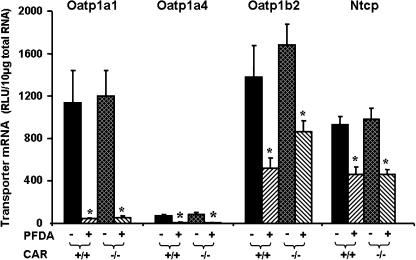
Effects of PFDA on Oatp1a1, 1a4, 1b2, and Ntcp mRNA Expression in WT and CAR-Null Mice
PFOA treatment markedly increased the mRNA expression of Cyp2B15, which is a CAR responsive gene in rats (Guruge et al., 2006; Slatter et al., 2006). Therefore, the wild-type and CAR-null mice were used to determine whether the PFDA-induced down-regulation of Oatp1a1, 1a4, 1b2, and Ntcp is via CAR. As depicted in Figure 6, PFDA decreased Oatp1a1, 1a4, 1b2, and Ntcp mRNA levels in the livers of wild-type mice. PFDA produced similar down-regulation of each transporter in the CAR-null mice, indicating CAR-independent mechanisms.
FIG. 6.
Effects of PFDA on mouse Oatp1a1, 1a4, and 1b2, as well as Ntcp mRNA expression in wild-type and CAR-null mice. The mouse treatments are separated into four groups: CAR(+/+)+CONT, wild-type mice treated with the vehicle, propylene glycerol:water (1:1, vol/vol); CAR(+/+) + PFDA, wild-type mice treated with PFDA at dose 80 mg/kg of body weight; CAR(−/−) + CONT, CAR-null mice treated with the vehicle; CAR(−/−) + PFDA, CAR-null mice treated with PFDA at dose 80 mg/kg. The mice were given a single i.p. administration. After 2 days, livers were removed. Total RNA from treated mouse livers was analyzed by the bDNA assay. All data are expressed as mean ± SEM of five mice for each treatment. Asterisk indicates statistically significant difference between treated and control mice (p < 0.05).
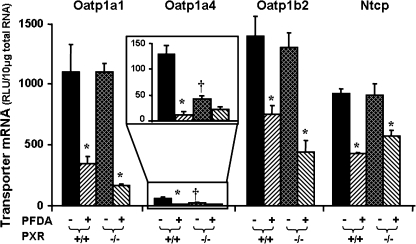
Effects of PFDA on Oatp1a1, 1a4, 1b2, and Ntcp mRNA Expression in WT and PXR-Null Mice
Oatp1a4 is a well-known PXR target gene in mice and rats (Cheng and Klaassen, 2006; Guo et al., 2002b). Therefore, the wild-type and PXR-null mice were used to determine whether the PFDA-induced down-regulation of Oatp1a4 and other Oatps (Oatp1a1 and 1b2), as well as Ntcp is via PXR. As shown in Figure 7, disruption of PXR, as demonstrated in the PXR-null mice, decreased the constitutive expression of Oatp1a4 mRNA, but did not alter the constitutive expression of Oatp1a1, 1b2, or Ntcp mRNA. PFDA treatment decreased mRNA expression of Oatp1a1, 1b2, and Ntcp in both wild-type and PXR-null mice. PFDA decreased Oatp1a4 mRNA expression in the wild-type mice, and tended to decrease Oatp1a4 mRNA in the PXR-null mice, but this was not statistically significant.
FIG. 7.
Effects of PFDA on mouse Oatp1a1, 1a4, and 1b2, as well as Ntcp mRNA expression in wild-type and PXR-null mice. The mouse treatments are separated into four groups: PXR(+/+) + CONT, wild-type mice treated with the vehicle, propylene glycerol:water (1:1, vol/vol); PXR(+/+) + PFDA, wild-type mice treated with PFDA at dose 80 mg/kg of body weight; PXR(−/−) + CONT, PXR-null mice treated with the vehicle; PXR(−/−) + PFDA, PXR-null mice treated with PFDA at dose 80 mg/kg. The mice were given a single i.p. administration. After 2 days, livers were removed. Total RNA from untreated or treated mouse livers was analyzed by the bDNA assay. All data are expressed as mean ± SEM of five mice for each treatment. Asterisk indicates statistically significant difference between PFDA-treated and control mice (p < 0.05); single dagger (†) represents statistically significant differences (p < 0.05) between the vehicle-treated wild-type male mice and the vehicle-treated PXR-null male mice.
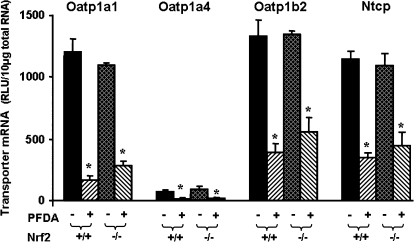
Effects of PFDA on Oatp1a1, 1a4, and 1b2, as well as Ntcp mRNA Expression in WT and Nrf2-Null Mice
PFCAs cause oxidative stress, which can be sensed by the transcription factor Nrf2 (Cai et al., 1995; Itoh et al., 1997; Maher et al., 2007). Therefore, the wild-type and Nrf2-null mice were used to determine whether the PFDA-induced down-regulation of Oatp1a1, 1a4, 1b2, and Ntcp is via Nrf2. As shown in Figure 8, PFDA decreased Oatp1a1, 1a4, and 1b2, as well as Ntcp mRNA levels in wild-type mouse livers. Similar down-regulation of Oatp1a1, 1a4, 1b2, and Ntcp was observed in the Nrf2-null mice.
FIG. 8.
Effects of PFDA on mouse Oatp1a1, 1a4, and 1b2, as well as Ntcp mRNA expression in wild-type and Nrf2-null mice. The mouse treatments are separated into four groups: Nrf2(+/+) + CONT, wild-type mice treated with the vehicle, propylene glycerol:water (1:1, vol/vol); Nrf2(+/+) + PFDA, wild-type mice treated with PFDA at dose 80 mg/kg of body weight; Nrf2(−/−) + CONT, Nrf2-null mice treated with the vehicle; Nrf2(−/−) + PFDA, Nrf2-null mice treated with PFDA at dose 80 mg/kg. The mice were given a single i.p. administration. After 2 days, livers were removed. Total RNA from treated mouse livers was analyzed by the bDNA assay. All data are expressed as mean ± SEM of five mice for each treatment. Asterisk indicates statistically significant difference between treated and control mice (p < 0.05).
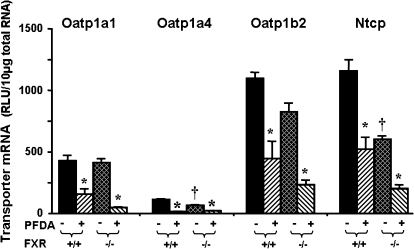
Effects of PFDA on Oatp1a1, 1a4, and 1b2, as well as Ntcp mRNA Expression in WT and FXR-Null Mice
PFDA is a perfluorinated fatty acid. Fatty acids have been shown to be ligands of FXR (Zhao et al., 2004). Therefore, it was determined whether down-regulation of hepatic Oatps and Ntcp by PFDA is via FXR activation. As shown in Figure 9, disruption of FXR, as demonstrated in the FXR-null mice, decreased the constitutive expression of Oatp1a4 (45%) and Ntcp (50%) mRNA, but did not alter the constitutive expression of Oatp1a1 and 1b2 mRNA. PFDA treatment decreased mRNA expression of Oatp1a1 (64%), 1a4 (88%), 1b2 (60%), and Ntcp (55%) in the wild-type mice. In comparison, PFDA decreased mRNA expression of Oatp1a1 (88%), 1a4 (70%), 1b2 (72%), and Ntcp (68%) in the FXR-null mice. Therefore, in FXR-null mice, PFDA decreased the mRNA expression of Oatps and Ntcp even more than that in wild-type mice.
FIG. 9.
Effects of PFDA on Oatp1a1, 1a4, and 1b2, as well as Ntcp mRNA expression in wild-type and FXR-null mice. The black and the dark-latticed bars represent regulation of each transporter by vehicle treatment; the striated bars depict regulation of each transporter by PFDA treatment. Adult C57BL/6 wild-type or FXR-null male mice were treated with the vehicle, propylene glycerol:water (1:1, vol/vol) or PFDA at dose 80 mg/kg of body weight. The mice were treated for 2 days. Total RNA from untreated or treated mouse livers was analyzed by the bDNA assay. All data are expressed as mean ± SEM of five mice for each treatment. Asterisk indicates statistically significant difference between PFDA-treated and control mice (p < 0.05). Single dagger (†) represents statistically significant differences (p < 0.05) between the vehicle-treated wild-type male mice and the vehicle-treated FXR-null male mice.
DISCUSSION
The present study demonstrates that PFOA did not alter serum BA concentrations, but PFDA increased serum BA concentrations 300% 2 days after a single i.p. administration. Both PFOA and PFDA markedly decreased the expression of liver-predominant Oatp1a1, 1a4, and 1b2 at both protein and mRNA levels (Figs. 2 and 3). PFDA, but not PFOA decreased Ntcp mRNA and protein expression in mouse liver. Furthermore, PFDA treatment caused a dose-dependent down-regulation of mRNA expression of mouse Oatp1a1, 1a4, 1b2, and Ntcp (Fig. 4), with a decrease of Oatp1a4 being observed at lower doses than the other three transporters.
The present data indicate that activation of the nuclear receptor PPAR-α is the main mechanism by which PFDA decreases the expression of all hepatic uptake transporters examined at the doses evaluated in this study, namely Oatp1a1, 1a4, 1b2, and Ntcp (Fig. 5). PPAR-α is a nuclear receptor that is critical in mediating both peroxisome proliferation and tumorigenesis in livers of mice exposed to PPAR-α agonists. PPARs, including PPAR-α, can influence gene expression indirectly, and usually negatively, through competition with other transcription factors (Semple et al., 2006). PPAR-α can be activated by endogenous ligands, such as polyunsaturated fatty acids, and by synthetic agonists such as the fibrate drugs. PPAR-α is expressed mainly in liver, kidney, and skeletal muscle and is involved in fatty acid oxidation (Gouni-Berthold and Krone, 2005). PFCAs structurally mimic fatty acids, thus they bind and subsequently activate PPAR-α (Vanden Heuvel et al., 2006). The present data are consistent with previous reports that activation of PPAR-α decreases mouse Oatp1a1 mRNA and protein expression, and Ntcp protein levels (Cheng et al., 2005b; Kok et al., 2003).
The present study indicates that PPAR-α is not only involved in chemical regulation of mouse Oatps and Ntcp, but also the constitutive expression of Oatps and Ntcp. As shown in Figure 5, elimination of PPAR-α increased the basal expression of Oatp1a1, 1a4, 1b2, and Ntcp. Previous studies showed a tendency for increase in Oatp1a1 and Ntcp mRNA expression in PPAR-α-null mice, but the increases were not statistically significant (Kok et al., 2003).
The current data suggest that PPAR-α activation plays an important role in PFDA-induced down-regulation of Oatp and Ntcp expression. However, the theory does not apply to the regulation of Ntcp expression by PFOA, another PPAR-α agonist (Takacs and Abbott, 2007). Unlike down-regulation of Ntcp by PFDA, PFOA did not alter Ntcp expression (Figs. 2 and 3). Moreover, previous reports showed that some other PPAR-α ligands (ciprofibrate, clofibrate, and diethylhexylphthalate) did not decrease Oatps and Ntcp mRNA expression as did PFDA (Cheng et al., 2005b, 2007). Our recent unpublished data showed that (1) compared to ciprofibrate, clofibrate, and diethylhexylphthalate, PFDA is a much more effective PPAR-α activator; and (2) another potent PPAR-α activator, Wy-14643, decreased Oatp1a1, 1a4, 1b2, and Ntcp mRNA expression in mouse liver. Therefore, diverse PPAR-α ligands differently regulate the expression of Oatps and Ntcp, possibly due to variation of intensity of PPAR-α activation.
In addition to activation of PPAR-α, other mechanisms may contribute to regulation of individual hepatic uptake transporters by PFDA. For example, PFDA decreased Oatp1a1 mRNA 76% in wild-type mice, and somewhat less (56%) in PPAR-α-null mice. In the promoter of mouse Oatp1a1, there is a DR-1 DNA fragment “TGACCTaTGATCT,” which is a putative PPAR-α response element. Therefore, down-regulation of Oatp1a1 by PFDA is only partially PPAR-α dependent (Fig. 5). It has been shown that PFDA causes testicular atrophy in rats (Olson and Andersen, 1983), and interferes with testosterone biotransformation and secretion, leading to lower levels of serum androgens in rats (Bookstaff et al., 1990; Boujrad et al., 2000). Oatp1a1 expression is androgen-dependent in mouse liver and kidney (Cheng et al., 2006; Isern et al., 2001). Thus, in addition to PPAR-α, PFDA-induced decrease in serum androgen concentrations might also contribute to decreased Oatp1a1 expression.
In addition to PPAR-α-null mice, numerous transcription factor-null mouse models including CAR-, PXR-, Nrf2-, and FXR-null mice were used to characterize the underlying regulatory mechanisms for the downregulation of Ntcp and Oatps by PFDA. However, except for PPAR-α, downregulation of Ntcp and Oatps by PFDA are not dependent on CAR, PXR, Nrf2, and FXR activity (Figs. 6–9). PFOA has been recently suggested to activate CAR (Rosen et al., 2008). However, CAR activation is not necessary for regulation of Ntcp and Oatps by PFDA (Fig. 6).
Alteration in the regulation of hepatic uptake transporters can have physiological effects. For example, in the present study, PFDA increased BA concentrations in mouse serum about 300% 2 days after a single i.p. administration, but PFOA did not alter BA concentrations in mouse serum. Recent unpublished data from this laboratory also showed that after 2 weeks following a single i.p. administration, 100 μmol/kg of PFDA increased serum BA concentrations more than 20-fold in mice, but 100 μmol/kg of PFOA did not. BAs can be taken up into liver by Ntcp and Oatps, but mainly by Ntcp. The decrease in Ntcp by PFDA most likely is responsible for the increased BA concentrations in mouse serum by PFDA.
Taken together, PFDA clearly decreases expression of Ntcp and three liver-predominant Oatps, namely Oatp1a1, 1a4, and 1b2 in mouse liver, but PFOA only decreases Oatps in mouse liver. PPAR-α plays a central role in regulating these uptake transporters by PFDA. The current studies provide important insight into the underlying regulatory mechanisms of drug transporters by these PFCAs.
FUNDING
National Institutes of Health grants (ES013714, ES09649, ES09716, and RR021940).
Acknowledgments
We thank Drs Jonathan M. Maher, Lauren M. Aleksunes, Hong Lu, and Xiaohong (Lucy) Lei for their help with sample processing and comments on the manuscript.
References
- Abdellatif AG, Preat V, Taper HS, Roberfroid M. The modulation of rat liver carcinogenesis by perfluorooctanoic acid, a peroxisome proliferator. Toxicol. Appl. Pharmacol. 1991;111:530–537. doi: 10.1016/0041-008x(91)90257-f. [DOI] [PubMed] [Google Scholar]
- Adinehzadeh M, Reo NV. Effects of peroxisome proliferators on rat liver phospholipids: Sphingomyelin degradation may be involved in hepatotoxic mechanism of perfluorodecanoic acid. Chem. Res. Toxicol. 1998;11:428–440. doi: 10.1021/tx970155t. [DOI] [PubMed] [Google Scholar]
- Akiyama TE, Nicol CJ, Fievet C, Staels B, Ward JM, Auwerx J, Lee SS, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-alpha regulates lipid homeostasis, but is not associated with obesity: Studies with congenic mouse lines. J. Biol. Chem. 2001;276:39088–39093. doi: 10.1074/jbc.M107073200. [DOI] [PubMed] [Google Scholar]
- Aleksunes LM, Scheffer GL, Jakowski AB, Pruimboom-Brees IM, Manautou JE. Coordinated expression of multidrug resistance-associated proteins (Mrps) in mouse liver during toxicant-induced injury. Toxicol. Sci. 2006;89:370–379. doi: 10.1093/toxsci/kfi332. [DOI] [PubMed] [Google Scholar]
- Bookstaff RC, Moore RW, Ingall GB, Peterson RE. Androgenic deficiency in male rats treated with perfluorodecanoic acid. Toxicol. Appl. Pharmacol. 1990;104:322–333. doi: 10.1016/0041-008x(90)90306-f. [DOI] [PubMed] [Google Scholar]
- Borges T, Peterson RE, Pitot HC, Robertson LW, Glauert HP. Effect of the peroxisome proliferator perfluorodecanoic acid on the promotion of two-stage hepatocarcinogenesis in rats. Cancer Lett. 1993;72:111–120. doi: 10.1016/0304-3835(93)90019-6. [DOI] [PubMed] [Google Scholar]
- Boujrad N, Vidic B, Gazouli M, Culty M, Papadopoulos V. The peroxisome proliferator perfluorodecanoic acid inhibits the peripheral-type benzodiazepine receptor (PBR) expression and hormone-stimulated mitochondrial cholesterol transport and steroid formation in Leydig cells. Endocrinology. 2000;141:3137–3148. doi: 10.1210/endo.141.9.7678. [DOI] [PubMed] [Google Scholar]
- Brewster DW, Birnbaum LS. The biochemical toxicity of perfluorodecanoic acid in the mouse is different from that of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Appl. Pharmacol. 1989;99:544–554. doi: 10.1016/0041-008x(89)90161-0. [DOI] [PubMed] [Google Scholar]
- Cai Y, Appelkvist EL, DePierre JW. Hepatic oxidative stress and related defenses during treatment of mice with acetylsalicylic acid and other peroxisome proliferators. J. Biochem. Toxicol. 1995;10:87–94. doi: 10.1002/jbt.2570100205. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. Serum concentrations of 11 polyfluoroalkyl compounds in the u.s. population: Data from the national health and nutrition examination survey (NHANES) Environ. Sci. Technol. 2007a;41:2237–2242. doi: 10.1021/es062686m. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003-2004 and comparisons with NHANES 1999-2000. Environ. Health Perspect. 2007b;115:1596–1602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Buckley D, Klaassen CD. Regulation of hepatic bile acid transporters Ntcp and Bsep expression. Biochem. Pharmacol. 2007;74:1665–1676. doi: 10.1016/j.bcp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng X, Klaassen CD. Regulation of mRNA expression of xenobiotic transporters by the pregnane-X receptor (PXR) in mouse liver, kidney, and intestine. Drug Metab. Dispos. 2006;34:1863–1867. doi: 10.1124/dmd.106.010520. [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher J, Chen C, Klaassen CD. Tissue distribution and ontogeny of mouse organic anion transporting polypeptides (Oatps) Drug Metab. Dispos. 2005a;33:1062–1073. doi: 10.1124/dmd.105.003640. [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher JM, Dieter MZ, Klaassen CD. Regulation of mouse organic anion-transporting polypeptides (Oatps) in liver by prototypical microsomal enzyme inducers that activate distinct transcription factor pathways. Drug Metab. Dispos. 2005b;33:1276–1282. doi: 10.1124/dmd.105.003988. [DOI] [PubMed] [Google Scholar]
- Cheng X, Maher JM, Lu H, Klaassen CD. Endocrine regulation of gender-divergent mouse organic anion transporting polypeptide (Oatp) expression. Mol. Pharmacol. 2006;70:1291–1297. doi: 10.1124/mol.106.025122. [DOI] [PubMed] [Google Scholar]
- Cimini A, Cristiano L, Bernardo A, Farioli-Vecchioli S, Stefanini S, Ceru MP. Presence and inducibility of peroxisomes in a human glioblastoma cell line. Biochim. Biophys. Acta. 2000;1474:397–409. doi: 10.1016/s0304-4165(00)00036-2. [DOI] [PubMed] [Google Scholar]
- De Silva AO, Mabury SA. Isolating isomers of perfluorocarboxylates in polar bears (Ursus maritimus) from two geographical locations. Environ. Sci. Technol. 2004;38:6538–6545. doi: 10.1021/es049296p. [DOI] [PubMed] [Google Scholar]
- Falandysz J, Taniyasu S, Gulkowska A, Yamashita N, Schulte-Oehlmann U. Is fish a major source of fluorinated surfactants and repellents in humans living on the Baltic Coast? Environ. Sci. Technol. 2006;40:748–751. doi: 10.1021/es051799n. [DOI] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: A study within the Danish National Birth Cohort. Environ. Health Perspect. 2007;115:1677–1682. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon JTHR, Kaiser MA, Mueller T. Review II: Perfluorooctanoic acid (PFOA) in the environment. White paper by DuPont Wilmington. 2006 DE. Report Number: DuPont-19567. US EPA Administrative Record. AR-226–3679. [Google Scholar]
- George ME, Andersen ME. Toxic effects of nonadecafluoro-n-decanoic acid in rats. Toxicol. Appl. Pharmacol. 1986;85:169–180. doi: 10.1016/0041-008x(86)90110-9. [DOI] [PubMed] [Google Scholar]
- Gouni-Berthold I, Krone W. Peroxisome proliferator-activated receptor alpha (PPARalpha) and athero-sclerosis. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005;5:513–523. doi: 10.2174/156800605774962022. [DOI] [PubMed] [Google Scholar]
- Guo GL, Johnson DR, Klaassen CD. Postnatal expression and induction by pregnenolone-16alpha-carbonitrile of the organic anion-transporting polypeptide 2 in rat liver. Drug Metab. Dispos. 2002a;30:283–288. doi: 10.1124/dmd.30.3.283. [DOI] [PubMed] [Google Scholar]
- Guo GL, Staudinger J, Ogura K, Klaassen CD. Induction of rat organic anion transporting polypeptide 2 by pregnenolone-16alpha-carbonitrile is via interaction with pregnane X receptor. Mol. Pharmacol. 2002b;61:832–839. doi: 10.1124/mol.61.4.832. [DOI] [PubMed] [Google Scholar]
- Guruge KS, Yeung LW, Yamanaka N, Miyazaki S, Lam PK, Giesy JP, Jones PD, Yamashita N. Gene expression profiles in rat liver treated with perfluorooctanoic acid (PFOA) Toxicol. Sci. 2006;89:93–107. doi: 10.1093/toxsci/kfj011. [DOI] [PubMed] [Google Scholar]
- Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim. Biophys. Acta. 2003;1609:1–18. doi: 10.1016/s0005-2736(02)00633-8. [DOI] [PubMed] [Google Scholar]
- Harris MW, Uraih LC, Birnbaum LS. Acute toxicity of perfluorodecanoic acid in C57BL/6 mice differs from 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam. Appl. Toxicol. 1989;13:723–736. doi: 10.1016/0272-0590(89)90330-8. [DOI] [PubMed] [Google Scholar]
- Hartley DP, Klaassen CD. Detection of chemical-induced differential expression of rat hepatic cytochrome P450 mRNA transcripts using branched DNA signal amplification technology. Drug Metab. Dispos. 2000;28:608–616. [PubMed] [Google Scholar]
- Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC. Biological monitoring of polyfluoroalkyl substances: A review. Environ. Sci. Technol. 2006;40:3463–3473. doi: 10.1021/es052580b. [DOI] [PubMed] [Google Scholar]
- Isern J, Hagenbuch B, Stieger B, Meier PJ, Meseguer A. Functional analysis and androgen-regulated expression of mouse organic anion transporting polypeptide 1 (Oatp1) in the kidney. Biochim. Biophys. Acta. 2001;19(1518):73–78. doi: 10.1016/s0167-4781(01)00169-5. [DOI] [PubMed] [Google Scholar]
- Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Leffers H. Emerging endocrine disrupters: Perfluoroalkylated substances. Int. J. Androl. 2008;31:161–169. doi: 10.1111/j.1365-2605.2008.00870.x. [DOI] [PubMed] [Google Scholar]
- Johnson DR, Guo GL, Klaassen CD. Expression of rat multidrug resistance protein 2 (Mrp2) in male and female rats during normal and pregnenolone-16alpha-carbonitrile (PCN)-induced postnatal ontogeny. Toxicology. 2002;178:209–219. doi: 10.1016/s0300-483x(02)00231-7. [DOI] [PubMed] [Google Scholar]
- Johnson DR, Klaassen CD. Regulation of rat multidrug resistance protein 2 by classes of prototypical microsomal enzyme inducers that activate distinct transcription pathways. Toxicol. Sci. 2002;67:182–189. doi: 10.1093/toxsci/67.2.182. [DOI] [PubMed] [Google Scholar]
- Kim SK, Kannan K. Perfluorinated acids in air, rain, snow, surface runoff, and lakes: Relative importance of pathways to contamination of urban lakes. Environ. Sci. Technol. 2007;41:8328–8334. doi: 10.1021/es072107t. [DOI] [PubMed] [Google Scholar]
- Kok T, Bloks VW, Wolters H, Havinga R, Jansen PL, Staels B, Kuipers F. Peroxisome proliferator-activated receptor alpha (PPARalpha)-mediated regulation of multidrug resistance 2 (Mdr2) expression and function in mice. Biochem. J. 2003;369:539–547. doi: 10.1042/BJ20020981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Kawashima Y. Fish oil-feeding prevents perfluorooctanoic acid-induced fatty liver in mice. Toxicol. Appl. Pharmacol. 1997;145:285–293. doi: 10.1006/taap.1997.8186. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Stieger B, Hagenbuch B, Meier PJ. Hepatic transport of bile salts. Semin. Liver Dis. 2000;20:273–292. doi: 10.1055/s-2000-9426. [DOI] [PubMed] [Google Scholar]
- Langley AE. Effects of perfluoro-n-decanoic acid on the respiratory activity of isolated rat liver mitochondria. J. Toxicol. Environ. Health. 1990;29:329–336. doi: 10.1080/15287399009531395. [DOI] [PubMed] [Google Scholar]
- Lau C, Butenhoff JL, Rogers JM. The developmental toxicity of perfluoroalkyl acids and their derivatives. Toxicol. Appl. Pharmacol. 2004;198:231–241. doi: 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell. Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung L, Kwong M, Hou S, Lee C, Chan JY. Deficiency of the Nrf1 and Nrf2 transcription factors results in early embryonic lethality and severe oxidative stress. J. Biol. Chem. 2003;278:48021–48029. doi: 10.1074/jbc.M308439200. [DOI] [PubMed] [Google Scholar]
- Li N, Hartley DP, Cherrington NJ, Klaassen CD. Tissue expression, ontogeny, and inducibility of rat organic anion transporting polypeptide 4. J. Pharmacol. Exp. Ther. 2002;301:551–560. doi: 10.1124/jpet.301.2.551. [DOI] [PubMed] [Google Scholar]
- Maher JM, Dieter MZ, Aleksunes LM, Slitt AL, Guo G, Tanaka Y, Scheffer GL, Chan JY, Manautou JE, Chen Y, et al. Oxidative and electrophilic stress induces multidrug resistance-associated protein transporters via the nuclear factor-E2-related factor-2 transcriptional pathway. Hepatology. 2007;46:1597–1610. doi: 10.1002/hep.21831. [DOI] [PubMed] [Google Scholar]
- Meier PJ, Eckhardt U, Schroeder A, Hagenbuch B, Stieger B. Substrate specificity of sinusoidal bile acid and organic anion uptake systems in rat and human liver. Hepatology. 1997;26:1667–1677. doi: 10.1002/hep.510260641. [DOI] [PubMed] [Google Scholar]
- Meier PJ, Stieger B. Bile salt transporters. Annu. Rev. Physiol. 2002;64:635–661. doi: 10.1146/annurev.physiol.64.082201.100300. [DOI] [PubMed] [Google Scholar]
- Ogura K, Choudhuri S, Klaassen CD. Full-length cDNA cloning and genomic organization of the mouse liver-specific organic anion transporter-1 (lst-1) Biochem. Biophys. Res. Commun. 2000;272:563–570. doi: 10.1006/bbrc.2000.2830. [DOI] [PubMed] [Google Scholar]
- Ogura K, Choudhuri S, Klaassen CD. Genomic organization and tissue-specific expression of splice variants of mouse organic anion transporting polypeptide 2. Biochem. Biophys. Res. Commun. 2001;281:431–439. doi: 10.1006/bbrc.2001.4387. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Church TR, Larson EB, van Belle G, Lundberg JK, Hansen KJ, Burris JM, Mandel JH, Zobel LR. Serum concentrations of perfluorooctanesulfonate and other fluorochemicals in an elderly population from Seattle, Washington. Chemosphere. 2004;54:1599–1611. doi: 10.1016/j.chemosphere.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Mair DC, Reagen WK, Ellefson ME, Ehresman DJ, Butenhoff JL, Zobel LR. Preliminary evidence of a decline in perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) concentrations in American Red Cross blood donors. Chemosphere. 2007;68:105–111. doi: 10.1016/j.chemosphere.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Olson CT, Andersen ME. The acute toxicity of perfluorooctanoic and perfluorodecanoic acids in male rats and effects on tissue fatty acids. Toxicol. Appl. Pharmacol. 1983;70:362–372. doi: 10.1016/0041-008x(83)90154-0. [DOI] [PubMed] [Google Scholar]
- Oono S, Matsubara E, Harada KH, Takagi S, Hamada S, Asakawa A, Inoue K, Watanabe I, Koizumi A. Survey of airborne polyfluorinated telomers in Keihan area, Japan. Bull. Environ. Contam. Toxicol. 2008;80:102–106. doi: 10.1007/s00128-007-9324-2. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Lee JS, Ren H, Vallanat B, Liu J, Waalkes MP, Abbott BD, Lau C, Corton JC. Toxicogenomic dissection of the perfluorooctanoic acid transcript profile in mouse liver: Evidence for the involvement of nuclear receptors PPAR alpha and CAR. Toxicol. Sci. 2008;103:46–56. doi: 10.1093/toxsci/kfn025. [DOI] [PubMed] [Google Scholar]
- Semple RK, Chatterjee VK, O'Rahilly S. PPAR gamma and human metabolic disease. J. Clin. Invest. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Sinclair E, Mayack DT, Roblee K, Yamashita N, Kannan K. Occurrence of perfluoroalkyl surfactants in water, fish, and birds from New York State. Arch. Environ. Contam. Toxicol. 2006;50:398–410. doi: 10.1007/s00244-005-1188-z. [DOI] [PubMed] [Google Scholar]
- Skutlarek D, Exner M, Farber H. Perfluorinated surfactants in surface and drinking waters. Environ. Sci. Pollut. Res. Int. 2006;13:299–307. doi: 10.1065/espr2006.07.326. [DOI] [PubMed] [Google Scholar]
- Slatter JG, Cheng O, Cornwell PD, de Souza A, Rockett J, Rushmore T, Hartley D, Evers R, He Y, Dai X, et al. Microarray-based compendium of hepatic gene expression profiles for prototypical ADME gene-inducing compounds in rats and mice in vivo. Xenobiotica. 2006;36:902–937. doi: 10.1080/00498250600861694. [DOI] [PubMed] [Google Scholar]
- Son HY, Kim SH, Shin HI, Bae HI, Yang JH. Perfluorooctanoic acid-induced hepatic toxicity following 21-day oral exposure in mice. Arch. Toxicol. 2008;82:239–246. doi: 10.1007/s00204-007-0246-x. [DOI] [PubMed] [Google Scholar]
- Starkov AA, Wallace KB. Structural determinants of fluorochemical-induced mitochondrial dysfunction. Toxicol. Sci. 2002;66:244–252. doi: 10.1093/toxsci/66.2.244. [DOI] [PubMed] [Google Scholar]
- Staudinger J, Liu Y, Madan A, Habeebu S, Klaassen CD. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab. Dispos. 2001a;29:1467–1472. [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, et al. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc. Natl. Acad. Sci. U. S. A. 2001b;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takacs ML, Abbott BD. Activation of mouse and human peroxisome proliferator-activated receptors (alpha, beta/delta, gamma) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol. Sci. 2007;95:108–117. doi: 10.1093/toxsci/kfl135. [DOI] [PubMed] [Google Scholar]
- Takagi A, Sai K, Umemura T, Hasegawa R, Kurokawa Y. Short-term exposure to the peroxisome proliferators, perfluorooctanoic acid and perfluorodecanoic acid, causes significant increase of 8-hydroxydeoxyguanosine in liver DNA of rats. Cancer Lett. 1991;57:55–60. doi: 10.1016/0304-3835(91)90063-n. [DOI] [PubMed] [Google Scholar]
- Tao L, Kannan K, Aldous KM, Mauer MP, Eadon GA. Biomonitoring of perfluorochemicals in plasma of New York State personnel responding to the World Trade Center disaster. Environ. Sci. Technol. 2008;42:3472–3478. doi: 10.1021/es8000079. [DOI] [PubMed] [Google Scholar]
- Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbuhler K. Estimating consumer exposure to PFOS and PFOA. Risk Anal. 2008;28:251–269. doi: 10.1111/j.1539-6924.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, Negishi M. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol. Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- Van Rafelghem MJ, Mattie DR, Bruner RH, Andersen ME. Pathological and hepatic ultrastructural effects of a single dose of perfluoro-n-decanoic acid in the rat, hamster, mouse, and guinea pig. Fundam. Appl. Toxicol. 1987;9:522–540. [PubMed] [Google Scholar]
- Vanden Heuvel JP, Kuslikis BI, Van Rafelghem MJ, Peterson RE. Disposition of perfluorodecanoic acid in male and female rats. Toxicol. Appl. Pharmacol. 1991a;107:450–459. doi: 10.1016/0041-008x(91)90308-2. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Kuslikis BI, Van Rafelghem MJ, Peterson RE. Tissue distribution, metabolism, and elimination of perfluorooctanoic acid in male and female rats. J. Biochem. Toxicol. 1991b;6:83–92. doi: 10.1002/jbt.2570060202. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty acids: A comparison of human, mouse, and rat peroxisome proliferator-activated receptor-alpha, -beta, and -gamma, liver X receptor-beta, and retinoid X receptor-alpha. Toxicol. Sci. 2006;92:476–489. doi: 10.1093/toxsci/kfl014. [DOI] [PubMed] [Google Scholar]
- Wei Y, Liu Y, Wang J, Tao Y, Dai J. Toxicogenomic analysis of the hepatic effects of perfluorooctanoic acid on rare minnows (Gobiocypris rarus) Toxicol. Appl. Pharmacol. 2008;226:285–297. doi: 10.1016/j.taap.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Wolf DC, Moore T, Abbott BD, Rosen MB, Das KP, Zehr RD, Lindstrom AB, Strynar MJ, Lau C. Comparative hepatic effects of perfluorooctanoic acid and WY 14,643 in PPAR-{alpha} knockout and wild-type mice. Toxicol. Pathol. 2008;36:632–639. doi: 10.1177/0192623308318216. [DOI] [PubMed] [Google Scholar]
- Wolters H, Elzinga BM, Baller JF, Boverhof R, Schwarz M, Stieger B, Verkade HJ, Kuipers F. Effects of bile salt flux variations on the expression of hepatic bile salt transporters in vivo in mice. J. Hepatol. 2002;37:556–563. doi: 10.1016/s0168-8278(02)00247-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Kawashima Y. Perfluorodecanoic acid enhances the formation of oleic acid in rat liver. Biochem. J. 1997;325(Pt 2):429–434. doi: 10.1042/bj3250429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao A, Yu J, Lew JL, Huang L, Wright SD, Cui J. Polyunsaturated fatty acids are FXR ligands and differentially regulate expression of FXR targets. DNA Cell. Biol. 2004;23:519–526. doi: 10.1089/1044549041562267. [DOI] [PubMed] [Google Scholar]