Abstract
Background
Rett syndrome, a common cause of mental retardation in females, is caused by mutations in the MECP2 gene. Most females with MECP2 mutations fulfil the established clinical criteria for Rett syndrome, but single cases of asymptomatic carriers have been described. It is therefore likely that there are individuals falling between these two extreme phenotypes.
Objective
To describe three patients showing only minor symptoms of Rett syndrome.
Findings
The patient with the best intellectual ability had predominantly psychiatric problems with episodes of uncontrolled aggression that have not been described previously in individuals with MECP2 mutations. All three patients had normal hand function, communicated well, and showed short spells of hyperventilation only under stress. Diagnosis in such individuals requires the identification of subtle signs of Rett syndrome in girls with a mild mental handicap. Analysis of the MECP2 gene revealed mutations that are often found in classical Rett syndrome. Skewed X inactivation was present in all three cases, which may explain the mild phenotype.
Conclusions
Because of skewed X inactivation, the phenotype of Rett patients may be very mild and hardly recognisable.
Keywords: Rett syndrome, mild disease, skewed X inactivation, aggression
Rett syndrome (MIN No 312750) is one of the most common causes of mental retardation in females.1 After a period of normal development that lasts between 8 and 18 months, girls with Rett syndrome show a regression of motor and mental abilities, especially language and hand function.2,3 Other hallmarks of the syndrome are hand stereotypies, episodic breathing abnormalities such as hyperventilation or prolonged apnoea, and a deceleration of head growth. After the period of regression the condition stabilises and the patients may reach old age. In 1999 the first mutations in the MECP2 gene, encoding the methyl‐CpG‐binding protein (MeCP2), were described in patients with Rett syndrome.4 Since then it has been found that most patients carry either point mutations in the coding region or deletions of the gene or parts of it.5 Even before the first mutations in the MECP2 gene were found, some patients with milder phenotypes had been described and called “forme fruste” or preserved speech variant.6 These patients had some residual language but otherwise fulfilled the criteria for classical Rett syndrome. In many of them mutations in the MECP2 gene were found.7 In this article we describe three patients who carry mutations in the MECP2 gene but do not fulfil the criteria for Rett syndrome. However, they are not asymptomatic and they do carry mutations that have been described in patients with classical Rett syndrome. To explain their very mild phenotypes we carried out X inactivation analyses.
Subjects
Patient 1 was born to a healthy family after an uneventful pregnancy. Her motor development was normal; she learned to sit at eight months and to walk at 13 months of age. Her speech development was normal. At the time of examination she had not lost any motor or mental abilities. At 17 years of age she could ride a bicycle, use inline skates, liked to swim, ate with a knife and fork, could read and write, and was capable of doing delicate handicrafts. However, a learning disability was revealed when she started school at the age of six years and as a result of this she could not cope in a regular school. She was currently attending a school for children with learning disabilities. She was able to copy written texts and count, and could do simple addition and substitution sums up to 10. Since she was 13, she had been prone to episodes of uncontrolled aggression. During these episodes, she screams for hours, hyperventilates, hits the wall with her hands, and becomes aggressive towards others. On physical examination she showed hyperventilation and hand stereotypies only under stress. Her head circumference (53 cm, 10th to 25th centile) and height were age appropriate, but her feet were relatively small. Her EEG was normal and she has never had seizures.
Patient 2 was the first child of healthy parents. She learned to walk at 12 months and spoke her first words at 13 months. Her speech development was unusually fast and at the age of two years she had a large vocabulary, sang songs, and counted up to 10. At the start of her third year of life she suddenly lost interest in social contact, spoke only single words, and her hand function deteriorated. After one year this autistic‐like phase ended. She regained the ability to speak in full and long sentences and her hand function improved. At the time of examination at the age of nine years she was able to communicate well, follow instructions, count up to 20, and liked to sing German and English songs. She could run, climb stairs, ride a scooter, and swim. She also ate and drank independently. On examination she showed short phases of hyperventilation and hand stereotypies. She had had one seizure at the age of seven years but her EEG was normal.
Patient 3 was born in the 42nd gestational week after an uneventful pregnancy. Her early motor development was normal but she did not walk until 19 months. At the age of six she lost her ability to run and started to walk with a slightly outwardly rotated right foot. Her fine motor abilities were very good for the first four years of life, but thereafter a decline in her drawing abilities was noted. Her language development started remarkably early. She spoke her first words at 10 months and full sentences at the age of two years. At age six years her vocabulary declined and she used shorter sentences. At the time of examination, at the age of 11 years, she showed preserved hand function, was able to eat with a knife and fork, and drank from a glass. She was still able to communicate well and could read short written texts. On examination she followed instructions and answered simple questions. Hand stereotypies and short phases of hyperventilation occurred only occasionally. Her head circumference was 56.3 cm (>97th centile).
Methods
Mutation analysis of the MECP2 gene
The entire coding region and flanking intron sequences were sequenced using the method described earlier.8
X inactivation studies
Patterns of X inactivation were determined in peripheral blood leucocytes by polymerase chain reaction analysis of a polymorphic CAG repeat in the first exon of the human androgen receptor gene (AR) as previously described (HUMARA assay).9 X inactivation was considered significantly skewed if the ratio exceeded 75:25.10
Results
Mutation analysis of the MECP2 gene
The sequence analysis of the MECP2 gene showed mutations in the coding region in all three patients: In patient 1 the deletion c.1164–1207del was detected. It is located within the deletion hot spot at the 3′ end of the gene. In patient 2 the missense mutation c.916C→T (R306C) was found. This mutation is frequently found in classical Rett syndrome patients and is located in the transcriptional repression domain. The missense mutation c.674C→G (P225R) revealed in patient 3 had also been described earlier (IRSA MECP2 variation database, http://mecp2.chw.edu.au/).
X inactivation studies
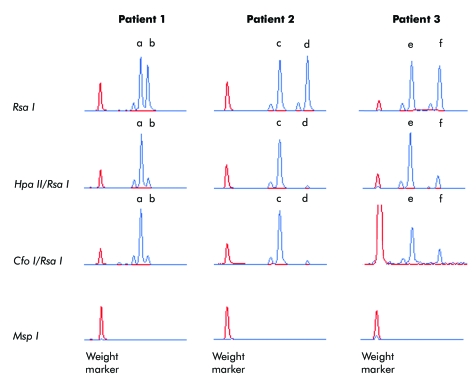
X inactivation analyses were undertaken in leucocytes and showed skewing in all three patients (fig 1). In patient 1 the X inactivation ratio was 84:16, in patient 2 had a ratio of 95:5, and patient 3, a ratio 76:24.
Figure 1 Skewed X inactivation in three patients with very mild phenotypes of Rett syndrome. Patterns of X inactivation were determined by polymerase chain reaction analysis of a polymorphic CAG repeat in the first exon of the human androgen receptor gene (AR). For each patient, four DNA aliquots were incubated with two methylation sensitive restriction enzymes (Hpa II and Cfo I), and two control enzymes (Rsa I and Msp I). Hpa II and Msp I show identical recognition sites but cleavage of the latter is unaffected by methylation of DNA. Therefore, the Msp I digest was used to ensure the complete digestion of genomic DNA. Rsa I was used to reduce a preferential amplification of shorter alleles. Patient 1 shows two different AR alleles (a and b) amplified from DNA predigested with Rsa I indicating the carrier's heterozygosity for the AR repeat (panel 1). Predigestion with the methylation sensitive restriction enzymes Hpa II (panel 2) and Cfo I (panel 3) revealed skewing of X inactivation (84:16) with a preferential inactivation of allele a. After predigestion with the restriction enzyme Msp I, no AR alleles can be amplified (panel 4) assuring that all genomic DNA was cut. Patient 2 was found to be heterozygous for the AR alleles c and d (panel 1). She showed skewing of X inactivation (95:5) with the AR allele d being active in most of her cells (panels 2 and 3). Patient 3 was heterozygous for the AR alleles e and f (panel 1). She showed skewing of X inactivation (76:24) with a preferential inactivation of AR allele e (panels 2 and 3).
Discussion
In this report we describe three girls with a very mild form of Rett syndrome. None of them met the established diagnostic criteria for classical Rett syndrome. These patients represent the link between classical Rett syndrome and asymptomatic carriers for mutations in the MECP2 gene. Patient 1, who was the least affected, had predominantly psychiatric problems, with uncontrolled aggression since her early teenage years. So far, she had not shown any signs of developmental regression. The only recognisable signs of Rett syndrome were occasional hand stereotypies and hyperventilation that appeared under stress. Patient 2 did show a phase of developmental regression in her past and during that phase she also showed autistic‐like features comparable to patients with Rett syndrome. However, at the time of writing she was able to communicate well and had normal hand function and gait. Like patient 1 she presented some hand stereotypies and had a tendency to hyperventilate only under stress. Patient 3 highlighted the importance of taking a detailed history in order to diagnose very mild cases of Rett syndrome. Her history showed only subtle signs of developmental regression, which occurred relatively late. On examination at the time of diagnosis she showed hand stereotypies infrequently, and otherwise her phenotype did not suggest Rett syndrome as the diagnosis.
The three mutations in the MECP2 gene found in our patients have been described previously in numerous patients with classical Rett syndrome (IRSA MECP2 variation database, http://mecp2.chw.edu.au/). Several studies on genotype/phenotype correlation in Rett syndrome have shown an influence of the type and location of mutations in MECP2 on the phenotype but this only accounts for differences within classical Rett syndrome.11,12,13,14 Patient 1 and 2 carry a late deletion and a missense mutation, respectively, that are associated with mild phenotypes but this does not explain the extremely mild phenotype in our patients. As Rett syndrome is an X linked trait and the MECP2 locus is subject to X inactivation different patterns of X inactivation may lead to different phenotypes within a group of patients who carry the same mutation.15 In order to explain the very mild Rett syndrome phenotype in our patients we analysed their patterns of X inactivation and found skewing in all three of them. Several studies have addressed the influence of X inactivation on the Rett syndrome phenotype. Most of these studies could not show a statistically significant influence on the phenotype.16,17,18 However, other investigators have described single cases with mild phenotypes of classical Rett syndrome and skewed X inactivation.12 Ishii and co‐workers reported a monozygotic twin pair with Rett syndrome in whom the milder phenotype was associated with skewing of X inactivation.19 Further evidence for an influence of the X inactivation on the severity of the phenotype came from the description of asymptomatic females carriers for MECP2 mutations with skewed X inactivation who have daughters with classical Rett syndrome.12,20,21 Studies in Mecp2−/+ mice showed a preferential expression of the Mecp2 wild type alleles in neurones, indicating a selective survival of these cells.22,23 The knock‐out mice with a larger percentage of neurones with an inactivated mutated Mecp2 allele were the least affected. Based on these data the authors speculated that there might be a group of Rett syndrome patients with milder phenotypes owing to skewed X inactivation, who have not so far been identified because of their atypical phenotypes.23 Our study describing three barely recognisable and extremely mild cases with confirmed skewed X inactivation supports this hypothesis. Nonetheless, in the majority of Rett syndrome patients X inactivation seems to be balanced, and the clinical phenotype in these patients is determined by the type and location of the MECP2 mutations.
In conclusion, our study extends the currently growing clinical spectrum of Rett syndrome by adding females with a very mild disease form who have skewed X inactivation and do not fulfil the established clinical criteria for Rett syndrome. These very mild cases may communicate well and have normal hand function and age appropriate motor skills. Psychiatric problems and subtle signs of Rett syndrome such as hand stereotypies, hyperventilation episodes provoked by stress, and a past period of developmental regression at some point in childhood may be clues to diagnosis.
Acknowledgement
Grand sponsor: the German Research Foundation, grant number HU 941/2‐1.
Footnotes
Conflicts of interest: none declared
References
- 1.Hagberg B. Rett's syndrome: prevalence and impact on progressive severe mental retardation in girls. Acta Paediatr Scand 198574405–408. [DOI] [PubMed] [Google Scholar]
- 2.Hagberg B. Rett syndrome: clinical peculiarities and biological mysteries. Acta Paediatr 199584971–976. [DOI] [PubMed] [Google Scholar]
- 3.Huppke P, Held M, Laccone F, Hanefeld F. The spectrum of phenotypes in females with Rett Syndrome. Brain Dev 200325346–351. [DOI] [PubMed] [Google Scholar]
- 4.Amir R E, Van den Veyver I B, Wan M, Tran C Q, Francke U, Zoghbi H Y. Rett syndrome is caused by mutations in X‐linked MECP2, encoding methyl‐CpG‐binding protein 2. Nat Genet 199923185–188. [DOI] [PubMed] [Google Scholar]
- 5.Huppke P, Gartner J. Molecular diagnosis of Rett syndrome. J Child Neurol 200520732–736. [DOI] [PubMed] [Google Scholar]
- 6.Hagberg B A, Skjeldal O H. Rett variants: a suggested model for inclusion criteria. Pediatr Neurol 1994115–11. [DOI] [PubMed] [Google Scholar]
- 7.Zappella M, Meloni I, Longo I, Hayek G, Renieri A. Preserved speech variants of the Rett syndrome: molecular and clinical analysis. Am J Med Genet 200110414–22. [DOI] [PubMed] [Google Scholar]
- 8.Huppke P, Laccone F, Kramer N, Engel W, Hanefeld F. Rett syndrome: analysis of MECP2 and clinical characterization of 31 patients. Hum Mol Genet 200091369–1375. [DOI] [PubMed] [Google Scholar]
- 9.Maier E M, Kammerer S, Muntau A C, Wichers M, Braun A, Roscher A A. Symptoms in carriers of adrenoleukodystrophy relate to skewed X inactivation. Ann Neurol 200252683–688. [DOI] [PubMed] [Google Scholar]
- 10.Racchi O, Mangerini R, Rapezzi D, Rolfo M, Gaetani G F, Ferraris A M. X chromosome inactivation patterns in normal females. Blood Cells Mol Dis 199824439–447. [DOI] [PubMed] [Google Scholar]
- 11.Cheadle J P, Gill H, Fleming N, Maynard J, Kerr A, Leonard H, Krawczak M, Cooper D N, Lynch S, Thomas N, Hughes H, Hulten M, Ravine D, Sampson J R, Clarke A. Long‐read sequence analysis of the MECP2 gene in Rett syndrome patients: correlation of disease severity with mutation type and location. Hum Mol Genet 200091119–1129. [DOI] [PubMed] [Google Scholar]
- 12.Hoffbuhr K, Devaney J M, LaFleur B, Sirianni N, Scacheri C, Giron J, Schuette J, Innis J, Marino M, Philippart M, Narayanan V, Umansky R, Kronn D, Hoffman E P, Naidu S. MeCP2 mutations in children with and without the phenotype of Rett syndrome. Neurology 2001561486–1495. [DOI] [PubMed] [Google Scholar]
- 13.Huppke P, Held M, Hanefeld F, Engel W, Laccone F. Influence of mutation type and location on phenotype in 123 patients with Rett syndrome. Neuropediatrics 20023363–68. [DOI] [PubMed] [Google Scholar]
- 14.Schanen C, Houwink E J, Dorrani N, Lane J, Everett R, Feng A, Cantor R M, Percy A. Phenotypic manifestations of MECP2 mutations in classical and atypical Rett syndrome. Am J Med Genet 2004126A129–140. [DOI] [PubMed] [Google Scholar]
- 15.D'Esposito M, Quaderi N A, Ciccodicola A, Bruni P, Esposito T, D'Urso M, Brown S D. Isolation, physical mapping, and northern analysis of the X‐linked human gene encoding methyl CpG‐binding protein, MECP2. Mamm Genome 19967533–535. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen J B, Henriksen K F, Hansen C, Silahtaroglu A, Schwartz M, Tommerup N. MECP2 mutations in Danish patients with Rett syndrome: high frequency of mutations but no consistent correlations with clinical severity or with the X chromosome inactivation pattern. Eur J Hum Genet 20019178–184. [DOI] [PubMed] [Google Scholar]
- 17.Auranen M, Vanhala R, Vosman M, Levander M, Varilo T, Hietala M, Riikonen R, Peltonen L, Jarvela I. MECP2 gene analysis in classical Rett syndrome and in patients with Rett‐like features. Neurology 200156611–617. [DOI] [PubMed] [Google Scholar]
- 18.Weaving L S, Williamson S L, Bennetts B, Davis M, Ellaway C J, Leonard H, Thong M K, Delatycki M, Thompson E M, Laing N, Christodoulou J. Effects of MECP2 mutation type, location and X‐inactivation in modulating Rett syndrome phenotype. Am J Med Genet 2003118A103–114. [DOI] [PubMed] [Google Scholar]
- 19.Ishii T, Makita Y, Ogawa A, Amamiya S, Yamamoto M, Miyamoto A, Oki J. The role of different X‐inactivation pattern on the variable clinical phenotype with Rett syndrome. Brain Dev 200123(suppl 1)S161–S164. [DOI] [PubMed] [Google Scholar]
- 20.Sirianni N, Naidu S, Pereira J, Pillotto R F, Hoffman E P. Rett syndrome: confirmation of X‐linked dominant inheritance, and localization of the gene to Xq28. Am J Hum Genet 1998631552–1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villard L, Kpebe A, Cardoso C, Chelly P J, Tardieu P M, Fontes M. Two affected boys in a Rett syndrome family: clinical and molecular findings. Neurology 2000551188–1193. [DOI] [PubMed] [Google Scholar]
- 22.Braunschweig D, Simcox T, Samaco R C, LaSalle J M. X‐Chromosome inactivation ratios affect wild‐type MeCP2 expression within mosaic Rett syndrome and Mecp2−/+ mouse brain. Hum Mol Genet 2004131275–1286. [DOI] [PubMed] [Google Scholar]
- 23.Young J I, Zoghbi H Y. X‐chromosome inactivation patterns are unbalanced and affect the phenotypic outcome in a mouse model of Rett syndrome. Am J Hum Genet 200474511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]