Abstract
Chlorpyrifos (CPS) is widely used in agricultural settings and residue analysis has suggested that children in agricultural communities are at risk of exposure. This has resulted in a large amount of literature investigating the potential for CPS-induced developmental neurotoxic effects. Two developmental routes of administration of CPS are orally in corn oil at a rate of 0.5 ml/kg and subcutaneously in dimethyl sulfoxide (DMSO) at a rate of 1.0 ml/kg. For comparison between these methods, rat pups were exposed daily from days 10 to 16 to CPS (5 mg/kg) either orally dissolved in corn oil or subcutaneously dissolved in DMSO, both at rates of either 0.5 or 1.0 ml/kg. A representative vehicle/route group was present for each treatment. Both the low and high volume CPS in DMSO subcutaneous groups were lower than that of the low and high volume CPS in oil oral groups. At 4 h following the final administration, serum carboxylesterase was inhibited > 90% with all treatments. For cholinesterase activity in the cerebellum, medulla-pons, forebrain, and hindbrain, and serum, inhibition in the CPS-oil groups was similar and inhibition in the CPS-DMSO groups was similar. However, significantly greater inhibition was present in the high volume CPS-DMSO group as compared to the CPS-oil groups. Inhibition in the low volume CPS-DMSO group was generally between that in the CPS-oil groups and the high volume CPS-DMSO group. These data suggest that using DMSO as a vehicle for CPS may alter the level of brain ChE inhibition.
Keywords: chlorpyrifos, cholinesterase inhibition, developmental neurotoxicity
In recent years, the environmental exposure of children to pesticides and its effects on the developing nervous systems has been of great concern. Over the past decade, the exposure rates and effects of organophosphate (OP) insecticides on developing neural pathways have been scrutinized closely (Andersen et al., 2008; Arcury et al., 2007; Brimijoin and Koenigsberger, 1999; Curl et al., 2002; Richardson and Chambers, 2005). Many OP insecticides such as chlorpyrifos (CPS) (trade name Dursban) have been removed from home usage due to the potential for neurotoxicity in children, but are still used agriculturally on crops such as cotton, corn, almonds, oranges, and apples. Due to environmental drift (air and water) and take-home exposure pathways, the exposure rate to CPS is still 80–90% in certain areas of the United States (Arcury et al., 2007; Curl et al., 2002; Fenske et al., 2002; Steenland et al., 2000). While up to 70% of CPS is metabolized and excreted in the urine, depending on the route of exposure, bioaccumulation has been observed in adipose tissue in humans (Barr and Angerer, 2006).
The active metabolite of CPS, chlorpyrifos-oxon (CPO), inhibits the enzyme acetylcholinesterase (AChE), and leads to the accumulation of acetylcholine (ACh) and hyperstimulation of the cholinergic system. Hyperstimulation of the cholinergic system causes the classic signs of acute OP toxicity: salivation, lacrimation, urination, and defecation, and can lead to death if the toxic insult is severe enough and not treated promptly. Long-term and repeated low dose exposure to CPS has been shown to affect mental processes such as memory and concentration, induce depression, irritability and insomnia, and cause nonspecific reactions such as flu-like illnesses (Barr and Angerer, 2006; Salvi et al., 2003). These nonspecific or atypical reactions to low and repeated doses of CPS are usually accompanied by or follow AChE inhibition, which indicates a perturbation in the normal patterns of cholinergic neuronal activity.
Many differences in the developmental and toxic responses to CPS can be found in the literature; most of these differences stem from the route of exposure and vehicle used in the studies. For example, Griffin et al. (1999) reported differences in metabolism and excretion of CPS following oral and dermal exposures. Chambers et al. (2007) reported dermal exposures in children exposed to dogs treated with flea medications. Bradman et al. (2005) and Andersen et al. (2008) reported occupational and secondhand exposures of pregnant women to OP insecticides in agricultural communities and some of the effects of these exposures on fetal development. In our developmental studies, CPS dissolved in corn oil is administered orally (per os or PO) (Betancourt and Carr, 2004; Betancourt et al., 2006, 2007; Carr et al., 2001; Guo-Ross et al., 2007; Tang et al., 1999) while many other developmental studies administer CPS dissolved in dimethyl sulfoxide (DMSO) by subcutaneous injection (SC) (e.g., Aldridge et al., 2004, 2005a, b; Auman et al., 2000; Dam et al., 1999, 2000, 2003; Meyer et al., 2004a, b, 2005; Slotkin and Seidler, 2007). DMSO has a relatively low toxicity in and of itself and is used medically as a topical analgesic, anti-inflammatory drug, and antioxidant (Santos et al., 2003). The primary use of DMSO stems from its ability to readily cross skin and other tissue barriers, and to carry other chemical compounds in tandem. Since different methods of administration could produce differences in the toxicokinetics (absorption, distribution, metabolism, excretion) of a chemical, it is possible that different methods of administration could also influence toxicity. This study was designed to investigate potential differences between these two exposure routes using cholinesterase (ChE) inhibition as an indicator of toxicological impact.
A comparison of the two vehicles/routes of exposure was performed using the 0.5 ml/kg administration rate, the rate used in our developmental OP studies with corn oil, and using the 1.0 ml/kg administration rate since this rate is used by the majority of the studies in the literature using subcutaneous injections of CPS dissolved in DMSO. The two vehicles/routes were compared in a 7-day repeated exposure scenario at both the 0.5 and 1.0 ml/kg administration rates with each vehicle.
METHODS AND MATERIALS
Chemicals.
CPS was a generous gift from DowElanco Chemical Company (Indianapolis, IN). All other chemicals were purchased from Sigma Chemical Co. (St Louis, MO).
Animal treatment.
Adult male and female Sprague Dawley rats (CD IGS) were obtained from Charles River Laboratories (Wilmington, MA) and used for breeding. All animals were housed in a temperature-controlled environment (22 ± 2°C) with a 12-h dark-light cycle with lights on between 0700 and 1900 h in an Association for Assessment and Accreditation of Laboratory Animal Care–accredited facility. LabDiet rodent chow and tap water were freely available during the experimentation. All procedures were approved by the Mississippi State University Institutional Animal Care and Use Committee. Following parturition, male and female rat pups within the same litter were assigned to different treatment groups. While not all treatments were present in each litter, there was always a representative control animal present for each sex and CPS treatment in each litter. For this project, seven litters were used.
CPS was dissolved either in corn oil and administered by oral gavage (per os) or in DMSO and administered by subcutaneous injection at every day from postnatal day 10 (PND 10) through PND 16 (the day of birth was considered as PND 0). The dosage selected (5 mg/kg) was that previously used in other developmental studies involving CPS (Aldridge et al., 2004, 2005a, b; Auman et al., 2000; Dam et al., 1999, 2000, 2003; Meyer et al., 2004a, b, 2005; Slotkin and Seidler, 2007). Oral gavage was performed using a 25-μl tuberculin syringe equipped with a 1-inch 24-gauge straight intubation needle (Popper and Sons, Inc., New Hyde Park, NY) to deliver the solution to the back of the throat. Subcutaneous injections were performed using a 25-μl tuberculin syringe and were made under the skin on the back of the neck. The treatment groups are described in Table 1.
TABLE 1.
Treatment Groups
| Treatment group | Abbreviation | Description |
| Oil low volume control | OLV-PO | Corn oil administered daily orally (per os) at 0.5 ml/kg |
| CPS-oil low volume | CPS-OLV-PO | 5.0 mg/kg CPS dissolved in corn oil administered orally (per os) at 0.5 ml/kg |
| Oil high volume control | OHV-PO | Corn oil administered daily orally (per os) at 1.0 ml/kg |
| CPS-oil high volume | CPS-OHV-PO | 5.0 mg/kg CPS dissolved in corn oil administered orally (per os) at 1.0 ml/kg |
| DMSO low volume control | DLV-SC | DMSO administered subcutaneously at 0.5 ml/kg |
| CPS-DMSO low volume | CPS-DLV-SC | 5.0 mg/kg CPS dissolved in DMSO administered subcutaneously at 0.5 ml/kg |
| DMSO high volume control | DHV-SC | DMSO administered subcutaneously at 1.0 ml/kg |
| CPS-DMSO low volume | CPS-DHV-SC | 5.0 mg/kg CPS dissolved in DMSO administered subcutaneously at 1.0 ml/kg |
Rat pups were sacrificed on PND 16 at 4 h after the last exposure to CPS, a time utilized in out previous studies (Betancourt et al., 2007) and one which falls into the range of peak blood CPS levels in PND 12 and PND 17 rats as reported by Timchalk et al. (2006). Blood was collected by trunk bleeding to obtain serum. It has been previously suggested that the effects of CPS on cholinergic markers can vary based on region (Dam et al., 1999) and thus, multiple regions were analyzed. All brains were rapidly removed, dissected on ice to obtain the cerebellum, medulla pons, forebrain (region anterior to the optic chiasma), and hindbrain (region posterior to the optic chiasma excluding the cerebellum and medulla pons), frozen, and maintained at −20°C until assay.
Esterase assays.
The brain regions were homogenized at 40 mg tissue weight/ml in cold 0.05M Tris-HCl buffer (pH 7.4 at 37°C) in a glass mortar using a Wheaton motorized tissue grinder and a Teflon pestle. Serum was diluted in cold 0.05M Tris-HCl buffer (pH 7.4 at 37°C). The activity of ChE in brain regions (1 mg tissue/ml final concentration) and serum (12.5 μl serum/ml final concentration) was measured spectrophotometrically using a modification (Chambers et al., 1988) of Ellman et al. (1961) using acetylthiocholine as the substrate (1mM final concentration) and 5,5′-dithiobis(nitrobenzoic acid) as the chromagen. Serum carboxylesterase (CbxE) (0.625 μl serum/ml final concentration) was measured spectrophotometrically using 4-nitrophenyl valerate as the substrate (0.5mM final concentration) and monitoring 4-nitrophenol, one of the hydrolysis products, as previously described (Carr and Chambers, 1991). Protein concentration of the homogenates was quantified with the Folin phenol reagent using bovine serum albumin as a standard (Lowry et al., 1951). Specific activities of ChE and CbxE were calculated and expressed as nmol product produced per min/mg protein.
Statistical analysis.
The sphericity of the body weight data was initially tested by ANOVA using the general linear model with a repeated measures paradigm and was found to violate the assumption of sphericity. Therefore, subsequent analysis by ANOVA using the mixed model (Littell et al., 1996) was conducted with a repeat measures paradigm with a Huynh-Feldt covariance structure (Huynh and Feldt, 1970) followed by separation of means using least significant difference. The analysis identified differences in the main effects (sex and treatment) and all possible interactions. This analysis included litter and sex × treatment × litter as random effects. Esterase-specific activities were analyzed by ANOVA using the mixed procedure to determine significant sex, treatment, and sex x treatment interactions. There were no differences between sex or sex × treatment interactions. Thus, males and females were pooled for subsequent analysis and mean separation was performed by LSD. The criterion for significance was set at p ≤ 0.05. For determination of statistical significance from control and for calculation of percent inhibition, each treatment group was compared to its respective control which consisted of the same volume and route.
RESULTS
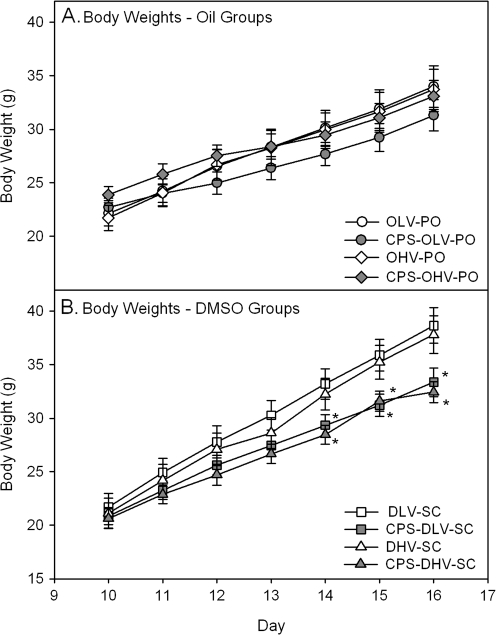
No signs of overt toxicity or cholinergic hyperstimulation, such as lacrimation, diarrhea, or tremors, were observed following CPS exposure regardless of the vehicle-route combination. As reported by Marty et al. (2007), we also observed signs of discomfort (“squirming”) in pups injected subcutaneously with DMSO. With respect to body weight, there were no significant differences in the pups treated orally with CPS in oil and their respective oil controls (Fig. 1A) while the body weights of pups treated subcutaneously with CPS in DMSO at administration rates of 0.5 ml/kg and 1.0 ml/kg were significantly reduced on days 5, 6, and 7 as compared to their respective controls (Fig. 1B). Within controls, there were no significant differences between any groups at any age with the exception of on day 7 when the body weights of the 0.5 ml/kg DMSO group were significantly greater than that of the 0.5 ml/kg and 1.0 ml/kg Oil groups. Within treated rats, a few random significant differences were present between the different treatment groups but no obvious pattern was present and no significant differences were present between any treatment groups on days 4–7.
FIG. 1.
Plots of body weights of rat pups exposed daily from PND 10 to 16 either (A) orally in oil or (B) subcutaneously in DMSO using the different administration paradigms as described in Table 1. Body weights were measured 24 h following the last exposure and values are expressed as mean body weight ± SE (n = 9–15). Values significantly different from their respective control (p ≤ 0.05) are indicated with an asterisk (*).
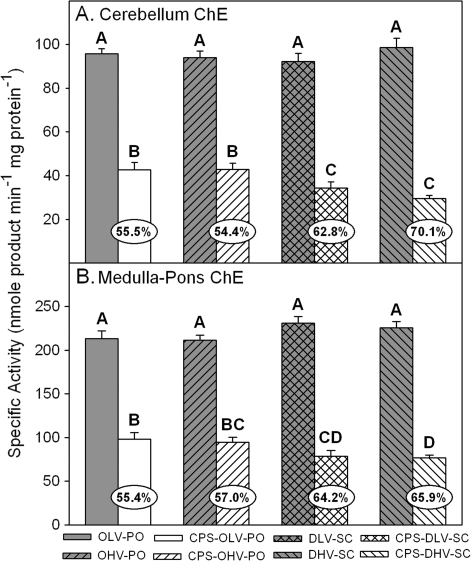
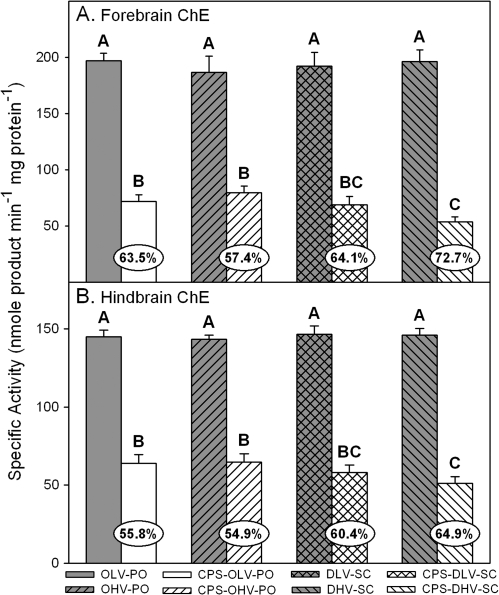
With respect to effects on brain ChE specific activity, there were no statistical differences in control ChE specific activity between the control groups regardless of the vehicle-route combination (Figs. 2 and 3). However, all CPS treatments resulted in significant inhibition as compared to their respective controls but there were differences in the level of inhibition between the different vehicle-route combinations within each respective brain region. In the cerebellum (Fig. 2A), inhibition in the rats treated orally with CPS in oil at 0.5 and 1.0 ml/kg was similar (54–55%) but significantly greater inhibition was present in the rats treated subcutaneously with CPS in DMSO at 0.5 (63%) and 1.0 ml/kg (70%). In the medulla (Fig. 2B), inhibition in the rats treated orally with CPS in oil at 0.5 and 1.0 ml/kg was also similar (54–57%) but significantly greater inhibition was present in the rats treated subcutaneously with CPS in DMSO at 1.0 ml/kg (66%). Inhibition in the rats treated subcutaneously with CPS in DMSO at 0.5 ml/kg (64%) was significantly different from the rats treated orally with CPS in oil at 0.5 ml/kg but was statistically similar to the rats treated orally with CPS in oil at 1.0 ml/kg and the rats treated subcutaneously with CPS in DMSO at 1.0 ml/kg. A similar pattern was present in the forebrain (Fig. 3A) and the hindbrain (Fig. 3B). Inhibition in the forebrain (57–64%) and in the hindbrain (55–56%) was similar in the rats treated orally with CPS in oil at 0.5 and 1.0 ml/kg. In the rats treated subcutaneously with CPS in DMSO at 0.5 ml/kg, inhibition in the forebrain (64%) and hindbrain (60%) was similar to that in the rats treated orally with CPS in oil at 0.5 and 1.0 ml/kg and to that in the rats treated subcutaneously with CPS in DMSO at 1.0 ml/kg in each respective brain region. However, significantly greater inhibition was present in the rats treated subcutaneously with CPS in DMSO at 1.0 ml/kg in both the forebrain (73%) and hindbrain (65%) than was present in the rats treated orally with CPS in oil at either administration rate.
FIG. 2.
Specific activity of (A) cerebellum ChE and (B) medulla-pons ChE from rat pups exposed daily from PND 10 through PND 16 using different administration paradigms as described in Table 1 and sacrificed 4 h after the last treatment on PND 16. Values are expressed as nmol/product min/mg protein ± SE (n = 5). Percent inhibition for each treatment group as compared to its respective control is presented in the oval overlaying the corresponding bar. Bars with different letters are statistically significant (p ≤ 0.05) from one another.
FIG. 3.
Specific activity of (A) forebrain ChE and (B) hindbrain ChE from rat pups exposed daily from PND 10 through PND 16 using different administration paradigms as described in Table 1 and sacrificed 4 h after the last treatment on PND 16. Values are expressed as nmol/product min/mg protein ± SE (n = 5). Percent inhibition for each treatment group as compared to its respective control is presented in the oval overlaying the corresponding bar. Bars with different letters are statistically significant (p ≤ 0.05) from one another.
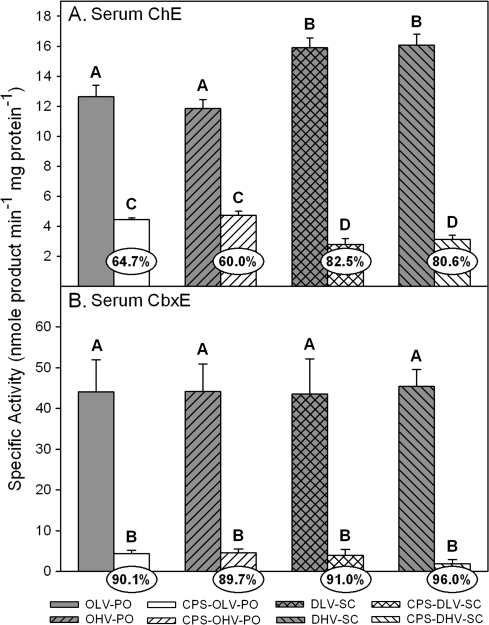
With respect to serum ChE (Fig. 4A), the DMSO treated controls had significantly greater specific activity than did the oil treated controls. While this increased specific activity in the DMSO controls contributed to a much greater level of inhibition in the rats treated subcutaneously with CPS in DMSO at 0.5 and 1.0 ml/kg (80–83%) than was present in the rats treated orally with CPS in oil at 0.5 ml/kg and 1.0 ml/kg (60–65%), the rats treated subcutaneously with CPS in DMSO at both administration rates had significantly lower specific activities than did the rats treated orally with CPS in oil at both administration rates. Unlike serum ChE, control serum CbxE specific activities (Fig. 4B) were similar regardless of the vehicle-route combination and treatment with CPS virtually eliminated activity (> 90%) in all groups.
FIG. 4.
Specific activity of (A) serum ChE and (B) serum CbxE from rat pups exposed daily from PND 10 through PND16 using different administration paradigms as described in Table 1 and sacrificed 4 h after the last treatment on PND16. Values are expressed as nmol product/min/mg protein ± SE (n = 5). Percent inhibition for each treatment group as compared to its respective control is presented in the oval overlaying the corresponding bar. Bars with different letters are statistically significant (p ≤ 0.05) from one another.
DISCUSSION
The main toxicological endpoint selected for study was the inhibition of ChE in the brain. Since the literature suggests that some of the developmental effects of CPS are mediated through noncholinergic mechanisms, ChE inhibition may not be reflective of all the possible negative effects associated with developmental CPS exposure. However, ChE inhibition is the traditional toxicological target of the OPs and therefore provides a dependable index of neurotoxic effects exerted by CPS. Secondly, a goal of this study was to compare the method of administration (oil-per os) utilized in our laboratory in juvenile rats (Betancourt and Carr, 2004; Betancourt et al., 2006, 2007; Carr et al., 2001; Guo-Ross et al., 2007; Tang et al., 1999) with the method of administration (DMSO-subcutaneous) frequently encountered in the literature (e.g., Aldridge et al., 2004, 2005a, b; Auman et al., 2000; Dam et al., 1999, 2000, 2003; Meyer et al., 2004a, b, 2005; Slotkin and Seidler, 2007). Therefore, the route of DMSO administered orally was not tested. In addition, the present study also did not include the route of oil administered subcutaneously which has also been used in developmental CPS studies in the literature (Karanth and Pope, 2000, 2003; Pope et al., 1991; Zhang et al., 2002). In comparison, at 4 h following a single exposure, no significant ChE inhibition was detected in either neonatal or juvenile rats exposed to a much higher dosage of CPS than that used in this study and by 24 h, significant inhibition was present (Karanth and Pope, 2000; Zhang et al., 2002). Thus, following a repeated exposure paradigm, such as the one used here, significant inhibition would more than likely be detected after multiple exposures. It would be interesting to compare the effects of repeated subcutaneous administration of CPS in oil to the routes used in this study.
With regards to pharmacokinetic differences between the two routes studied here, Marty et al. (2007) compared the pharmacokinetics of a single acute dosage of CPS in PND 5 rats administered either orally in corn oil at a rate of 5 ml/kg or subcutaneously in DMSO at a rate of 1 ml/kg. The time of maximum concentration of CPS in the blood was same (2 h) with both routes but the level of CPS following the oral administration in oil was five times higher than that following the subcutaneous administration in DMSO. While differences exist between the present study and that of Marty et al. including a repeated verses acute dosing paradigm, different rates of oil administration, and a different ages of rats, it is interesting that the pattern of ChE inhibition at 4 h following repeated exposures was not similar to the pattern of the levels of CPS in the blood at 2 h following a single exposure. Thus, the pharmacokinetics of CPS may differ following repeated exposures as compared to a single exposure. Marty et al. (2007) also reported that the majority of the CPS administered subcutaneously in DMSO remained at the injection site up to 2 h and stated that the fate of this CPS depot is not known, as 2 h was the last time point sampled, and that the effect of repeated subcutaneous injections in DMSO on CPS pharmacokinetics in unknown. Thus, while there are definitely differences in the pharmacokinetics of the two vehicles/routes, the role those differences play in the observed differences in inhibition of ChE following repeated exposure is not clear. It would be interesting to perform a time course for ChE inhibition to fully determine the pattern of ChE inhibition.
The literature reports that DMSO inhibits AChE in vitro (Jagota, 1992; Plummer et al., 1983; Watts and Hoogmoed, 1984) but we found no evidence that in vivo administration of DMSO, at the concentrations used in this study, leads to inhibition of brain ChE. In fact, we observed increased specific activity of serum ChE in the DMSO controls. However, the basis for this increase is not clear. It has been reported that DMSO can induce lysis of certain cell types including red blood cells (De Bruijne and Van Steveninck, 1972, 1974) and intravenous administration of DMSO in humans has been reported to cause transient hemolysis (Yellowlees et al., 1980). It is possible that the increased serum ChE could be the result of the release of soluble ChE from a small amount of hemolysis. However, the increased amount of serum ChE activity due to the presence of DMSO did not offer any additional protection as the specific activities of both the low and high volume CPS in DMSO subcutaneous groups were lower than that of the low and high volume CPS in oil oral groups.
With respect to oil, the amount of brain and serum ChE inhibition observed following oral CPS exposure was similar regardless of the volume of oil in which CPS was administered as indicated by the similar levels of inhibition present in both the rats treated with CPS in oil at 0.5 and at 1.0 ml/kg. This suggests that CPS is effectively absorbed through the gastrointestinal wall regardless of the volume of vehicle in the gut. It also appears that CPS dissolved in DMSO and administered subcutaneously will exert somewhat similar effects to those observed in the oral CPS dissolved in oil groups but only if a low volume of DMSO is used. However, statistically greater effects were observed with the 0.5 ml/kg CPS in DMSO group in a few brain regions, the medulla and cerebellum, but not in others, the forebrain or hindbrain. In contrast, raising the volume of DMSO administered (from 0.5 to 1.0 ml/kg) induced significantly greater ChE inhibition in all regions, at least at the time point measured in this study. A time course study of ChE would further clarify the potential for higher volumes of DMSO to impact ChE activity. It is possible that differences in the detoxication of CPS may exist between oral and subcutaneous routes and this could have contributed to the differences in ChE inhibition observed between the two routes since CPS administered orally would have to pass through the intestinal wall and liver initially and possibly allow a greater opportunity for detoxication. In contrast, CPS administered orally could also possibly allow greater opportunity for activation of CPS. However, in vitro studies with intestinal and hepatic microsomes have reported that detoxication is the preferred pathway as compared to activation (Poet et al., 2003). Whether this occurs in vivo is not totally clear. It is also not known how the balance between activation and detoxication of CPS would be affected by the different administration routes.
These data do not agree with previously published studies reporting that DMSO decreased the toxicity of ChE inhibitors (Kocsis et al., 1975). However, these data do indicate that DMSO can enhance the ability of CPS to exert at least some negative effects (i.e., ChE inhibition). Ballough et al. (2008) recently reported that the administration of DMSO, at the volumes used in this study, in combination with soman greatly increased both lethality and neuropathology induced by soman. As in this study, the basis for this increased anti-ChE toxicity associated with the presence of DMSO was not clear. In conclusion, it is not clear if using a 1 ml/kg DMSO administration volume will enhance the developmental neurotoxicity of CPS since many studies suggest that the developmental effects of CPS are mediated through unknown noncholinergic mechanisms. It may also be irrelevant that the presence of DMSO enhances the ability of CPS to yield ChE inhibition. A drawback of this study is that it investigated the effects of a single dosage at a single time point. Further studies which include different dosages, different time points, and possibly higher volumes will provide a better understanding of the effect of higher volumes of DMSO on CPS-induced toxicity and better determine its role as a potential confounder.
FUNDING
National Institute of Health (1P20-RR17661 and R01 ES 10386); Mississippi Agricultural and Forestry Experiment Station (MAFES) under MAFES project (#MISV-339010); and the College of Veterinary Medicine, Mississippi State University.
Acknowledgments
We wish to acknowledge the statistical expertise of Dr Sumalee Givaruangsawat. This paper is MAFES publication #J-11390 and Center for Environmental Health Sciences publication # 121.
References
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ. Health. Perspect. 2005a;113:1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Developmental exposure to terbutaline and chlorpyrifos: Pharmacotherapy of preterm labor and an environmental neurotoxicant converge on serotonergic systems in neonatal rat brain regions. Toxicol. Appl. Pharmacol. 2005b;203:132–144. doi: 10.1016/j.taap.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: Critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ. Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen HR, Schmidt IM, Grandjean P, Jensen TK, Budtz-Jørgensen E, Kjærstad MB, Bælum J, Nielsen JB, Skakkebaeæk NE, Main KM. Impaired reproductive development in sons of women occupationally exposed to pesticides during pregnancy. Environ. Health Perspect. 2008;116:566–572. doi: 10.1289/ehp.10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcury TA, Grzywacz JG, Barr DB, Tapia J, Chen H, Quandt SA. Pesticide urinary metabolite levels of children in eastern North Carolina farmworker households. Environ. Health Perspect. 2007;115:1254–1260. doi: 10.1289/ehp.9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auman JT, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure targets multiple proteins governing the hepatic adenylyl cyclase signaling cascade: Implications for neurotoxicity. Dev. Brain Res. 2000;121:19–27. doi: 10.1016/s0165-3806(00)00021-3. [DOI] [PubMed] [Google Scholar]
- Ballough GPH, Kan RK, Nicholson JD, Fath DM, Tompkins CP, Moffa GM, Filbert MG. Brain damage from soman-induced seizures is greatly exacerbated by dimethyl sulfoxide (DMSO): Modest neuroprotection by 2-aminoethyl diphenylborinate (2-APB), a transient receptor potential channel inhibitor and inositol 1,4,5-triphosphate receptor antagonist. J. Med. CBR Def. 2008;6:1–20. [Google Scholar]
- Barr DB, Angerer J. Potential uses of biomonitoring data: A case study using the organophosphorus pesticides chlorpyrifos and malathion. Environ. Health Perspect. 2006;114:1763–1769. doi: 10.1289/ehp.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betancourt AM, Burgess SC, Carr RL. Effect of developmental exposure to chlorpyrifos on the expression of neurotrophin growth factors and cell-specific markers in neonatal rat brain. Toxicol. Sci. 2006;92:500–506. doi: 10.1093/toxsci/kfl004. [DOI] [PubMed] [Google Scholar]
- Betancourt AM, Carr RL. The effect of chlorpyrifos and chlorpyrifos-oxon on brain neurotrophin levels in rats following early postnatal exposure. Toxicol. Sci. 2004;77:63–71. doi: 10.1093/toxsci/kfh003. [DOI] [PubMed] [Google Scholar]
- Betancourt AM, Filipov NM, Carr RL. Alteration of neurotrophins in the hippocampus and cerebral cortex of young rats exposed to chlorpyrifos and methyl parathion. Toxicol. Sci. 2007;100:445–455. doi: 10.1093/toxsci/kfm248. [DOI] [PubMed] [Google Scholar]
- Bradman A, Eskenazi B, Barr DB, Bravo R, Castorina R, Chevrier J, Kogut K, Harnly ME, McKone TE. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ. Health Perspect. 2005;113:1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimijoin S, Koenigsberger C. Cholinesterases in neural development: New findings and toxicologic implications. Environ. Health Perspect. 1999;107:59–64. doi: 10.1289/ehp.99107s159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr RL, Chambers JE. Acute effects of the organophosphate paraoxon on schedule-controlled behavior and esterase activity in rats: Dose-response relationships. Pharmacol. Biochem. Behav. 1991;40:929–936. doi: 10.1016/0091-3057(91)90108-e. [DOI] [PubMed] [Google Scholar]
- Carr RL, Chambers HW, Guarisco JA, Richardson JR, Tang J, Chambers JE. Effects of repeated oral postnatal exposure to chlorpyrifos on open-field behavior in juvenile rats. Toxicol. Sci. 2001;59:260–267. doi: 10.1093/toxsci/59.2.260. [DOI] [PubMed] [Google Scholar]
- Chambers JE, Boone JS, Davis MK, Moran JE, Tyler JW. Assessing transferable residues from intermittent exposure to flea control collars containing the organophosphate insecticide chlorpyrifos. J. Expo. Sci. Environ. Epidemiol. 2007;17:656–666. doi: 10.1038/sj.jes.7500570. [DOI] [PubMed] [Google Scholar]
- Chambers JE, Wiygul SM, Harkness JE, Chambers HW. Effects of acute paraoxon and atropine exposures on retention of shuttle avoidance behavior in rats. Neurosci. Res. Commun. 1988;3:85–92. [Google Scholar]
- Curl CL, Fenske RA, Kissel JC, Shirai JH, Moate TF, Griffith W, Coronado G, Thompson B. Evaluation of take-home organophosphorus pesticide exposure among agricultural workers and their children. Environ. Health Perspect. 2002;110:A787–A792. doi: 10.1289/ehp.021100787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam K, Garcia SJ, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure alters synaptic development and neuronal activity in cholinergic and catecholaminergic pathways. Dev. Brain Res. 1999;116:9–20. doi: 10.1016/s0165-3806(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Dev. Brain Res. 2000;121:179–187. doi: 10.1016/s0165-3806(00)00044-4. [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Transcriptional biomarkers distinguish between vulnerable periods for developmental neurotoxicity of chlorpyrifos: Implications for toxicogenomics. Brain Res. Bull. 2003;59:261–265. doi: 10.1016/s0361-9230(02)00874-2. [DOI] [PubMed] [Google Scholar]
- De Bruijne AW, Van Steveninck J. Lysis of yeast cells and erythrocytes by dimethylsulfoxide. Biochem. Pharmacol. 1972;21:153–162. doi: 10.1016/0006-2952(72)90265-1. [DOI] [PubMed] [Google Scholar]
- De Bruijne AW, Van Steveninck J. The influence of dimethylsulfoxide on the red cell membrane. Biochem. Pharmacol. 1974;23:3247–3258. doi: 10.1016/0006-2952(74)90647-9. [DOI] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fenske RA, Lu C, Barr D, Needham L. Children's exposure to chlorpyrifos and parathion in an agricultural community in central Washington State. Environ. Health Perspect. 2002;110:549–553. doi: 10.1289/ehp.02110549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin P, Mason H, Heywood K, Cocker J. Oral and dermal absorption of chlorpyrifos: A human volunteer study. Occup. Environ. Med. 1999;56:10–13. doi: 10.1136/oem.56.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo-Ross SX, Chambers JE, Meek EC, Carr RL. Altered muscarinic acetylcholine receptor subtype binding in neonatal rat brain following exposure to chlorpyrifos or methyl parathion. Toxicol. Sci. 2007;100:118–127. doi: 10.1093/toxsci/kfm195. [DOI] [PubMed] [Google Scholar]
- Huynh H, Feldt LS. Conditions under which mean square ratios in repeated measurement designs have exact F-distributions. J. Am. Stat. Assoc. 1970;65:1582–1589. [Google Scholar]
- Jagota SK. Inhibition of acetylcholinesterase of mice erythrocytes and synaptosomes by dimethylsulfoxide. Indian J. Med. Res. 1992;96:275–278. [PubMed] [Google Scholar]
- Karanth S, Pope C. Carboxylesterase and A-esterase activities during maturation and aging: Relationship to the toxicity of chlorpyrifos and parathion in rats. Toxicol. Sci. 2000;58:282–289. doi: 10.1093/toxsci/58.2.282. [DOI] [PubMed] [Google Scholar]
- Karanth S, Pope C. Age-related effects of chlorpyrifos and parathion on acetylcholine synthesis in rat striatum. Neurotoxicol. Teratol. 2003;25:599–606. doi: 10.1016/s0892-0362(03)00049-7. [DOI] [PubMed] [Google Scholar]
- Kocsis JJ, Harkaway S, Snyder R. Biological effects of the metabolites of dimethyl sulfoxide. Ann. N. Y. Acad. Sci. 1975;243:104–109. doi: 10.1111/j.1749-6632.1975.tb25349.x. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute; 1996. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marty MS, Domoradzki JY, Hansen SC, Timchalk C, Bartels MJ, Mattsson JL. The effect of route, vehicle, and divided doses on the pharmacokinetics of chlorpyrifos and its metabolite trichloropyridinol in neonatal Sprague–Dawley rats. Toxicol. Sci. 2007;100:360–373. doi: 10.1093/toxsci/kfm239. [DOI] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Aldridge JE, Slotkin TA. Developmental exposure to terbutaline alters cell signaling in mature rat brain regions and augments the effects of subsequent neonatal exposure to the organophosphorus insecticide chlorpyrifos. Toxicol. Appl. Pharmacol. 2005;203:154–166. doi: 10.1016/j.taap.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Aldridge JE, Tate CA, Cousins MM, Slotkin TA. Critical periods for chlorpyrifos-induced developmental neurotoxicity: Alterations in adenylyl cyclase signaling in adult rat brain regions after gestational or neonatal exposure. Environ. Health Perspect. 2004a;112:295–301. doi: 10.1289/ehp.6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A, Seidler FJ, Slotkin TA. Developmental effects of chlorpyrifos extend beyond neurotoxicity: Critical periods for immediate and delayed-onset effects on cardiac and hepatic cell signaling. Environ. Health Perspect. 2004b;112:170–178. doi: 10.1289/ehp.6690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer JM, Greenberg MJ, Lehman HK, Watts JA. Competitive inhibition by dimethylsulfoxide of molluscan and vertebrate acetylcholinesterase. Biochem. Pharmacol. 1983;32:151–158. doi: 10.1016/0006-2952(83)90668-8. [DOI] [PubMed] [Google Scholar]
- Poet TS, Wu H, Kousba AA, Timchalk C. In vitro rat hepatic and intestinal metabolism of the organophosphate pesticides chlorpyrifos and diazinon. Toxicol. Sci. 2003;72:193–200. doi: 10.1093/toxsci/kfg035. [DOI] [PubMed] [Google Scholar]
- Pope CN, Chakraborti TK, Chapman ML, Farrar JD, Arthun D. Comparison of in vivo cholinesterase inhibition in neonatal and adult rats by three organophosphorothioate insecticides. Toxicology. 1991;68:51–61. doi: 10.1016/0300-483x(91)90061-5. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Chambers JE. Effects of repeated oral postnatal exposure to chlorpyrifos on cholinergic neurochemistry in developing rats. Toxicol. Sci. 2005;84:352–359. doi: 10.1093/toxsci/kfi081. [DOI] [PubMed] [Google Scholar]
- Salvi RM, Lara DR, Ghisolfi ES, Portela LV, Dias RD, Souza DO. Neuropsychiatric evaluation in subjects chronically exposed to organophosphate pesticides. Toxicol. Sci. 2003;72:267–271. doi: 10.1093/toxsci/kfg034. [DOI] [PubMed] [Google Scholar]
- Santos NC, Figueira-Coelho J, Martins-Silva J, Saldanha C. Multidisciplinary utilization of dimethyl sulfoxide: Pharmacological, cellular, and molecular aspects. Biochem. Pharmacol. 2003;65:1035–1041. doi: 10.1016/s0006-2952(03)00002-9. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Developmental exposure to terbutaline and chlorpyrifos, separately or sequentially, elicits presynaptic serotonergic hyperactivity in juvenile and adolescent rats. Brain Res. Bull. 2007;73:301–309. doi: 10.1016/j.brainresbull.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Dick RB, Howell RJ, Chrislip DW, Hines CJ, Reid TM, Lehman E, Laber P, Krieg EF, Jr, Knott C. Neurologic function among termiticide applicators exposed to chlorpyrifos. Environ. Health Perspect. 2000;108:293–300. doi: 10.1289/ehp.00108293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Carr RL, Chambers JE. Changes in rat brain acetylcholinesterase activity and muscarinic receptor density during and after repeated oral exposure to chlorpyrifos in early postnatal development. Toxicol. Sci. 1999;51:265–272. doi: 10.1093/toxsci/51.2.265. [DOI] [PubMed] [Google Scholar]
- Timchalk C, Poet TS, Kousba AA. Age-dependent pharmacokinetic and pharmacodynamic response in preweanling rats following oral exposure to the organophosphorus insecticide chlorpyrifos. Toxicology. 2006;220:13–25. doi: 10.1016/j.tox.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Watts JA, Hoogmoed RP. Dimethyl sulfoxide: Inhibition of acetylcholinesterase in the mammalian heart. Biochem. Pharmacol. 1984;33:365–369. doi: 10.1016/0006-2952(84)90227-2. [DOI] [PubMed] [Google Scholar]
- Yellowlees P, Greenfield C, McIntyre N. Dimethylsulphoxide-induced toxicity. Lancet. 1980;2:1004–1006. doi: 10.1016/s0140-6736(80)92158-3. [DOI] [PubMed] [Google Scholar]
- Zhang H, Liu J, Pope CN. Age-related effects of chlorpyrifos on muscarinic receptor-mediated signaling in rat cortex. Arch. Toxicol. 2002;75:676–684. doi: 10.1007/s00204-001-0309-3. [DOI] [PubMed] [Google Scholar]