Abstract
Neurofibromatosis 1 (NF1) is a tumour suppressor gene syndrome characterized by multiple cutaneous and plexiform neurofibromas. Focal osseous abnormalities, short stature, and decreased bone mineral density are also frequent in people with NF1. We measured serum 25‐hydroxyvitamin D concentrations in 55 patients with NF1 and 58 healthy controls, and correlated the findings in the patients with NF1 with their estimated number of dermal neurofibromas. Geometric mean (SD) serum 25‐hydroxyvitamin D concentration was 14.0 (1.6) ng/mL among the patients with NF1 compared with 31.4 (1.7) ng/mL among healthy controls (p<<0.0001). The serum vitamin D concentration and number of dermal neurofibromas reported by patients with NF1 were inversely correlated (Spearman's ρ = −0.572, p<0.00001). The occurrence of low serum vitamin D concentrations in people with NF1, especially those with many dermal neurofibromas, may provide new pathogenic insights and have important therapeutic implications.
Keywords: neurofibromatosis 1, vitamin D, neurofibroma
Neurofibromatosis 1 (NF1) is an autosomal dominant disease caused by mutations of the NF1 gene on chromosome 17.1,2,3 Multiple café au lait spots and cutaneous neurofibromas occur in almost all affected individuals. Less frequent but more serious manifestations include plexiform neurofibromas, central nervous system gliomas, malignant peripheral nerve sheath tumours (MPNSTs), and NF1 vasculopathy.4,5 At least 10% of people with NF1 have characteristic focal bony abnormalities such as sphenoid wing lesions, dystrophic scoliosis, or tibial pseudarthrosis. In addition, people with NF1 frequently have a generalized skeletal abnormality, evidenced by mild short stature6 and decreased bone mineral density.7,8,9,10
Vitamin D regulates calcium homeostasis and plays a key role in bone metabolism.11 Patients with NF1 with severe osteomalacia respond favourably to vitamin D therapy,12 and topical vitamin D treatment has been reported to reduce the pigmentation in café au lait spots, one of the cardinal features of NF1.13 We measured serum 25‐hydroxyvitamin D concentrations in a series of patients with NF1 and assessed the relationship of these concentrations to the number of dermal neurofibromas reported by the patients.
METHODS
This study was approved by the ethics committee of Hamburg University. We measured serum 25‐hydroxyvitamin D concentrations in 69 unselected adults with NF1 diagnosed by standard clinical criteria.14 For comparison, we also measured serum 25‐hydroxyvitamin D levels in 58 apparently healthy employees of the testing laboratory. After assessment by the laboratory medical officer, fasting blood samples were taken and stored at −80°C until analysis. Individuals with diseases of the gastrointestinal tract, liver, kidneys, parathyroid glands, or skin which can affect vitamin D metabolism were excluded from both groups, as were individuals known to be taking supplemental vitamin D or to have unusually high ultraviolet light exposure. Serum concentrations of 25‐hydroxyvitamin D were measured by an automated chemiluminescence protein binding assay (Nichols Institute Diagnostics, San Clemente, CA, USA). Data on the crossreactivity of the vitamin D binding protein, based on spike and recovery studies, revealed an equimolar 100% recovery of 25‐hydroxyvitamin D3 and 25‐hydroxyvitamin D2. Although some patient samples containing 25‐hydroxyvitamin D2 may exhibit reduced recovery with this assay, vitamin D2 originates entirely from dietary sources, and none of the tested control subjects or patients with NF1 was receiving vitamin D2 supplementation.
All 58 control subjects and 55 of the 69 patients with NF1 were tested in the autumn or winter. The serum 25‐hydroxyvitamin D concentrations in the other 14 patients with NF1 were measured in the summer. Because serum vitamin D concentrations are known to be substantially higher in summer than in winter,15 these 14 patients with NF1 were excluded from the analyses. However, the results were very similar if the14 patients tested in the summer were included and the analysis was adjusted for the season of the year in which the serum was obtained.
Of the 55 patients with NF1 included in the present study, 45 (61 of the total 69 patients with NF1 tested) completed a questionnaire regarding the approximate number of skin tumours present, and this estimate agreed well with the number counted by one of us (ML) on physical examination of 19 of the patients.
Statistical analysis
Geometric mean serum vitamin D concentrations in patients with NF1 and controls were compared using Student's t test. Discrete comparisons were assessed with χ2 test. Correlations between number of dermal neurofibromas (categorized as 10–99, 100–999, or ⩾1000) and the log serum hydroxyvitamin D concentration were tested with Spearman's ρ. The geometric mean vitamin D concentration in patients with NF1 with different numbers of neurofibromas were compared using analysis of variance. Again, inclusion of the patients with NF1 whose sera were collected in the summer for vitamin D measurement produced very similar results if the analysis was adjusted for seasonality.
RESULTS
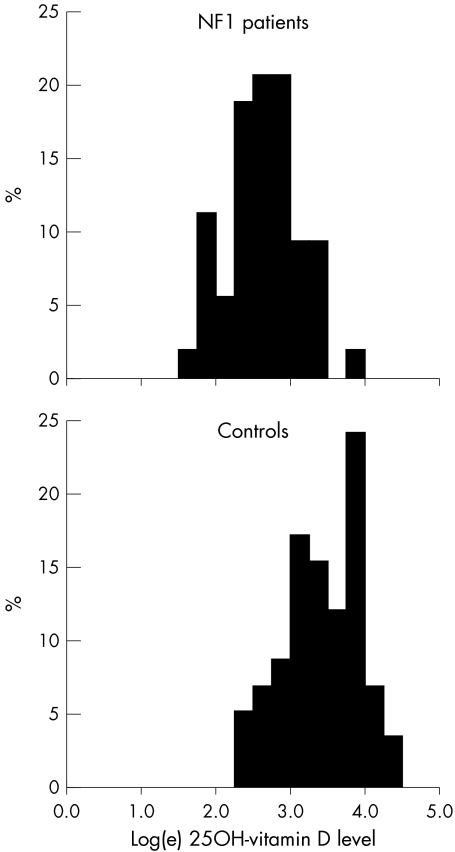
Table 1 compares the findings in 55 patients with NF1 and 58 controls whose serum 25‐hydroxyvitamin D concentrations were measured in autumn or winter. The groups were similar for sex ratio, but the patients with NF1 were, on average, about 4 years older than the controls. The distribution of serum 25‐hydroxyvitamin D concentrations was log normal (fig 1), and age was not related to the serum vitamin D concentration in either the patients or controls (data not shown). The geometric mean (SD) in patients with NF1 (14.0 (1.6) ng/mL) was much lower than that in controls (31.4 (1.7) ng/mL) (table 1). Nearly three quarters (72%) of the patients with NF1 but only 21% of the healthy comparison group had serum 25‐hydroxyvitamin D concentrations <20 ng/mL, which is considered to be the minimum requirement for normal physiological function.11,16 These differences are very highly statistically significant (table 1).
Table 1 Comparison of findings in patients with NF1 and controls.
| Patients | Controls | Statistical test of difference | ||||
|---|---|---|---|---|---|---|
| Number studied | 55 | 58 | ||||
| Age in years (mean (SD)) | 40.3 (10.4) | 36.0 (8.8) | t111 = 2.38, p = 0.019 | |||
| Female:male ratio | 33:22 | 38:20 | NS | |||
| Vitamin D* (ng/mL) | ||||||
| Mean (SD) | 15.7 (7.5) | 35.5 (17.1) | t111 = 7.91, p<<0.0001 | |||
| Geometric mean (SD) | 14.0 (1.6) | 31.4 (1.7) | t111 = 8.66, p<<0.0001 | |||
| Proportion <20 ng/mL | 72% | 21% | χ2 = 30.8, p<<0.0001 |
*Serum 25‐hydroxyvitamin D concentration. NS, not significant.
Figure 1 Distribution of 25‐hydroxyvitamin D concentrations in the sera of 55 NF1 patients and 58 healthy control subjects.
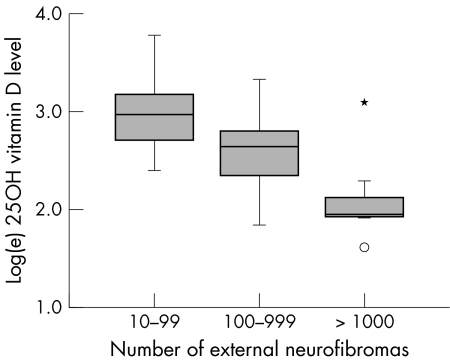
The estimated number of dermal neurofibromas in the 47 patients with NF1 who reported this information ranged from 14 to “more than a thousand”. For analysis, these estimates were converted into orders of magnitude: 10–99, 100–999, and ⩾1000. The number of dermal neurofibromas was associated with age, as expected (table 2). We also observed a very strong and highly significant inverse correlation between the serum vitamin D concentration and the number of dermal neurofibromas reported by the patients with NF1 (fig 2) (Spearman's ρ = −0.572, p<0.00001). The geometric mean serum vitamin D concentrations of the three groups (10–99, 100–999, and ⩾1000 external neurofibromas) were very significantly different after adjustment for age (p = 0.0003, table 2).
Table 2 Comparison of findings among patients with NF1 with different estimated numbers of dermal neurofibromas.
| Number of external neurofibromas | ||||||
|---|---|---|---|---|---|---|
| 10–99 | 100–999 | ⩾1000 | ||||
| Number of patients | 16 | 24 | 7 | |||
| Age in years (mean (SD)) | 37.2 (10.7) | 43.2 (7.5) | 50.4 (7.8) | |||
| Statistical significance | F2,44 = 5.7, p = 0.006 | |||||
| Vitamin D* (ng/mL) | ||||||
| Geometric mean (SD) | 19.5 (1.4) | 13.1 (1.5) | 8.2 (1.6) | |||
| Statistical significance | F2,44 = 12.7, p<0.0001 (unadjusted) | |||||
| F2,43 = 9.7, p = 0.0003 (adjusted for age) | ||||||
| Proportion <20 ng/mL | 9/16 (56%) | 20/24 (83%) | 6/7 (86%) | |||
*Serum 25‐hydroxyvitamin D concentration.
Figure 2 Association between serum 25‐hydroxyvitamin D concentration and estimated number of dermal neurofibromas in 47 patients with NF1. In the box plots, the median is shown as a solid horizontal line, the 25th and 75th centiles define the upper and lower limits of the box, and the distance between them (that is, the height of the box) is the interquartile distance. The whiskers show the most extreme value that is less than 1.5 times the interquartile distance above and below the box. Outliers, indicated by small circles, lie 1.5–3 times the interquartile distance above or below the box, while extreme values, indicated by *, lie more than 3 times the interquartile distance above or below the box.
DISCUSSION
Our observation of much lower serum vitamin D concentrations in patients with NF1 than controls is consistent with, and may be pathogenically related to, the decreased bone mineral density that we and others have observed among people with NF1.7,8,9,10 Osteomalacia and associated low serum vitamin D concentrations have previously been described in people with NF1 in case reports and small clinical series,12 but the frequency with which this occurs has not been determined. The fact that patients with NF1 and osteomalacia respond well to treatment with pharmacological doses of vitamin D raises the possibility that similar treatment may help to prevent the development of osteomalacia and osteoporosis in people with NF1. Other interventions such as increased weight bearing exercise may also be beneficial.
We measured vitamin D as the serum 25‐hydroxyvitamin D concentration. Although 1,25‐dihydroxyvitamin D is the active form, its measurement is not useful in determining vitamin D status.16 The major circulating form, 25‐hydroxyvitamin D, is converted to active 1,25‐dihydroxyvitamin D by the kidney. When vitamin D deficiency occurs, parathyroid hormone secretion increases, which in turn increases renal 1‐α‐hydroxylase activity, converting more 25‐hydroxyvitamin D to 1,25‐dihydroxyvitamin D. Sunlight induced cutaneous vitamin D synthesis can produce all of the vitamin D needed to maintain normal physiological functions.11 We do not know why the serum vitamin D concentrations in people with NF1 are low. We are not aware of any studies of vitamin D intake, absorption, synthesis, transport, or catabolism in people with NF1. Thus this issue needs to be investigated.
The strong correlation we observed between the number of skin neurofibromas and serum vitamin D concentrations was unexpected. It is possible that patients with NF1 with multiple dermal neurofibromas are more likely to cover their skin and thus receive less sunlight than patients with NF1 who have fewer dermal tumours. None of the subjects we studied was reclusive, and none habitually wore a scarf over the head or face. We do not have information on the amount of cutaneous hyperpigmentation in the patients studied, but this is not usually severe in people with NF1.
Our analysis was limited to patients tested in the autumn or winter, so a major difference in sun exposure seems unlikely to account for the very large effect we observed. It seems more likely that the presence of large numbers of neurofibromas causes vitamin D deficiency in patients with NF1 in some other manner, that adequate serum concentrations of vitamin D inhibit the development of neurofibromas, that low serum vitamin D concentrations promote the development of neurofibromas, or that both low serum vitamin D concentration and large numbers of neurofibromas are related to NF1 haploinsufficiency in some other way.
Although cutaneous neurofibromas may occur in very large numbers in people with NF1, these benign tumours very rarely, if ever, undergo transformation to MPNSTs. In contrast, plexiform neurofibromas may give rise to MPNSTs, which occur in about 10% of patients with NF117 and are a frequent cause of premature death.18,19 Plexiform neurofibromas occur in at least 40% of adults with NF120,21 but often are not apparent without imaging studies. We do not know whether the development of plexiform neurofibromas or MPNSTs is associated with low serum vitamin D concentrations in people with NF1.
Several molecular mechanisms might be responsible for the association of low serum vitamin D levels and the occurrence of neurofibromas in people with NF1. Neurofibromin, the protein product of the NF1 gene, is a negative regulator of the Ras signal transduction pathway,22,23 which is involved in normal development and maintenance of bone.24,25 Vitamin D treatment inhibits the growth of some cell lines in vitro, but this effect can be blocked through activation of the Ras signalling pathway,26 which occurs as a result of NF1 haploinsufficiency. Alternatively, vitamin D and neurofibromin could interact at the level of cellular proliferation; reduction in the normal antiproliferative and proapoptotic effects of vitamin D27 in patients with NF1 and vitamin deficiency might foster the tendency to tumour development associated with neurofibromin haploinsufficiency.
In any case, our findings provide a new perspective on the pathogenesis of NF1. Systematic studies of vitamin D metabolism and its relationship to bone mineral density, osteoporosis, focal osseous lesions, and tumour development in people with NF1 are clearly needed. There are also obvious clinical implications; we need to explore the possibility that treatment with vitamin D may inhibit tumour development as well as improving bone mineralization in patients with NF1.
Abbreviations
MPNSTs - malignant peripheral nerve sheath tumours
NF1 - neurofibromatosis 1
Footnotes
Competing interests: there are no competing interests.
References
- 1.Cawthon R M, Weiss R, Xu G F, Viskochil D, Culver M, Stevens J, Robertson M, Dunn D, Gesteland R, O'Connell P, White R. A major segment of neurofibromatosis type 1 gene: cDNA sequence, genomic structure and point mutations. Cell 199062193–201. [DOI] [PubMed] [Google Scholar]
- 2.Viskochil D, Buchberg A M, Xu G, Cawthon R M, Stevens J, Wolff R K, Culver M, Carey J C, Copeland N G, Jenkins N A, White R, O'Connell P. Deletions and translocations interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell 199062187–192. [DOI] [PubMed] [Google Scholar]
- 3.Wallace M R, Marchuk D A, Andersen L B, Letcher R, Odeh H M, Saulino A M, Fountain J W, Brereton A, Nicholson J, Mitchell A L, Brownstein B H, Collins F S. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science 1990249181–186. [DOI] [PubMed] [Google Scholar]
- 4.Huson S. Neurofibromatosis 1: a clinical and genetical overview. In Huson S, Hughes R, eds. The neurofibromatoses:a pathogenetic and clinical overview. London Chapman and Hall Medical, 1994160–203.
- 5.Friedman J M, Gutmann D H, MacCollin M, Riccardi V M. eds. Neurofibromatosis: phenotype, natural history, and pathogenesis. Baltimore: Johns Hopkins University Press, 1999
- 6.Szudek J, Birch P, Friedman J. Growth in North American white children with neurofibromatosis 1 (NF1). J Med Genet 200037933–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Illes T, Halmai V, de Jonge T, Dubousset J. Decreased bone mineral density in neurofibromatosis‐1 patients with spinal deformities. Osteoporosis Int 200112823–827. [DOI] [PubMed] [Google Scholar]
- 8.Brunetti‐Pierri N, Phan K, Carter S, Lewis R A, Plon S, Ellis K J, O'Brian S E, Lee B. Generalized osteopenia in neurofibromatosis type 1 patients points to an underlying disorder of skeletal homeostasis and mineralization. Am J Hum Genet200373(suppl)s261 [Google Scholar]
- 9.Kuorilehto T, Poyhonen M, Bloigu R, Heikkinen J, Vaananen K, Peltonen J. Decreased bone mineral density and content in neurofibromatosis type 1: lowest local values are located in the load‐carrying parts of the body. Osteoporos Int 200416928–936. [DOI] [PubMed] [Google Scholar]
- 10.Lammert M, Kappler M, Mautner V F, Lammert K, Storkel S, Friedman J M, Atkins D. Decreased bone mineral density in patients with neurofibromatosis 1. Osteoporos Int 2005161161–1166. [DOI] [PubMed] [Google Scholar]
- 11.Holick M. Sunlight and vitamin D for bone health and prevention of autoimmune disease, cancers and cardiovascular disease. Am J Clin Nutr 200480(suppl)1678–88S. [DOI] [PubMed] [Google Scholar]
- 12.Konishi K, Nakamura M, Yamakawa H, Suzuki H, Saruta T, Hanaoka H, Davatchi F. Hypophosphatemic osteomalacia in von Recklinghausen neurofibromatosis. Am J Med Sci 1991301322–328. [DOI] [PubMed] [Google Scholar]
- 13.Nakayama J, Kiryu H, Urabe K, Matsuo S, Shibata S, Koga T, Furue M. Vitamin D3 analogues improve cafe au lait spots in patients with von Recklinghausen's disease: experimental and clinical studies. Eur J Dermatol 19999202–206. [PubMed] [Google Scholar]
- 14.Gutmann D H, Aylsworth A, Carey J C, Korf B, Marks J, Pyeritz R E, Rubenstein A, Viskochil D. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA 199727851–57. [PubMed] [Google Scholar]
- 15.Calvo M, Whiting S. Prevalence of vitamin D insufficiency in Canada and the United States: importance to health status and efficacy of current food fortification and dietary supplement use. Nutr Rev 200361107–113. [DOI] [PubMed] [Google Scholar]
- 16.Holick M F. Vitamin D: important for prevention of osteoporosis, cardiovascular heart disease, type 1 diabetes, autoimmune diseases, and some cancers. South Med J 2005981024–1027. [DOI] [PubMed] [Google Scholar]
- 17.Evans D G, Baser M E, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet 200239311–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zoller M, Rembeck B, Akesson H O, Angervall L. Life expectancy, mortality and prognostic factors in neurofibromatosis type 1. A twelve‐year follow‐up of an epidemiological study in Goteborg, Sweden. Acta Derm Venereol 199575136–140. [DOI] [PubMed] [Google Scholar]
- 19.Rasmussen S, Yang Q, Friedman J. Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am J Hum Genet 2001681110–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tonsgard J H, Kwak S M, Short M P, Dachman A H. CT imaging in adults with neurofibromatosis‐1: frequent asymptomatic plexiform lesions. Neurology 1998501755–1760. [DOI] [PubMed] [Google Scholar]
- 21.Thakkar S, Feigen U, Mautner V. Spinal tumours in neurofibromatosis type 1: an MRI study of frequency, multiplicity and variety. Neuroradiology 199941625–629. [DOI] [PubMed] [Google Scholar]
- 22.Cichowski K, Jacks T. NF1 tumor suppressor gene function: narrowing the GAP. Cell 2001104593–604. [DOI] [PubMed] [Google Scholar]
- 23.Dasgupta B, Gutmann D. Neurofibromatosis 1: closing the GAP between mice and men. Curr Opin Genet Dev 20031320–27. [DOI] [PubMed] [Google Scholar]
- 24.Kuorilehto T, Nissinen M, Koivunen J, Benson M D, Peltonen J. NF1 tumor suppressor protein and mRNA in skeletal tissues of developing and adult normal mouse and NF1‐deficient embryos. J Bone Miner Res 200419983–989. [DOI] [PubMed] [Google Scholar]
- 25.Yu X, Chen S, Potter O L, Murthy S M, Li J, Pulcini J M, Ohashi N, Winata T, Everett E T, Ingram D, Clapp W D, Hock J M. Neurofibromin and its inactivation of Ras are prerequisites for osteoblast functioning. Bone 200536793–802. [DOI] [PubMed] [Google Scholar]
- 26.Goltzman D, White J, Kremer R. Studies of the effects of 1,25‐dihydroxyvitamin D on skeletal and calcium homeostasis and on inhibition of tumor cell growth. J Steroid Biochem Mol Biol 20017643–47. [DOI] [PubMed] [Google Scholar]
- 27.Dusso A, Brown A, Slatopolsky E. Vitamin D. Am J Physiol Renal Physiol 2005289F8–28. [DOI] [PubMed] [Google Scholar]