Abstract
Current evidence suggests that matrix metalloproteinases (MMPs) have a role in early atherosclerosis, plaque rupture and myocardial infarction. Polymorphisms in MMP genes have been examined for associations with atherosclerosis, but interpretation is complicated by methodological issues. This article presents a systematic review of these association studies and a meta‐analysis of available data for polymorphisms where a sufficient number of studies was available. The 5A allele of the MMP3 5A/6A polymorphism was associated with acute myocardial infarction (odds ratio (OR) 1.26, 95% confidence interval (CI) 1.1 to 1.4, p<0.001), suggesting its role in plaque rupture. There was no association with the functional MMP9 −1562C/T polymorphism (OR 1.11, 95% CI 1.0 to 1.3, p = 0.18). Current data provide evidence for the role of MMP3 polymorphism in plaque destabilisation, but elucidation of the role of other MMP gene variants in atherosclerosis will depend on better study design, including a larger sample size, extensive screening of individual genes with haplotype analysis and replication of studies to avoid publication bias.
Atherosclerosis is the major cause of coronary artery disease (CAD) and stroke. Although much of the risk for this condition is explained by conventional risk factors, a great deal remains unexplained. Gene–environment interactions may be particularly relevant. Most studies have looked at associations between polymorphic variants in candidate genes and atherosclerosis, quantified by imaging or cardiovascular end points. A potential candidate gene system is the matrix metalloproteinase (MMP) family.
The MMPs are proteolytic enzymes that degrade the extracellular matrix, leading to connective tissue remodelling during normal biological processes. Vascular remodelling is currently recognised as a determinant of major vascular pathologies including atherosclerosis and restenosis,1 and it is now widely accepted that deregulation of the MMP system has a pivotal role in vascular remodelling and atherosclerosis (table 1).2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28
Table 1 Roles of matrix metalloproteinases in atherosclerosis.
| Subclinical atherosclerosis | Early migration and proliferation of SMC5,8,9,10,11,12,13 |
| Infiltration of leucocytes14 | |
| Intimal thickening and growth of atherosclerotic lesions15,16 | |
| Delay of flow‐limiting stenosis by expansive remodelling of plaques17 | |
| Clinical atherosclerosis | Carotid plaque instability18,19,20,21,22,23 |
| Coronary plaque rupture6,24,25 | |
| Development of aneurysms7,26,27,28 |
SMC, smooth‐muscle cell.
Studying genetic variants in the MMPs, which are associated with lifelong changes in MMP activity, offers the possibility of determining whether such relationships are really causal. Using genetic variants in this way is referred to as “mendelian randomisation”, and has recently been used to determine causality in cardiovascular and other complex diseases.29
In this article, we present a critical examination of the association between MMP polymorphisms and atherosclerosis, focusing on coronary and carotid atherosclerosis.
Search strategy
We performed a computer‐based search using the PubMed database with the following search terms: “matrix metalloproteinases” and “atherosclerosis”, “coronary artery disease”, “carotid stenosis”, “intima‐media thickness”, “polymorphism” and “genetics”. References from the retrieved articles were also screened for additional papers. Publications in English until November 2005 were included. Papers on aneurysms were not reviewed.
We reviewed all association studies on polymorphic variants in the MMP genes in patient groups with subclinical atherosclerosis or clinical coronary or carotid atherosclerosis. For each study, several markers of quality were determined, including details of clinical phenotyping, degree of controlling for ethnicity and other cardiovascular risk factors. Where data from ⩾3 similar studies were available, meta‐analysis was performed using the Mantel–Haenszel method. This was possible for case–control studies of the MMP3 5A/6A and MMP9 −1562C/T polymorphisms. As MMPs may have different roles in early atherosclerosis and in late disease, we analysed studies with the end point of atherosclerotic stenosis on imaging separately from those with the end point of acute symptomatic events (myocardial infarction).
Polymorphisms of MMPs tested for associations with coronary and carotid atherosclerosis
Genetic association studies with cardiovascular disease have used a range of phenotypes. Clinical end points such as myocardial infarction, angina or stroke, and intermediate phenotypes of subclinical atherosclerosis can both be used. The intermediate phenotypes have been measured using high‐resolution duplex ultrasound of the carotid arteries or angiographic imaging of the coronary arteries. The use of intermediate phenotypes is statistically more powerful because continuous variables are used, and because the phenotypes overcome the problem of subclinical disease, in which a control, in a case–control study, may have presymptomatic atherosclerosis. It is important to remember that intermediate phenotypes deal with only part of the pathogenic process, and therefore associations may occur with clinical events but not with intermediate phenotypes. For example, a gene associated with plaque rupture leading to myocardial infarction may not be associated with early arteriosclerosis detected on carotid ultrasound. A popular intermediate phenotype is intima–media thickness (IMT) and carotid plaque on carotid ultrasound, which is non‐invasive and can be applied in large population studies. Numerous studies have shown that IMT is an independent predictor of risk for myocardial infarction and stroke,30,31,32,33 and seems to represent early arteriosclerosis.34 However, it may also represent remodelling changes in response to vascular risk factors such as hypertension.
The MMP3 5A/6A polymorphism
Coronary artery disease
An association between the MMP3 5A/6A promoter polymorphism and atherosclerosis was first described in 1995: the 6A6A genotype was associated with greater progression of coronary atherosclerosis.35 Functional studies showed that the 6A allele was associated with twofold lower transcriptional activity.36
Several investigators have used the clinical end point of coronary atherosclerosis or its progression, and >6000 people have been included in such analyses. The 6A6A genotype was associated with a greater progression of CAD after angioplasty.37 In a prospective drug trial comparing gemfibrozil with placebo in 371 patients with prior coronary artery bypass graft, the 6A allele was associated with atheroma progression during the 32‐month follow‐up in placebo‐treated patients, whereas in the gemfibrozil arm, changes were less marked.38 In 1240 Caucasians, the number of coronary arteries with >50% stenosis increased with increasing frequency of the 6A6A genotype, whereas the 5A5A genotype increased the risk of myocardial infarction among those with at least one artery with >50% stenosis.39 In 1011 Japanese people with CAD, the 6A allele was an independent risk factor for CAD in women without cardiovascular risk factors.40 However, not all studies have found the 6A allele to be associated with CAD. A small study on 131 Koreans with CAD and 117 controls found an association between the 5A allele and stable angina.41 In 3333 patients with CAD treated with either percutaneous coronary interventions or stent, no association was reported with angiographic restenosis,42 consistent with the results of a case–control study of 204 Caucasians with CAD and 267 controls.43
Other studies have used the end point of myocardial infarction; here an association could represent a role in atherosclerosis, or plaque instability and rupture. The high‐activity 5A allele is suggested to predispose a patient to plaque rupture, a hypothesis supported by positive associations reported with acute myocardial infarction in young (<45 years) Han‐Chinese patients from Taiwan,44 Han‐Chinese patients from China45 and two separate Japanese cohorts.46,47 By contrast, a genomewide association study looking at 112 polymorphisms in 71 candidate genes in 4152 Japanese people found an association between the 6A allele and myocardial infarction, although this was confined to women.48 Similarly, a recent study showed that serum concentration of MMP3 is influenced by the MMP3 genotype and associated with myocardial infarction, although this association was seen between myocardial infarction and the 6A allele, particularly among men.49 A possible interaction between the 5A allele and smoking was reported in a Taiwanese population with acute myocardial infarction,44 and a similar interaction was reported in a cohort study of 2743 British middle‐aged men.50 Of the 125 who developed acute coronary events, 6A6A carriers had a higher relative risk of acute CAD events among non‐smokers, whereas among smokers an association was found with the 5A5A genotype. A possible interaction has also been reported with the pleiotropic effects of pravastatin in the Regression Growth Evaluation Statin Study trial.51 Treatment with pravastatin reduced the clinical event rate and the need for repeated angioplasties in those with the 6A allele. This effect was independent of the lipid‐lowering effects of pravastatin.
The 5A allele was associated with calcified coronary lesions in an autopsy series of Finnish men, although no association was found with complicated lesions or myocardial infarction.52 In a recent publication using the same autopsy series, this group showed that the combined MMP3 5A/6A and MMP9 −1652C/T genotype status represented a major risk factor for coronary complicated plaques, with the combination of both high‐activity alleles showing a considerable association with the area of the complicated plaque.53 The MMP3 polymorphism has also been explored in relation to aortic stiffening. The 5A and 6A homozygotes aged >60 years had greater aortic stiffness, whereas heterozygotes showed more elastic aortas and a level of gene and protein expression intermediate between the two homozygotes.54 Finally, the 5A allele was associated with coronary aneurysms in patients with CAD,26 suggesting that a proteolytic balance in favour of extracellular matrix destruction may underlie the pathological occurrence of aneurysms.
Carotid atherosclerosis
Four studies have examined the role of the 5A/6A polymorphism in carotid atherosclerosis, and all have shown association with the 6A allele. Three of them studied associations with IMT and one with degree of carotid stenosis. In healthy Caucasian men without major risk factors for atherosclerosis, the 6A allele was associated with increased carotid IMT, enlarged arterial lumen and a local reduction in wall shear stress.55 This association was confirmed in a further study on healthy men, and additive effects were found with the interleukin 6 (−174G/C) promoter polymorphism.56 Similar associations were found in 87 Hispanic people from the Northern Manhattan Prospective Cohort Study, who had had no stroke,57 and with degree of carotid stenosis in an Italian study.58 The combination of the MMP3 6A6A genotype and the MMP1 2G2G genotype (MMP1 −1607 1G/2G polymorphism) was an independent risk factor for internal carotid artery stenosis.58
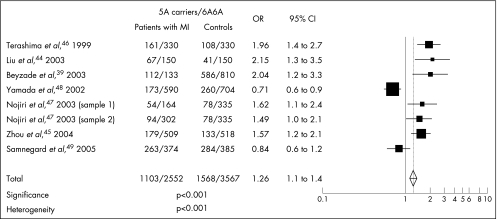
In summary, some studies have shown associations between the low‐activity 6A allele and both coronary and carotid atherosclerosis and increased IMT. This suggests that people with the 6A6A genotype, who produce less MMP3, show a change in matrix turnover, favouring deposition of matrix and accelerated atherosclerosis. By contrast, several studies have shown an association between the high‐activity 5A allele and acute coronary events, suggesting its role in plaque rupture due to increased proteolysis. We pooled together data from seven studies that had looked at associations with myocardial infarction.39,44,45,46,47,48,49 The meta‐analysis showed a moderate genetic influence of the 5A allele as a plaque‐disrupting risk factor (odds ratio (OR) 1.26, 95% confidence interval (CI) 1.1 to 1.4, p<0.001; fig 1). However, heterogeneity was highly significant across studies (p<0.001). Heterogeneity is possibly suggested by the study of Yamada et al,48 where a large group of Japanese patients with myocardial infarction was investigated. In this study, an association was seen between the 6A allele and myocardial infarction only in women. Patients in Yamada et al's study were older than those in the other studies. A possible explanation for the heterogeneity might be that in older women, myocardial infarction develops from severe flow‐limiting stenoses rather than from complicated lesions.
Figure 1 Odds, for patients with myocardial infarction (MI) versus controls, of carrying the 5A allele (matrix metalloproteinase 3 5A/6A polymorphism). x axis: odds ratio (OR) and 95% CI; y axis: studies included in the meta‐analysis.
Other MMP3 polymorphisms
Beyzade et al39 genotyped 1240 Caucasians for six MMP3 polymorphisms (−1986T/C, −1612 5A/6A, −1346A/C, −709A/C, −376G/C and +802A/G) and found none of them, except 5A/6A, significantly associated with CAD (p<0.05). A recent case–control study on three MMP3 polymorphisms (5A/6A, −376C/G and the coding Gly45Lys) through haplotype analysis showed an association between the 5A–G–Lys haplotype and an increased risk of myocardial infarction, although the effect of the haplotype was mainly due to the 5A/6A polymorphism.45
MMP9 polymorphisms
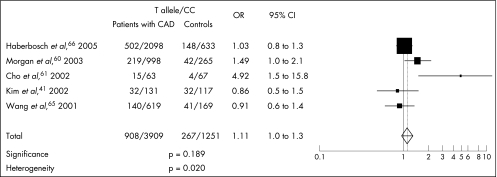
Zhang et al59 described a functional −1562C/T polymorphism in the promoter region of MMP9. Transfection experiments and DNA–protein interaction assays indicated that the T allele had higher activity; an association with severity of coronary atherosclerosis measured by the number of coronary arteries showing >50% stenosis was reported, although no association was seen with myocardial infarction. Others have confirmed this association with CAD.60,61 The T allele has also been associated with complicated coronary lesions,62 and carriers of the T allele had greater levels of MMP9 mRNA and protein, and stiffer large arteries.63 In a prospective study,64 the T allele was associated with raised plasma MMP9 levels, which themselves predicted cardiovascular mortality in patients with CAD, although the T allele itself was not associated with mortality. Three published studies on 788 Caucasians, 248 Koreans and 2731 German men with angiographically documented CAD failed to confirm an association with the T allele.41,65,66 We carried out a meta‐analysis of five studies41,60,61,65,66 and found no association between the T allele and angiographically documented coronary atherosclerosis (OR 1.11, 95% CI 1.0 to 1.3, p = 0.18; fig 2).
Figure 2 Odds of carrying the T allele (matrix metalloproteinase MMP9 –1562C/T polymorphism) for angiographically documented patients with coronary artery disease (CAD). x axis: odds ratio (OR) and 95% CI; y axis: studies included in the meta‐analysis.
Three more MMP9 polymorphisms have been studied: a CA repeat, +6C/T and the coding R279Q. In one study, the 279Q allele was associated with increased MMP9 levels and the combined end point of cardiovascular death and non‐fatal myocardial infarction,64 whereas in another, neither R279Q nor +6C/T was associated with CAD.60 The CA repeat and the R279Q polymorphisms were not associated with coronary aneurysms and stable angina in two other studies.26,41
Other MMP polymorphisms
Ye et al43 showed a reduced risk of CAD in 471 Caucasians with the MMP1 2G2G genotype (MMP1 −1607 1G/2G polymorphism). However, in a study on 164 patients with myocardial infarction and 335 controls, no association was seen with this polymorphism.47 In another study, no significant associations of MMP2 −1306C/T and MMP12 −82A/G were reported with aneurysmal CAD.26 A case–control study on patients with triple‐vessel CAD looked at four MMP2 promoter polymorphisms (−1575G/A, −1306C/T, −790T/G and −735C/T), and showed a twofold higher risk of triple‐vessel disease for the MMP2 –790T allele.67 Two common functional polymorphisms have been identified in the promoter region of the MMP7 gene,68 and both were associated with smaller luminal diameters but only among people with hypercholesterolaemia, suggesting either an allele‐specific effect of cholesterol on MMP7 expression or MMP7 expression only under hypercholesterolaemic conditions. The MMP12 −82A/G polymorphism was also studied in 367 patients with established CAD, and the G allele, which shows lower transcriptional activity in vitro, was associated with a greater luminal diameter in patients with diabetes undergoing angioplasty with stent implantation.69 Finally, no linkage was reported between loci of tissue inhibitor of metalloproteinases 1, 2 and 3 and premature CAD.70
Conclusions
Considerable evidence, including data from genetically engineered mice, expression studies on atherosclerotic tissue and measurement of circulating markers, has implied a role of MMPs in atherosclerosis. Evidence from expression studies and circulating markers cannot prove causality, because changes could be secondary to the disease process itself rather than having a causal role. The study of polymorphisms, which are associated with changes in MMP activity, are present since birth and therefore give an estimate of lifelong exposure, enables further information on causality to be obtained. Several genetic association studies with MMP variants have been performed, although conclusions from many of these are complicated by poor methodology, particularly small sample sizes, and by the possibility of publication bias. The most studied polymorphisms are the MMP3 5A/6A and the MMP9 −1562C/T. The MMP3 6A6A genotype is suggested to be associated with atherosclerosis, and the 5A allele with plaque rupture. On meta‐analysis of published studies, we found a marked association between the 5A allele and acute events (myocardial infarction), suggesting its possible role in plaque rupture. A meta‐analysis of studies associating the MMP9 polymorphism with CAD found no evidence of an association. The number of studies examining the association with polymorphisms in other MMP genes is limited, and no meta‐analysis of these data was possible.
In summary, genetic association studies suggest a role of MMP3 in plaque rupture. However, conclusions are limited by poor methodology in many studies. Further progress will depend on improved methods, including larger sample sizes, genotyping of haplotypes rather than single‐nucleotide polymorphisms, better matching of cases and controls, controlling for conventional cardiovascular risk factors and, importantly, replicating positive findings in a second independent population before publication.
Acknowledgements
We thank Peter Rothwell and Enrico Flossmann for the meta‐analysis software.
Abbreviations
CAD - coronary artery disease
IMT - intima–media thickness
MMP - matrix metalloproteinase
Footnotes
Competing interests: None.
References
- 1.Gibbons G H, Dzau V J. The emerging concept of vascular remodelling. N Engl J Med 19943301431–1438. [DOI] [PubMed] [Google Scholar]
- 2.Galis Z S, Khatri J J. Matrix metalloproteinases in vascular remodeling and atherogenesis. The good, the bad, and the ugly. Circ Res 200290251–262. [PubMed] [Google Scholar]
- 3.Galis Z, Muszynski M, Sukhova G K, Simon‐Morrisey E, Libby P. Enhanced expression of vascular matrix metalloproteinases induced in vitro by cytokines and in regions of human atherosclerotic lesions. Ann N Y Acad Sci 1995748501–507. [DOI] [PubMed] [Google Scholar]
- 4.Moreau M, Brocheriou I, Petit L, Ninio E, Chapman M J, Rouis M. Interleukin‐8 mediates downregulation of tissue inhibitor of metalloproteinase‐1 expression in cholesterol‐loaded human macrophages: relevance to stability of atherosclerotic plaque. Circulation 199999420–426. [DOI] [PubMed] [Google Scholar]
- 5.Zempo N, Kenagy R D, Au Y P, Bendeck M, Clowes M M, Reidy M A, Clowes A W. Matrix metalloproteinases of vascular wall cells are increased in balloon‐injured rat carotid artery. J Vasc Surg 199420209–217. [DOI] [PubMed] [Google Scholar]
- 6.Brown D L, Hibbs M S, Kearney M, Loushin C, Isner J M. Identification of 92‐kD gelatinase in human coronary atherosclerotic lesions: association of active enzyme synthesis with unstable angina. Circulation 1995912125–2131. [DOI] [PubMed] [Google Scholar]
- 7.Pyo R, Lee J K, Shipley J M, Curci J A, Mao D, Ziporin S J, Ennis T L, Shapiro S D, Senior R M, Thompson R W. Targeted gene disruption of matrix metalloproteinase‐9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest 20001051641–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bendeck M P, Zempo N, Clowes A W, Galardy R E, Reidy M A. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res 199475539–545. [DOI] [PubMed] [Google Scholar]
- 9.Forough R, Koyama N, Hasenstab D, Lea H, Clowes M, Nikkari S T, Clowes A W. Overexpression of tissue inhibitor of matrix metalloproteinase‐1 inhibits vascular smooth muscle cell functions in vitro and in vivo. Circ Res 199679812–820. [DOI] [PubMed] [Google Scholar]
- 10.Mason D P, Kenagy R D, Hasenstab D, Bowen‐Pope D F, Seifert R A, Coats S, Hawkins S M, Clowes A W. Matrix metalloproteinase‐9 overexpression enhances vascular smooth muscle cell migration and alters remodeling in the injured rat carotid artery. Circ Res 1999851179–1185. [DOI] [PubMed] [Google Scholar]
- 11.Bendeck M P, Irvin C, Reidy M A. Inhibition of matrix metalloproteinase activity inhibits smooth muscle cell migration but not neointimal thickening after arterial injury. Circ Res 19967838–43. [DOI] [PubMed] [Google Scholar]
- 12.Pauly R R, Passaniti A, Bilato C, Monticone R, Cheng L, Papadopoulos N, Gluzband Y A, Smith L, Weinstein C, Lakatta E G, Crow M T. Migration of cultured vascular smooth muscle cells through a basement membrane barrier requires type IV collagenase activity and is inhibited by cellular differentiation. Circ Res 19947541–54. [DOI] [PubMed] [Google Scholar]
- 13.Aguilera C M, George S J, Johnson J L, Newby A C. Relationship between type IV collagen degradation, metalloproteinase activity and smooth muscle cell migration and proliferation in cultured human saphenous vein. Cardiovasc Res 200358679–688. [DOI] [PubMed] [Google Scholar]
- 14.Romanic A M, Madri J A. The induction of 72‐kD gelatinase in T cells upon adhesion to endothelial cells is VCAM‐1 dependent. J Cell Biol 19941251165–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prescott M F, Sawyer W K, Von Linden‐Reed J, Jeune M, Chou M, Caplan S L, Jeng A Y. Effect of matrix metalloproteinase inhibition on progression of atherosclerosis and aneurysm in LDL receptor‐deficient mice overexpressing MMP‐3, MMP‐12 and MMP‐13 and on restenosis in rats after balloon injury. Ann N Y Acad Sci 1999878179–190. [DOI] [PubMed] [Google Scholar]
- 16.Coats W D, Jr, Whittaker P, Cheung D T, Curier J W, Han B, Faxon D P. Collagen content is significantly lower in restenotic versus nonrestenotic vessels after balloon angioplasty in the atherosclerotic rabbit model. Circulation 1997951293–1300. [DOI] [PubMed] [Google Scholar]
- 17.Pasterkamp G, Schoneveld A H, Hijnen D J, de Leijn D P, Teepen H, van der Valt A C, Borst C. Atherosclerotic arterial remodeling and the localization of macrophages and matrix metalloproteases 1, 2 and 9 in the human coronary artery. Atherosclerosis 2000150245–253. [DOI] [PubMed] [Google Scholar]
- 18.Loftus I M, Naylor A R, Goodall S, Crowther M, Jones L, Bell P R, Thompson M M. Increased matrix metalloproteinase‐9 activity in unstable carotid plaques. A potential role in acute plaque disruption. Stroke 20003140–47. [DOI] [PubMed] [Google Scholar]
- 19.Molloy K J, Thompson M M, Jones J L, Schwalbe E C, Bell P R, Naylor A R, Loftus I M. Unstable carotid plaques exhibit raised matrix metalloproteinase‐8 activity. Circulation 2004110337–343. [DOI] [PubMed] [Google Scholar]
- 20.Morgan A R, Rerkasem K, Gallagher P J, Zhang B, Morris G E, Calder P C, Grimble R F, Eriksson P, McPheat W L, Shearman C P, Ye S. Differences in matrix metalloproteinase‐1 and matrix metalloproteinase‐12 transcript levels among carotid atherosclerotic plaques with different histopathological characteristics. Stroke 2004351310–1315. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez B, Ruiz C, Chacon P, Alvarez‐Sabin J, Matas M. Serum values of metalloproteinase‐2 and metalloproteinase‐9 as related to unstable plaque and inflammatory cells in patients with greater than 70% carotid artery stenosis. J Vasc Surg 200440469–475. [DOI] [PubMed] [Google Scholar]
- 22.Bicknell C D, Peck D, Alkhamesi N A, Cowling M G, Clark M W, Goldin R, Foale R, Jenkins M P, Wolfe J H, Darzi A W, Cheshire N J. Relationship of matrix metalloproteinases and macrophages to embolization during endoluminal carotid interventions. J Endovasc Ther 200411483–493. [DOI] [PubMed] [Google Scholar]
- 23.Nikkari S T, O'Brien K D, Ferguson M, Hatsukami T, Welgus H G, Alpers C E, Clowes A W. Interstitial collagenase (MMP‐1) expression in human carotid atherosclerosis. Circulation 1995921393–1398. [DOI] [PubMed] [Google Scholar]
- 24.Galis Z S, Sukhova G K, Lark M W, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest 1994942493–2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kai H, Ikeda H, Yasukawa H, Kai M, Seki Y, Kuwahara F, Ueno T, Sugi K, Imaizumi T. Peripheral blood levels of matrix metalloproteases‐2 and ‐9 are elevated in patients with acute coronary syndromes. J Am Coll Cardiol 199832368–372. [DOI] [PubMed] [Google Scholar]
- 26.Lamblin N, Bauters C, Hermant X, Lablanche J M, Helbecque N, Amouyel P. Polymorphisms in the promoter regions of MMP‐2, MMP‐3, MMP‐9 and MMP‐12 genes as determinants of aneurysmal coronary artery disease. J Am Coll Cardiol 20024043–48. [DOI] [PubMed] [Google Scholar]
- 27.Nollendorfs A, Greiner T C, Nagase H, Baxter B T. The expression and localization of membrane type‐1 matrix metalloproteinase in human abdominal aortic aneurysms. J Vasc Surg 200134316–322. [DOI] [PubMed] [Google Scholar]
- 28.Longo G M, Xiong W, Greiner T C, Zhao Y, Fiotti N, Baxter B T. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest 2002110625–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davey Smith G, Ebrahim S. “Mendelian randomization”: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003321–22. [DOI] [PubMed] [Google Scholar]
- 30.Simon A, Gariepy J, Chironi G, Megnien J L, Levenson J. Intima‐media thickness: a new tool for diagnosis and treatment of cardiovascular risk. J Hypertens 200220159–169. [DOI] [PubMed] [Google Scholar]
- 31.Kablak‐Ziembicka A, Tracz W, Przewlocki T, Pieniazek P, Sokolowski A, Konieczynska M. Association of increased carotid intima‐media thickness with the extent of coronary artery disease. Heart 2004901286–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van der Meer I M, Bots M L, Hofman A, del Sol A I, van der Kuip D A, Witteman J C. Predictive value of noninvasive measures of atherosclerosis for incident myocardial infarction: the Rotterdam Study. Circulation 20041091089–1094. [DOI] [PubMed] [Google Scholar]
- 33.Hodis H N, Mack W J, LaBree L, Selzer R H, Liu C R, Liu C H, Azen S P. The role of carotid arterial intima‐media thickness in predicting clinical coronary events. Ann Intern Med 1998128262–269. [DOI] [PubMed] [Google Scholar]
- 34.de Groot E, Hovingh G K, Wiegman A, Duriez P, Smit A J, Fruchart J C, Kastelein J J. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation 2004109III33–III38. [DOI] [PubMed] [Google Scholar]
- 35.Ye S, Watts G F, Mandalia S, Humphries S E, Henney A M. Preliminary report: genetic variation in the human stromelysin promoter is associated with progression of coronary atherosclerosis. Br Heart J 199573209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye S, Eriksson P, Hamsten A, Kurkinen M, Humphries S E, Henney A M. Progression of coronary atherosclerosis is associated with a common genetic variant of the human stromelysin‐1 promoter which results in reduced gene expression. J Biol Chem 199627113055–13060. [DOI] [PubMed] [Google Scholar]
- 37.Humphries S, Bauters C, Meirhaeghe A, Luong L, Bertrand M, Amouyel P. The 5A6A polymorphism in the promoter of the stromelysin‐1 (MMP3) gene as a risk factor for restenosis. Eur Heart J 200223721–725. [DOI] [PubMed] [Google Scholar]
- 38.Humphries S E, Luong L A, Talmud P J, Frick M H, Kesaniemi Y A, Pasternak A, Taskinen M R, Syvanne M. The 5A/6A polymorphism in the promoter of the stromelysin‐1 (MMP‐3) gene predicts progression of angiographically determined coronary artery disease in men in the LOCAT gemfibrozil study. Atherosclerosis 199813949–56. [DOI] [PubMed] [Google Scholar]
- 39.Beyzade S, Zhang S, Wong Y K, Day I N, Eriksson P, Ye S. Influences of matrix metalloproteinase‐3 gene variation on extent of coronary atherosclerosis and risk of myocardial infarction. J Am Coll Cardiol 2003412130–2137. [DOI] [PubMed] [Google Scholar]
- 40.Hirashiki A, Yamada Y, Murase Y, Suzuki Y, Kataoka H, Morimoto Y, Tajika T, Murohara T, Yokota M. Association of gene polymorphisms with coronary artery disease in low‐ or high‐risk subjects defined by conventional risk factors. J Am Coll Cardiol 2003421429–1437. [DOI] [PubMed] [Google Scholar]
- 41.Kim J S, Park H Y, Kwon J H, Im E K, Choi D, Jang Y, Cho S Y. The roles of stromelysin‐1 and the gelatinase B gene polymorphism in stable angina. Yonsei Med J 200243473–481. [DOI] [PubMed] [Google Scholar]
- 42.Hoppmann P, Koch W, Schomig A, Kastrati A. The 5A/6A polymorphism of the stromelysin‐1 gene and restenosis after percutaneous coronary interventions. Eur Heart J 200425335–341. [DOI] [PubMed] [Google Scholar]
- 43.Ye S, Gale C R, Martyn C N. Variation in the matrix metalloproteinase‐1 gene and risk of coronary heart disease. Eur Heart J 2003241668–1671. [DOI] [PubMed] [Google Scholar]
- 44.Liu P Y, Chen J H, Li Y H, Wu H L, Shi G Y. Synergistic effect of stromelysin‐1 (matrix metallo‐proteinase‐3) promoter 5A/6A polymorphism with smoking on the onset of young acute myocardial infarction. Thromb Haemost 200390132–139. [PubMed] [Google Scholar]
- 45.Zhou X, Huang J, Chen J, Su S, Chen R, Gu D. Haplotype analysis of the matrix metalloproteinase‐3 gene and myocardial infarction in a Chinese Han population. The Beijing Atherosclerosis Study. Thromb Haemost 200492867–873. [DOI] [PubMed] [Google Scholar]
- 46.Terashima M, Akita H, Kanazawa K, Inoue N, Yamada S, Ito K, Matsuda Y, Takai E, Iwai C, Kurogane H, Yoshida Y, Yokoyama M. Stromelysin promoter 5A/6A polymorphism is associated with acute myocardial infarction. Circulation 1999992717–2719. [DOI] [PubMed] [Google Scholar]
- 47.Nojiri T, Morita H, Imai Y, Maemura K, Ohno M, Ogasawara K, Aizawa T, Saito A, Hayashi D, Hirata Y, Sugiyama T, Yamasaki T, Nagai R. Genetic variations of matrix metalloproteinase‐1 and ‐3 promoter regions and their associations with susceptibility to myocardial infarction in Japanese. Int J Cardiol 200392181–186. [DOI] [PubMed] [Google Scholar]
- 48.Yamada Y, Izawa H, Ichihara S, Takatsu F, Ishihara H, Hirayama H, Sone T, Tanaka M, Yokota M. Prediction of the risk of myocardial infarction from polymorphisms in candidate genes. N Engl J Med 20023471916–1923. [DOI] [PubMed] [Google Scholar]
- 49.Samnegard A, Silveira A, Lundman P, Boquist S, Odeberg J, Hulthe J, McPheat W, Tornvall P, Bergstrand L, Ericsson C G, Hamsten A, Eriksson P. Serum matrix metalloproteinase‐3 concentration is influenced by MMP‐3 –1612 5A/6A promoter genotype and associated with myocardial infarction. J Int Med 2005258411–419. [DOI] [PubMed] [Google Scholar]
- 50.Humphries S E, Martin S, Cooper J, Miller G. Interaction between smoking and the stromelysin‐1 (MMP3) gene 5A/6A promoter polymorphism and risk of coronary heart disease in healthy men. Ann Hum Genet 200266343–352. [DOI] [PubMed] [Google Scholar]
- 51.de Maat M P, Jukema J W, Ye S, Zwinderman A H, Moghaddam P H, Beekman M, Kastelein J J, van Boven A J, Bruschke A V, Humphries S E, Kluft C, Henney A M. Effect of the stromelysin‐1 promoter on efficacy of pravastatin in coronary atherosclerosis and restenosis. Am J Cardiol 199983852–856. [DOI] [PubMed] [Google Scholar]
- 52.Pollanen P J, Lehtimaki T, Ilveskoski E, Mikkelsson J, Kajander O A, Laippala P, Perola M, Goebeler S, Penttila A, Mattila K M, Syrjakoski K, Koivula T, Nikkari S T, Karhunen P J. Coronary artery calcification is related to functional polymorphism of matrix metalloproteinase 3: the Helsinki Sudden Death Study. Atherosclerosis 2002164329–335. [DOI] [PubMed] [Google Scholar]
- 53.Pollanen P J, Lehtimaki T, Mikkelsson J, Ilveskoski E, Kunnas T, Perola M, Penttila A, Mattila K M, Nikkari S T, Syrjakoski K, Karhunen P J. Matrix metalloproteinase 3 and 9 gene promoter polymorphisms: joint action of two loci as a risk factor for coronary artery complicated plaques. Atherosclerosis 200518073–78. [DOI] [PubMed] [Google Scholar]
- 54.Medley T L, Kingwell B A, Gatzka C D, Pillay P, Cole T J. Matrix metalloproteinase‐3 genotype contributes to age‐related aortic stiffening through modulation of gene and ‐protein expression. Circ Res 2003921254–1261. [DOI] [PubMed] [Google Scholar]
- 55.Gnasso A, Motti C, Irace C, Carallo C, Liberatoscioli L, Bernardini S, Massoud R, Mattioli P L, Federici G, Cortese C. Genetic variation in human stromelysin gene promoter and common carotid geometry in healthy male subjects. Arterioscler Thromb Vasc Biol 2000201600–1605. [DOI] [PubMed] [Google Scholar]
- 56.Rauramaa R, Vaisanen S B, Luong L A, Schmidt‐Trucksass A, Penttila I M, Bouchard C, Toyry J, Humphries S E. Stromelysin‐1 and interleukin‐6 gene promoter polymorphisms are determinants of asymptomatic carotid artery atherosclerosis. Arterioscler Thromb Vasc Biol 2000202657–2662. [DOI] [PubMed] [Google Scholar]
- 57.Rundek T, Elkind M S, Pittman J, Boden‐Albala B, Martin S, Humphries S E, Juo S H, Sacco R L. Carotid intima‐media thickness is associated with allelic variants of stromelysin‐1, interleukin‐6, and hepatic lipase genes: the Northern Manhattan Prospective Cohort Study. Stroke 2002331420–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ghilardi G, Biondi M L, DeMonti M, Turri O, Guagnellini E, Scorza R. Matrix metalloproteinase‐1 and matrix metalloproteinase‐3 gene promoter polymorphisms are associated with carotid artery stenosis. Stroke 2002332408–2412. [DOI] [PubMed] [Google Scholar]
- 59.Zhang B, Ye S, Herrmann S M, Eriksson P, de Maat M, Evans A, Arveiler D, Luc G, Cambien F, Hamsten A, Watkins H, Henney A M. Functional polymorphism in the regulatory region of gelatinase B gene in relation to severity of coronary atherosclerosis. Circulation 1999991788–1794. [DOI] [PubMed] [Google Scholar]
- 60.Morgan A R, Zhang B, Tapper W, Collins A, Ye S. Haplotypic analysis of the MMP‐9 gene in relation to coronary artery disease. J Mol Med 200381321–326. [DOI] [PubMed] [Google Scholar]
- 61.Cho H J, Chae I H, Park K W, Ju J R, Oh S, Lee M M, Park Y B. Functional polymorphism in the promoter region of the gelatinase B gene in relation to coronary artery disease and restenosis after percutaneous coronary intervention. J Hum Genet 20024788–91. [DOI] [PubMed] [Google Scholar]
- 62.Pollanen P J, Karhunen P J, Mikkelsson J, Laippala P, Perola M, Penttila A, Mattila K M, Koivula T, Lehtimaki T. Coronary artery complicated lesion area is related to functional polymorphism of matrix metalloproteinase 9 gene: an autopsy study. Arterioscler Thromb Vasc Biol 2001211446–1450. [DOI] [PubMed] [Google Scholar]
- 63.Medley T L, Cole T J, Dart A M, Gatzka C D, Kingwell B A. Matrix metallaproteinase‐9 genotype influences large artery stiffness through effects on aortic gene and protein expression. Arterioscler Thromb Vasc Biol 2004241479–1484. [DOI] [PubMed] [Google Scholar]
- 64.Blankenberg S, Rupprecht H J, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambien F, Tiret L, AtheroGene Investigators Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation 20031071579–1585. [DOI] [PubMed] [Google Scholar]
- 65.Wang J, Warzecha D, Wilcken D, Wang X L. Polymorphism in the gelatinase B gene and the severity of coronary arterial stenosis. Clin Sci (London) 200110187–92. [PubMed] [Google Scholar]
- 66.Haberbosch W, Gardemann A. Gelatinase B (C–1652 T) polymorphism in relation to ischaemic heart disease. Scand J Clin Lab Invest 200565513–522. [DOI] [PubMed] [Google Scholar]
- 67.Vasku A, Goldbergova M, Izakovicova Holla L, Siskova L, Groch L, Beranek M, Tschoplova S, Znojil V, Vacha J. A haplotype constituted of four MMP‐2 promoter polymorphisms (−1575G/A, −1306C/T, −790T/G and −735C/T) is associated with coronary triple‐vessel disease. Matrix Biol 200422585–591. [DOI] [PubMed] [Google Scholar]
- 68.Jormsjo S, Whatling C, Walter D H, Zeiher A M, Hamsten A, Eriksson P. Allele‐specific regulation of matrix metalloproteinase‐7 promoter activity is associated with coronary artery luminal dimensions among hypercholesterolemic patients. Arterioscler Thromb Vasc Biol 2001211834–1839. [DOI] [PubMed] [Google Scholar]
- 69.Jormsjo S, Ye S, Moritz J, Walter D H, Dimmeler S, Zeiher A M, Henney A, Hamsten A, Eriksson P. Allele‐specific regulation of matrix metalloproteinase‐12 gene activity is associated with coronary artery luminal dimensions in diabetic patients with manifest coronary artery disease. Circ Res 200086998–1003. [DOI] [PubMed] [Google Scholar]
- 70.Dorsch M F, Barrett J A, Lawrance R A, Maqbool A, Durham N P, Ellis S, Samani N J, Bishop T, Ball S G, Balmforth A J, Hall A S. Premature coronary artery disease shows no evidence of linkage to loci encoding for tissue inhibitors of matrix metalloproteinases. J Hum Genet 200348508–513. [DOI] [PubMed] [Google Scholar]