Abstract
Background
Posterior polar cataract is a clinically distinctive opacity located at the back of the lens. It is commonly acquired in age related cataract, and may infrequently occur in pedigrees with congenital cataract. To date, five loci for autosomal dominant congenital posterior polar cataract have been identified. These include two genes, CRYAB and PITX3, on chromosomes 11q and 10q respectively, and three loci with as yet unknown genes on chromosomes 1p, 16q and 20p.
Purpose
To find the chromosomal location of a gene causing autosomal dominant congenital posterior polar cataract in three Moroccan Jewish families.
Methods
A whole genome scan was performed using microsatellite markers spaced at approximately 10 cM intervals. For fine mapping, five additional microsatellite markers were genotyped. Two‐point lod scores were calculated using MLINK software, from the LINKAGE program package. After linkage was established, several positional candidate genes were assessed by PCR based DNA sequencing.
Results
The new cataract locus was mapped to an 11.3 cM interval between D14S980 and D14S1069 on chromosome 14q22‐23. A maximum two point lod score of 5.19 at θ = 0 was obtained with the markersD14S274. The positional and functional candidate genes SIX1, SIX4, SIX6, OTX2, and ARHJ were excluded as the cause of cataract in these families.
Conclusion
An as yet unidentified gene associated with posterior polar cataract maps to the long arm of chromosome 14q22‐23.
Keywords: posterior polar cataract, mapping
Posterior polar cataracts (CTPP; MIM #116600) are opacities at the back of the lens that have a marked effect on visual acuity because of their axial position and proximity to the optical centre of the eye. The slit lamp biomicroscopic appearance of these cataracts may vary, subdividing them further into vacuolar or plaque‐like morphology, and they may also be subdivided regarding posterior polar, subcapsular, or cortical involvement.1,2 However, there is considerable overlap between these subdivisions. Ultrastructural changes seen in CTPP include swelling of the lens fibres, accumulation of granular extracellular material, and the presence of abnormal bladder‐like Wedl cells. These all result in the disruption of normal lens architecture and light transmission properties at the back of the lens.2,3
The prevalence of posterior polar cataracts may vary among different populations and geographic areas.4 CTTP are commonly associated with a variety of systemic and localised ocular conditions.2 Not infrequently, posterior lens opacities can be acquired in adult patients with senility4 or diabetes, and those treated with steroids.5,6 Rarely these lens opacities may accompany monogenic mendelian syndromes such as neurofibromatosis type II7 or retinitis pigmentosa.8 Early development of CTPP may rarely be seen in families, inherited as an autosomal dominant trait. Molecular studies of these families may provide insight into the underling mechanism involved in the more common forms of posterior lens opacities. To date, five loci for autosomal dominant (ad) CTPP have been identified. These include three loci on chromosomes 1p,9 16q,10 and 20p12‐q1211 with as yet unknown genes, and identified mutations in the CRYAB and PITX3 genes.12,13 Cataracts associated with 16q, 20p12‐q12 and PITX3 are described as progressive, whereas cataracts in families with linkage to the 1p locus and the CRYAB gene mutation do not progress to other regions of the lens with age. Despite these advances, our understanding of the mechanism underlying CTPP remains poor.14
In this study, we have mapped a novel locus for autosomal dominant posterior polar cataract in three Moroccan Jewish families to chromosome 14q22‐23, and we also report our initial evaluation of candidate genes within this region.
METHODS
Families and DNA specimens
Three Moroccan Jewish families with autosomal dominant posterior polar cataract (56021A/B and 56031) were recruited at Sapir Medical Center, Kfar Saba, and the Kaplan Medical Center, Rehovot, Israel (fig 1). A detailed family history obtained from older family members could not establish a connection between the different family founders. Interestingly, affected siblings 5602107 and 5602108 from the two branches of family 56021A and 56021B were married, and had three affected offspring who could have inherited the disease allele from either or both parents.
Figure 1 Family pedigrees and haplotypes. Nine microsatellite marker readings on the long arm of chromosome 14, and the G→T change at position 69 of the second intron of the ARHJ gene (allele 2) are shown. The marker order is shown to the left of each generation. Black bars, disease associated haplotype common to both families; grey bars, alleles for markers not showing recombination but not common to both families; white bars, non‐disease associated alleles; narrow lines, indeterminate inheritance.
Participants gave informed consent to the study protocol, which was approved by the institutional review boards of Sheba Medical centre and the National Eye Institute, and conformed to the tenets of the Declaration of Helsinki.
In total, 28 family members underwent ophthalmic examinations including slit lamp biomicroscopy, review of medical records, and, where possible, photography of cataracts. Heparinised blood was obtained from each participant for genomic DNA isolation and genotyping.
Genotyping
A genome wide scan using 382 fluorescently labelled microsatellite markers (Linkage Mapping Set MD‐10; Applied Biosystems Inc., Foster City, CA, USA) was performed with samples from family 56021. Multiplex PCR was carried out as previously described.15 PCR products from each DNA sample were pooled and mixed with a loading cocktail (Applied Biosystems), and loading dye, and separated on a 5% denaturating polyacrylamide gel on an automated sequencer (377; Applied Biosystems). The GENSCAN 3.1 and GENOTYPER 2.1 software packages (Applied Biosystems) were used to analyse the alleles. For markers showing lod scores >1.5, fine mapping was carried out using both families 56021 and 56031 with additional closely spaced markers.
Linkage analysis
Two point linkage analysis was performed using the FASTLINK implementation16 of the MLINK program from the LINKAGE program package.17 Maximum lod scores were calculated using ILINK, assuming an autosomal dominant model of inheritance with 100% penetrance in both sexes and a disease gene frequency of 0.0001. The marker order and distances (fig 1, table 1) were taken from the Genethon database (www.genethon.fr/) and the National Center for Biotechnology Information chromosome 14 sequence map (www.ncbi.nlm.nih.gov/mapview/). Equal allele frequencies were assumed for the genome wide screening. Allele frequencies for markers used in fine mapping (table 1) were estimated from an analysis of 30 unrelated and unaffected individuals of Moroccan Jewish ethnicity. Haplotypes were constructed so as to minimise recombination.
Table 1 Two point lod scores for autosomal dominant posterior polar cataract (adCTPP) in families 56021 and 56031 and chromosome 14q markers.
| Marker | Position | Lod scores at theta = | Zmax | Θ | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cM | Mb | 0 | 0.01 | 0.05 | 0.1 | 0.2 | 0.3 | 0.4 | ||||||||||||||
| D14S288 | 39.1 | 41.93 | −∞ | −0.9 | 1.8 | 2.1 | 2.1 | 1.7 | 1 | 2.12 | 0.093 | |||||||||||
| D14S276 | 47 | 54.7 | 4.7 | 4.7 | 4.3 | 3.8 | 3.7 | 2.7 | 1.6 | 4.77 | 0 | |||||||||||
| D14S980 | 50.9 | 56.22 | 3.5 | 3.5 | 3.3 | 2.9 | 2.8 | 2.1 | 1.2 | 3.56 | 0 | |||||||||||
| D14S274 | 53.8 | 56.72 | 5.1 | 5.1 | 4.7 | 4.2 | 4 | 2.9 | 1.7 | 5.19 | 0 | |||||||||||
| D14S1038 | 56.7 | 58.69 | 3.3 | 3.3 | 3 | 2.7 | 2.6 | 1.9 | 1.1 | 3.33 | 0 | |||||||||||
| D14S290 | 58.5 | 62.6 | 3.5 | 3.5 | 3.3 | 2.9 | 2.8 | 2 | 1.2 | 3.59 | 0 | |||||||||||
| D14S63 | 59 | 63.72 | 3.4 | 3.4 | 3.2 | 2.8 | 2.7 | 2 | 1.2 | 3.49 | 0 | |||||||||||
| D14S1069 | 62.2 | 67.45 | −∞ | 1.5 | 2.7 | 2.7 | 2.6 | 2 | 1.2 | 2.79 | 0.051 | |||||||||||
| D14S258 | 65.8 | 69.65 | −∞ | −0.9 | 1.8 | 2.1 | 2.1 | 1.7 | 1 | 2.11 | 0.089 | |||||||||||
Candidate genes analysis
The sine oculis homebox (SIX) genes cluster family, consisting of SIX1 (MIM #601205), SIX4 (MIM #606342), SIX6 (MIM #606326), and the orthodenticle Drosophila homologue 2 gene (OTX2; MIM #600037) are implicated in normal development of the Drosophila visual system18,19,20 and are located within the disease gene interval, in the present families. The Ras homologue gene family member J (ARHJ; MIM #607653) has recently been related with lens cytoskeleton organisation and membrane integrity.21 Each of the coding exons and the flanking DNA sequence of these genes was amplified from genomic DNA of two affected patients and one unaffected individual, and subjected to direct DNA sequencing as previously described.15 Primers and amplification conditions are available upon request.
RESULTS
Phenotype
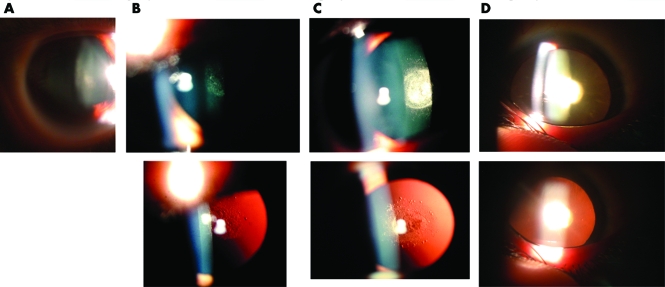
Ophthalmic examination with particular attention to the lens and cataract morphology along with review of available clinical records reveals that the CTPP phenotype shows slow progression with age. The opacity is initially evident at 3–4 years of age as a barely detectable mat reflex of the posterior capsule (fig 2A). This haziness worsens during the following years into a well demarcated 2–3 mm in diameter disc, confined to the posterior lens pole (fig 2B,C). Subsequently, this well defined zone becomes a dense opaque plaque (fig 2D). Although the main pathology is evident at the posterior pole, the process is accompanied with variable pathology in adjacent lens compartments. Scattered cortical white punctuate opacities are evident at early stages (fig 2c). Later, posterior cortical extensions develop and progress to form a pre‐senile nuclear sclerotic cataract by 40–50 years of age (fig 2D). At this time, the best corrected visual acuity does not exceed 20/50. Neither slit lamp biomicroscopy nor sonography show evidence of posterior lenticonus or high myopia, and there is no family history of other ocular or systemic abnormalities. Full recovery of normal visual acuity is the general outcome following a successful cataract extraction surgery.
Figure 2 Slit lamp (top) and retroillumination (bottom) photographs of the adCTPP lens opacities taken from patients 5602125 at (A) 4 and (B) 5 years of age, (B) 5602126 (at 7 years), and (D) 5602108 (at 56 years).
Linkage and haplotype analysis
A genome wide scan carried out using 22 members of family 56021 (fig 1) yielded lod scores <1.5 for all markers, except for a group of four consecutive markers on chromosome 14: D14S288 (Zmax = 1.53 at θ = 0.118), D14S276 (3.64 at θ = 0.0), D14S63 (3.23 at θ = 0.0) and D14S258 (1.73 at θ = 0.107). Fine mapping with calculated allele frequencies, and additional markers from the Genethon database (D14S980, D14S274, D14S1038, D14S290, D14S1069) was further pursued on DNA samples from both families 56021 and 56031. Summed lod scores from the three families for these markers are as listed in table 1. All of these markers show evidence for linkage to this region on chromosome 14, and a maximum lod score of 5.19 at θ = 0.0 was obtained with the marker D14S274. Lod score values of –∞ at θ = 0.0 reflect obligate recombination events.
Visual inspection of the haplotypes cosegregating with CTPP in the families confirmed the linkage data and suggests a common founder for the three families. Telomeric obligate recombination events have taken place in affected individuals 10 of family 56021 between the single nucleotide polymorphism (SNP) rs‐10145453 and D14S258 and in individual 7 of family 56021 between the marker D14S63 and D14S1069, setting the telomeric boundary of the interval at D14S1069. Centromeric obligate recombination events have taken place in affected individuals 2, 25 and 26 of family 56021 between D14S288 and D14S276. A common haplotype is preserved for four consecutive microsatellite markers (D14S274, D14S1038, D14S290, D14S63), and an SNP change in all affected family members (fig 1, black bar). This suggests an historic recombination event between D14S980 and D14S274, thus setting D14S980 as the centeromeric boundary of an 11.3 cM (11.23 Mb) critical interval between D14S980 and D14S1069 (fig 1). Of interest, affected individual 5602111 inherited the disease chromosomes from both parents, thus showing homozygosity for the carrier haplotype. However, we were unable to detect any clinical difference in this patient's disease status compared with patients who had inherited a single disease chromosome.
Sequencing of candidate genes
The D14S980‐D14S1069 interval includes the SIX cluster of genes, the OTX2 gene, and the ARHJ gene, which have been shown to play a significant role in normal development of the lens and Drosophila visual system.18,19,20,21SIX1, SIX4, SIX6, OTX2, and ARHJ were sequenced to search for mutations but no causative mutations were found. Two known polymorphic amino acid changes: histidine to proline at position 584 of the SIX4 protein (SIX4; H584P), and histidine to asparagine at position 141 of the SIX6 protein (SIX6;H141N), and a known G\T SNP at position 69 of intron 2 of ARHJ (provided as accession numbers rs3742636, gi:12230578, and rs10145453 respectively, in the public domain by the National Center for Biotechnology, Bethesda, MD), were identified in both affected and unaffected members of the CTPP families (data not shown).
DISCUSSION
In this study an autosomal dominant posterior polar cataract seen in three Moroccan Jewish families is assigned to a novel locus residing in an 11.3 cM (11.2 Mb) interval on chromosome 14q22‐23. The common haplotype found in these families suggests that the cataract mutation originated as a single mutation in a common founder. Sequencing of five genes within our linkage area on 14q22‐23, including the OTX2 and ARHJ genes and three members of the SIX gene family, shows no disease associated mutations.
This is the sixth genetic locus reported to date for adCTPP. It seems likely that individual 5602111 is homozygous for the disease haplotype. Homozygosity for a dominant allele is rare and often results in a more severe phenotype.22 However, examination and retrospective review of the medical records of this patient showed that she did not have a more severe phenotype than heterozygous affected family members in terms of age of onset, pace of progression, age of surgery, and postoperative visual outcome of 20/20 in both eyes. Therefore, it seems that adCTPP is an example of a true dominant disease in which a single copy of the mutated allele results in the same degree of involvement as in the homozygous state of the mutated gene.
The disease gene region is exceedingly rich in genes, containing more than 70 known or predicted transcripts, none of which is so far a known cataract associated gene. Of the five different loci involved in adCTPP, only the CRYAB gene and the developmental transcription factor PITX3 gene have been identified.12,13 The SIX and OTX2 are thought to interact in a genetic network of transcriptional factors during eye formation18,19,20 and were therefore sequenced. However, no causative mutations were found in any of these genes. Clues for prioritising other candidate genes for further investigation may come from better understanding of the metabolic pathways in which CRYAB is involved. Initially believed to play only a role in refraction of light, αB‐crystallin has since been shown to have numerous properties including extralenticular expression, autokinase activity phosphorylation patterns, chaperone‐like activity, anti‐apoptotic activity, an ability to protect cells against various stresses,23,24,25 and actin polymerisation‐depolymerisation dynamics26,27 by binding to actin filaments.28 Based on these properties, we assessed the involvement of ARHJ21 by sequencing. No pathogenic mutation was identified in the coding regions or in the exon‐intron boundaries. However the possibility of a mutation in the non‐coding regions, a large deletion, or a rearrangement has not been ruled out.
Future steps in trying to identify the new cataract causing gene will include attempts to trace additional families and family members, which may enable further linkage refinement, and sequencing of more candidate genes within this region. These new candidates include the dapper antagonist of beta‐catenin (DACT1) gene (MIM 607861), and the heat shock protein 2 (HSPA2) gene (MIM 140560), both of which may have a potential role in lens homeostasis and development.29,30
The identification of genes and protein products responsible for CTPP may help to define the underlying biochemical abnormalities responsible for both inherited and sporadic forms of this prevalent form of cataract. Defining gene defects that predispose to drug related adult onset forms of CTPP5,6 will help identify individuals at risk for the disease, thus allowing for appropriate treatment and prevention of cataract related blindness.
ACKNOWLEDGEMENTS
We thank the family members for taking part in the study. This work is supported in part by the Claire and Amedee Martier Institute for the Study of Blindness and Visual Disorders, and by the Vladimir Schreiber Fund for Medical Research.
Abbreviations
adCTTP - autosomal dominant posterior polar cataract
ARHJ - Ras homologue gene family member J
CTTP - posterior polar cataract
OTX2 - orthodenticle Drosophila homologue 2
SIX - sine oculis homebox
SNP - single nucleotide polymorphism
Footnotes
Competing interests: there are no competing interests
References
- 1.Amaya L, Taylor D, Russell‐Eggitt I, Nischal K K, Lengyel D. The morphology and natural history of childhood cataracts. Surv Ophthalmol 200348125–144. [DOI] [PubMed] [Google Scholar]
- 2.Eshagian J. Human posterior subcapsular cataracts. Trans Ophthalmol Soc UK 1982102364–368. [PubMed] [Google Scholar]
- 3.Al‐Ghoul K J, Novak L A, Kuszak J R. The structure of posterior subcapsular cataracts in the Royal College of Surgeons (RCS) rats. Exp Eye Res 199867163–177. [DOI] [PubMed] [Google Scholar]
- 4.Klein B E, Klein R, Lee K E. Incidence of age‐related cataract: the Beaver Dam Eye Study. Arch Ophthalmol 1998116219–225. [DOI] [PubMed] [Google Scholar]
- 5.Hennis A, Wu S Y, Nemesure B, Leske M C. Risk factors for incident cortical and posterior subcapsular lens opacities in the Barbados Eye Studies. Arch Ophthalmol 2004122525–530. [DOI] [PubMed] [Google Scholar]
- 6.Skalka H W, Prchal J T. The effect of diabetes mellitus and diabetic therapy on cataract formation. Ophthalmology 198188117–125. [DOI] [PubMed] [Google Scholar]
- 7.Ragge N K, Baser M E, Klein J, Nechiporuk A, Sainz J, Pulst S M, Riccardi V M. Ocular abnormalities in neurofibromatosis 2. Am J Ophthalmol 1995120634–641. [DOI] [PubMed] [Google Scholar]
- 8.Fishman G A, Anderson R J, Lourenco P. Prevalence of posterior subcapsular lens opacities in patients with retinitis pigmentosa. Br J Ophthalmol 198569263–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ionides A C, Berry V, Mackay D S, Moore A T, Bhattacharya S S, Shiels A. A locus for autosomal dominant posterior polar cataract on chromosome 1p. Hum Mol Genet 1997647–51. [DOI] [PubMed] [Google Scholar]
- 10.Richards J, Maumenee I H, Rowe S, Lourien E W. Congenital cataract possibly linked to haptoglobin. Cytogenet Cell Genet 198437570 [Google Scholar]
- 11.Yamada K, Tomita H, Yoshiura K, Kondo S, Wakui K, Fukushima Y, Ikegawa S, Nakamura Y, Amemiya T, Niikawa N. An autosomal dominant posterior polar cataract locus maps to human chromosome 20p12‐q12. Eur J Hum Genet 20008535–539. [DOI] [PubMed] [Google Scholar]
- 12.Berry V, Francis P, Reddy M A, Collyer D, Vithana E, MacKay I, Dawson G, Carey A H, Moore A, Bhattacharya S S, Quinlan R A. Alpha‐B crystallin gene (CRYAB) mutation causes dominant congenital posterior polar cataract in humans. Am J Hum Genet 2001691141–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry V, Yang Z, Addison P K, Francis P J, Ionides A, Karan G, Jiang L, Lin W, Hu J, Yang R, Moore A, Zhang K, Bhattacharya S S. Recurrent 17 bp duplication in PITX3 is primarily associated with posterior polar cataract (CPP4). J Med Genet 200441e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veromann S. Theoretical considerations regarding the study “alpha‐B crystallin gene (CRYAB) mutation causes dominant congenital posterior polar cataract in humans”. Am J Hum Genet 200271684–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pras E, Raz J, Yahalom V, Frydman M, Garzozi H J, Pras E, Hejtmancik J F. A nonsense mutation in the glucosaminyl (N‐acetyl) transferase 2 gene (GCNT2): association with autosomal recessive congenital cataracts. Invest Ophthalmol Vis Sci 2004451940–1945. [DOI] [PubMed] [Google Scholar]
- 16.Schaffer A A, Gupta S K, Shriram K, Cottingham R W. Avoiding recomputation in genetic linkage analysis. Hum Hered 199444225–237. [DOI] [PubMed] [Google Scholar]
- 17.Lathrop G M, Lalouel J M. Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet 198436460–465. [PMC free article] [PubMed] [Google Scholar]
- 18.Gallardo M E, Lopez‐Rios J, Fernaud‐Espinosa I, Granadino B, Sanz R, Ramos C, Ayuso C, Seller M J, Brunner H G, Bovolenta P, Rodriguez de Cordoba S. Genomic cloning and characterization of the human homeobox gene SIX6 reveals a cluster of SIX genes in chromosome 14 and associates SIX6 hemizygosity with bilateral anophthalmia and pituitary anomalies. Genomics 19996182–91. [DOI] [PubMed] [Google Scholar]
- 19.Kawakami K, Sato S, Ozaki H, Ikeda K. Six family genes—structure and function as transcription factors and their roles in development. BioEssays 200022616–626. [DOI] [PubMed] [Google Scholar]
- 20.Zuber M E, Gestri G, Viczian A S, Barsacchi G, Harris W A. Specification of the vertebrate eye by a network of eye field transcription factors. Development 20031305155–5167. [DOI] [PubMed] [Google Scholar]
- 21.Khurana R N, Maddala R L, Shimokawa H, Samuel Zigler J, Epstein D L, Vasantha Rao P. Inhibition of Rho‐kinase induces alphaB‐crystallin expression in lens epithelial cells. Biochem Biophys Res Commun 2002294981–987. [DOI] [PubMed] [Google Scholar]
- 22.Zlotogora J. Dominance and homozygosity. Am J Med Genet 199768412–416. [DOI] [PubMed] [Google Scholar]
- 23.Klemenz R, Frohli E, Steiger R H, Schafer R, Aoyama A. Alpha B‐crystallin is a small heat shock protein. Proc Natl Acad Sci USA 1991883652–3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantorow M, Piatigorsky J. Alpha‐crystallin/small heat shock protein has autokinase activity. Proc Natl Acad Sci USA 1994913112–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andley U P, Song Z, Wawrousek E F, Fleming T P, Bassnett S. Differential protective activity of alpha A‐ and alphaB‐crystallin in lens epithelial cells. J Biol Chem 200027536823–36831. [DOI] [PubMed] [Google Scholar]
- 26.Wang K, Spector A. alpha‐crystallin stabilizes actin filaments and prevents cytochalasin‐induced depolymerization in a phosphorylation‐dependent manner. Eur J Biochem 199624256–66. [DOI] [PubMed] [Google Scholar]
- 27.Wieske M, Benndorf R, Behlke J, Dolling R, Grelle G, Bielka H, Lutsch G. Defined sequence segments of the small heat shock proteins HSP25 and alphaB‐crystallin inhibit actin polymerization. Eur J Biochem 20012682083–2090. [DOI] [PubMed] [Google Scholar]
- 28.Gopalakrishnan S, Takemoto L. Binding of actin to lens alpha crystallins. Curr Eye Res 199211929–933. [DOI] [PubMed] [Google Scholar]
- 29.Stump R J, Ang S, Chen Y, von Bahr T, Lovicu F J, Pinson K, de Iongh R U, Yamaguchi T P, Sassoon D A, McAvoy J W. A role for Wnt/beta‐catenin signaling in lens epithelial differentiation. Dev Biol 200325948–61. [DOI] [PubMed] [Google Scholar]
- 30.Piao C S, Kim S W, Kim J B, Lee J K. Co‐induction of alphaB‐crystallin and MAPKAPK‐2 in astrocytes in the penumbra after transient focal cerebral ischemia. Exp Brain Res 2005163421–449. [DOI] [PubMed] [Google Scholar]