Abstract
Macrophage apoptosis is an important process in the pathophysiology of atherosclerosis. Oxidized low-density lipoproteins (OxLDL) are a major component of lesions and potently induce macrophage apoptosis. Cannabinoid receptor 2 (CB2), the predominant macrophage cannabinoid receptor, modulates several macrophage processes associated with ongoing atherosclerosis; however, the role of CB2 in macrophage apoptosis is unknown. To determine if CB2 influences a macrophage apoptotic pathway relevant to atherosclerosis, we examined the effect of CB2 deficiency on OxLDL-induced macrophage apoptosis. In situ terminal transferase-mediated dUTP nick end labeling (TUNEL) analysis of resident peritoneal macrophages detected significantly fewer apoptotic CB2−/− macrophages than CB2+/+ macrophages after incubation with OxLDL (27.9 ± 4.7% vs. 61.9 ± 8.5%, P < 0.001) or 7-ketocholesterol (7KC) (18.9 ± 10.5% vs. 54.1 ± 6.9%, P < 0.001), an oxysterol component of OxLDL. Caspase-3 activity; proteolytic conversion of procaspase-3; and cleavage of a caspase-3 substrate, PARP, were also diminished in 7KC-treated CB2−/− macrophages. Furthermore, the deactivation of the prosurvival kinase, Akt, in response to 7KC was impaired in CB2−/− macrophages. These results suggest that CB2 expression increases the susceptibility of macrophages to OxLDL-induced apoptosis, in part, by modulating the effect of oxysterols on the Akt survival pathway and that CB2 may influence atherosclerosis by modulating lesional macrophage apoptosis.
Keywords: 7-ketocholesterol, Akt, atherosclerosis, caspase-3
Atherosclerosis is a chronic inflammatory disease of the vascular wall (1) during which macrophages in the vascular intima ingest atherogenic lipoproteins, such as modified LDLs, and transform into cholesteryl ester-laden foam cells (2). Apoptotic foam cells have been well documented within atherosclerotic lesions, but until recently the consequences of apoptosis in the pathophysiology of atherosclerosis was unknown. Work by Liu et al. (3) and Arai et al. (4) showed that macrophage apoptosis represses lesion formation in mice, indicating that, at least in early lesions, macrophage apoptosis is antiatherogenic. In advanced lesions, there is strong evidence that apoptosis of macrophage-derived foam cells is a proatherogenic factor contributing to plaque instability and rupture (5, 6). Thus, the mechanisms regulating macrophage apoptosis are significant to the development of lesions and the progression toward events that may directly trigger the acute clinical manifestations of atherosclerosis.
Oxidized low-density lipoproteins (OxLDL) is the most widely studied modified LDL. It is present in circulating plasma and atherosclerotic lesions (7, 8), and is a potent inducer of apoptosis in cultured macrophages as well as other vascular cell types (9–12). The oxysterol constituents of OxLDL are responsible for a large portion of its apoptotic activity (13–15). Of the oxysterols present in OxLDL, 7-ketocholesterol (7KC) is one of the most abundant and cytotoxic (16, 17). In vitro, treatment of macrophages with 7KC at concentrations near the upper range of that of oxysterols found in plasma of hyperlipidemic humans (10–30 μM) (18, 19) induces apoptosis via the mitochondrial pathway (20, 21). The induction of apoptosis occurs, at least in part, by a mechanism dependent upon an influx of extracellular Ca2+, activation of cytosolic phospholipase A2 (cPLA2) resulting in release of arachidonic acid, and esterification of the oxysterol(s) with arachidonate mediated by acyl-CoA:cholesterol acyltransferase (ACAT) (21–24). These observations have led us to propose that arachidonyl oxysterols, particularly 3-arachidonyl 7KC, are the critical inducers of macrophage apoptosis formed in response to OxLDL (24).
The only known signaling lipid containing an arachidonyl ester as a structural feature is the endocannabinoid, 2-arachidonyl glycerol (25). Cannabinoids, such as Δ9-tetrahydrocannabinol (THC), and their endogenous counterparts, the endocannabinoids, function by binding and activating a pair of G-protein coupled receptors known as cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2) (26, 27). Despite sharing ∼44% homology, CB1 and CB2 appear to be functionally distinct. CB1 is expressed predominantly in the central and peripheral nervous system, modulates synaptic signaling, and is responsible for the psychotropic and analgesic effects of cannabinoids (28). CB2 is mainly expressed in cells of the peripheral immune system, including monocytes/macrophages (29), and is responsible for the anti-inflammatory and immunosuppressive effects of cannabinoids (30).
Evidence of a possible role for CB2 in atherosclerosis has been reported. In mice, CB2 is present in lesions but absent from adjacent nondiseased areas of the vascular wall, and activation of CB2 can be shown to diminish lesion progression (31). In vitro, CB2 impacts on several processes associated with the pathogenesis of atherosclerosis, including macrophage proliferation, chemotaxis, and apoptosis (32, 33). The antiatherosclerotic effects of macrophage apoptosis in the vasculature have been noted by our lab and others (3, 4). The intersection of observations of a physiological significance of CB2 in induction of macrophage apoptosis and candidate ligands for CB2 in the arachidonyl oxysterols formed in response to oxysterols motivated us to determine whether CB2 deficiency would alter the apoptotic response of macrophages to OxLDL. The potentially pharmacologically exploitable role of CB2 in atherosclerosis is also intriguing. In the current study, we observed that macrophages lacking CB2 have a diminished capacity to undergo OxLDL- and 7KC-induced apoptosis and to dephosphorylate Akt in response to 7KC.
MATERIALS AND METHODS
Materials
Oxidized human LDL produced by incubation with 20 μM copper sulfate for 24 h was purchased from Biomedical Technologies Inc. (Stoughton, MA). The lipid peroxidation of the OxLDL, as quantified by assaying for thiobarbituric acid reactive substances using malondialdehyde as the standard, was found to be 15.9 nmoles of malondialdehyde/mg protein. 7-ketocholesterol was obtained from Steraloids (Newport, RI) and staurosporine from Sigma Chemical Co. (St. Louis, MO). [9,10-3H]oleate was purchased from American Radiolabeled Chemicals (St. Louis, MO).
Mice
C57BL/J6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). CB2 receptor null mice on a C57BL/6 background (34) were kindly provided by Dr. Nancy Buckley (California State Polytechnic University, Pomona, CA). Genotyping was performed by PCR of genomic DNA isolated from tail clips using specific primers as described (34). All mice were housed in the Animal Research Facility at East Tennessee State University, and all procedures performed were in accordance with the guidelines administered by the East Tennessee State University Institutional Animal Care and Usage Committee.
Isolation of resident peritoneal macrophages
Resident mouse peritoneal macrophages (MPMs) were isolated by lavage and cultured as previously described (24).
TUNEL assay
MPMs isolated from four CB2 −/− and four CB2 +/+ mice (age 8–10 weeks) were pooled by genotype and seeded at ∼1 × 105 cells per chamber in 8-well glass chamber slides (Nalge, Naperville, IL) in DMEM containing 10% FBS (DMEM10). After attaching, the MPMs were rinsed twice with PBS and refed DMEM10 supplemented with various concentrations of OxLDL. Control wells received equivalent amounts of unmodified LDL. After a 16 h incubation, terminal transferase-mediated dUTP nick end labeling (TUNEL) assays were performed using an in situ cell death detection kit (Roche Diagnostics, Indianapolis, IN) as described previously (24). For each condition, the percentage of TUNEL-positive cells in 10 randomly chosen fields (70–100 cells/field) was determined. Each experiment was performed independently at least twice.
Caspase-3 activity assay
Caspase-3 and caspase-3-like activity (DEVDase activity) was measured using a fluorogenic tetrapeptide, Ac-DEVD-AFC, which is an efficient substrate for caspase-3, as well as caspase 7 (35, 36). Briefly, MPMs isolated from groups (n ⩾ 8) of CB2 null and wild-type mice were pooled according to genotype and seeded at 2 × 106 cells/well in 12-well culture plates in DMEM10. After an overnight incubation, the MPMs were rinsed with PBS twice and refed fresh DMEM10. 7-ketocholesterol was added directly to the wells from 2 mg/ml ethanol stock solutions. Untreated controls were adjusted to receive an equivalent volume of vehicle (ethanol). After a 16 h incubation, the attached and unattached cells were collected by scraping followed by centrifugation at 1,000 g for 5 min. The cells were rinsed in ice-cold PBS, resuspended in lysis buffer (10 mM Tris (pH 7.5), 130 mM NaCl, 1% Triton X-100, 10 mM sodium Pi, and 10 mM sodium PPi), incubated on ice for 10 min and centrifuged at 12,000 g for 15 min at 4°C. The supernatants were then assayed for DEVDase activity in the presence and absence of a caspase-3 inhibitor, Ac-DEVD-CHO, as described previously (21). The net caspase-3 activity of each sample was calculated by subtracting the relative fluorescence measured in the presence of the inhibitor from the relative fluorescence measured in the absence of the inhibitor and normalized to the protein concentration of the sample. All treatments were preformed in triplicate and the data presented as the mean ± SD.
Measurement of arachidonate release
MPMs were plated at 1 × 106 per 60 mm dish in DMEM10 and allowed to attach. The cells were rinsed twice with PBS and incubated for 24 h in serum-free DMEM containing 0.1% fatty acid-free BSA and 1 μCi/ml [3H]arachidonate after which the cells were rinsed thrice with PBS containing 0.1% fatty acid-free BSA and refed DMEM10. After 1 h incubation, the cells were either refed medium containing 7KC or an equivalent volume of vehicle (ethanol), and the incubation continued for various time periods. The medium was collected, centrifuged at 1,000 g for 5 min, and the radioactivity in the medium was determined by liquid scintillation counting. The radioactivity remaining in the cells was determined by liquid scintillation counting of cell lysates prepared with 0.1 N NaOH. The percent release of arachidonate was calculated as [medium dpm/(cells + medium) dpm] × 100 and was normalized to the value of unstimulated controls.
Determination of ACAT activity
ACAT activity was determined by incorporation of [3H]oleate into cellular cholesteryl and oxysteryl esters. Isolated MPMs were seeded at 2 × 106 per 60 mm dish, allowed to attach overnight, rinsed with PBS twice, and then incubated for 6 h in DMEM containing 0.1% fatty acid-free BSA and [3H]oleate (1.0 μCi/ml) in the presence or absence of 7KC. The cells were rinsed three times with PBS, and the membrane phospholipids and neutral lipids were extracted with hexane:isopropanol (3:2), brought to dryness under a stream of N2, and resuspended in 30 μl of hexane containing cholesteryl oleate (1 mg/ml) and 7-ketocholesteryl oleate (1 mg/ml). The residue was spotted on Whatman silica gel 60 plates and developed in hexane:diethyl ether:acetic acid (80:20:1). The cholesteryl oleate and 7KC-oleate bands were visualized with iodine vapor, scraped, and subjected to liquid scintillation counting. The extracted cells were dissolved in 0.1 N NaOH, and the protein concentration as determined by micro-BCA assay (Pierce, Rockford IL) was used to normalize the data. Treatments were performed in triplicate and, in the case of cholesteryl oleate formation, the data presented as mean fold induction ± SD for three independent experiments.
Immunoblotting
Macrophages were seeded at 2 × 106 per 35 mm plate in DMEM10 and allowed to attach for 24 h. 7KC was then added to media as described above for caspase-3 activity assays, and the incubation continued for 16 h. The cells were washed twice with PBS, collected in ice-cold 1 × cell lysis buffer (Cell Signaling, Danvers, MA) supplemented with Halt Proteinase Inhibitor cocktail (Pierce, Rockford, IL), incubated on ice for 20 min, and centrifuged at 14,000 g for 15 min at 4°C. The protein concentration was determined by micro-BCA assay (Pierce). Cell lysates were subjected to SDS-PAGE on 4–12% Bis-Tris NuPAGE gels (Invitrogen, Carlsbad, CA) and transferred to polyvinylidene fluoride membranes. The membranes were blocked in tris buffered saline containing 0.2% Tween-20 (TBS-T) and 5% nonfat dry milk for 1 h followed by overnight incubation at 4°C in TBS-T containing a 1:1000 dilution of a primary antibody to CB2, caspase-3, poly (ADP-ribose) polymerase (PARP) or total Akt1/2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) or a 1:5000 dilution of phospho-Akt[Ser473] antibody (Biosource International). The membranes were washed with TBS-T and then incubated with an HRP-conjugated secondary antibody for 1 h. The bands were visualized using a Supersignal West Pico ECL kit (Pierce). Phospho-Akt[Ser473] immunoblots were developed with a Supersignal West Femto ECL kit (Pierce). To control for equal protein loading and transfer, the membranes were rinsed with TBS-T and subjected to the immunoblotting procedure using a 1:10,000 dilution of Hsc70 antibody (Stressgen, Ann Arbor, MI). Immunoblots were quantified using ImageJ processing and analysis software (37).
Statistical analysis
Statistical differences between groups were determined by two-tailed, unpaired Student's t-test using SigmaPlot 2000 software (SPSS Inc.) with P < 0.05 considered statistically significant.
RESULTS
CB2 deficient macrophages are resistant to OxLDL-induced DNA fragmentation
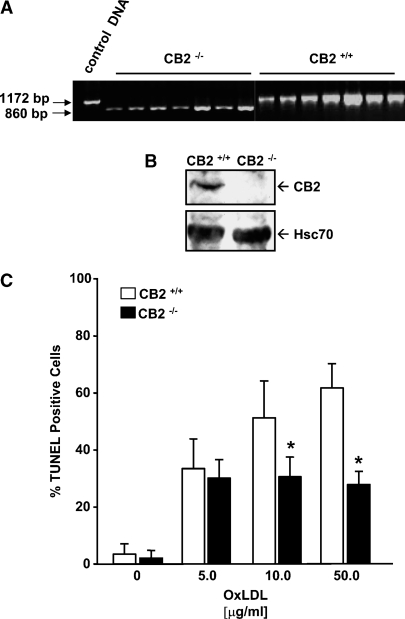
Representative results from PCR and immunoblot analysis confirming the CB2 null genotype and phenotype of the CB2−/− mice are shown in Fig. 1A, B, respectively. TUNEL analysis of cellular DNA fragmentation is a widely used sensitive and relatively specific method for quantitation of apoptosis in cell cultures (38), and in our prior studies, OxLDL-induced apoptosis in MPMs was reliably detected by TUNEL analysis (24). We, therefore, performed TUNEL analysis of CB2−/− and CB2+/+ MPMs incubated for 16 h with increasing amounts of OxLDL to examine the effect of CB2 deficiency in macrophages on OxLDL-induced apoptosis (Fig. 1C). Treatment with 5 μg/ml OxLDL resulted in no significant difference between the percentage of TUNEL-positive CB2+/+ macrophages and TUNEL-positive CB2−/− macrophages (33.6 ± 10.4% vs. 30.3 ± 6.5%, respectively). However, the percentage of TUNEL-positive macrophages produced by treatment with 10 μg/ml OxLDL was ∼1.7-fold higher in CB2+/+ macrophages compared with CB2−/− macrophages (51.4 ± 13.0% vs. 30.7 ± 7.0%, P < 0.001). This difference increased slightly to ∼2.2-fold with 50 μg/ml OxLDL (61.9 ± 8.5% vs. 27.9 ± 4.7%, P < 0.001). Compared with wild-type MPMs, CB2−/− MPMs did not display a reduced ability to uptake modified LDL (data not shown).
Fig. 1.
Oxidized low-density lipoproteins (OxLDL)-induced DNA fragmentation is reduced in cannabinoid receptor 2 (CB2)−/− macrophages. A: Genomic DNA isolated from seven randomly selected CB2 null and seven randomly selected CB2+/+ mice was subjected to PCR analysis. The expected size of the wild-type and CB2 null PCR amplification products are as indicated. BTotal cell lysates prepared from CB2−/− and CB2+/+ MPMs were subjected to SDS-PAGE and immunoblotting using a CB2 specific antibody. The immunoblots were also probed with an antibody against Hsc70 as a control for equal loading. C:: Induction of DNA fragmentation in CB2+/+ and CB2−/− MPMs after a 16 h treatment with increasing concentrations of OxLDL was determined by in situ terminal transferase-mediated dUTP nick end labeling (TUNEL) analysis as described in Materials and Methods. C: The graph shows the mean percentage ± SD of TUNEL stained nuclei observed for each treatment for three independent experiments. * P < 0.001 between similarly treated CB2 −/− and CB2+/+ macrophages.
CB2 deficient macrophages are resistant to 7-ketocholesterol-induced DNA fragmentation
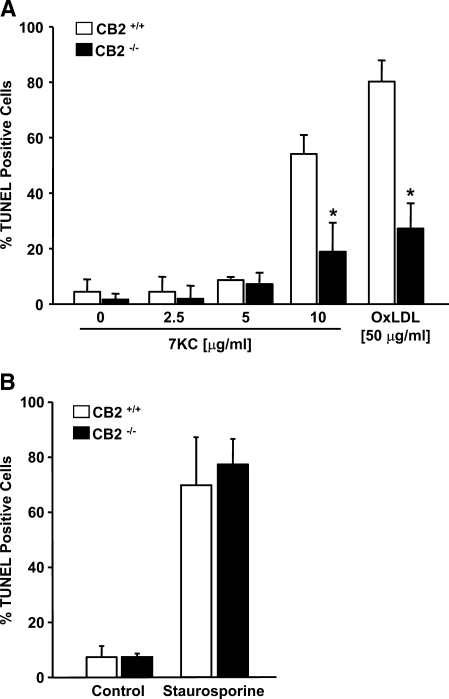
The apoptotic potential of OxLDL is, primarily, a consequence of its oxysterol constituents. Therefore we also performed TUNEL analysis to compare the apoptotic response of CB2−/− and CB2+/+ MPMs treated with varying concentrations of 7KC, one of the most abundant and cytotoxic oxysterols present in OxLDL (Fig. 2A). Treatment of macrophages with 7KC at concentrations below 10 μg/ml did not significantly induce DNA fragmentation in either CB2−/− or CB2+/+ macrophages. However, CB2−/− and CB2+/+ macrophages both showed an increase in DNA fragmentation following treatment with 10 μg/ml 7KC, a concentration nearing the upper limit of oxysterols detected in human plasma (19). In CB2−/− or CB2+/+ MPMs, the degree of apoptosis induced by treatment with 10 μg/ml 7KC was similar to that induced by treatment with 50 μg/ml OxLDL. When CB2−/− and CB2+/+ MPMs were treated with higher concentrations of 7KC (⩾20 μg/ml), extensive necrosis and cell detachment was observed such that quantitative analysis was not possible. Similar to the results with OxLDL treatment (Fig. 1C), the percentage of TUNEL positive CB2+/+ MPMs observed after treatment with 10 μg/ml 7KC was greater (∼2.9-fold) than that observed for similarly treated CB2−/− MPMs (54.1 ± 6.9% vs. 18.9 ± 10.5%, respectively, P < 0.001) (Fig. 2A). In contrast, no significant difference between the percentage of TUNEL-positive CB2−/− and CB2+/+ MPMs was observed following treatment with staurosporine, a highly effective inducer of apoptosis in a variety of cell types (39) (Fig. 2B).
Fig. 2.
DNA fragmentation induced by 7-ketocholesterol (7KC), but not staurosporine, is diminished by CB2 deficiency in macrophages. A: In situ TUNEL analysis of CB2+/+ and CB2−/− MPMs after 16 h in the presence of increasing concentrations of 7-ketocholesterol (7KC) as indicated. The MPMs were also treated with 50 μg/ml OxLDL as a positive control. B: TUNEL analysis of CB2+/+ and CB2−/− MPMs incubated for 6 h in the presence or absence of 1 μM staurosporine. The graph shows the mean percentage ± SD of TUNEL stained nuclei observed for each treatment performed in triplicate for two independent experiments. * P < 0.001 between similarly treated CB2−/− and CB2+/+ macrophages.
CB2 null macrophages are resistant to 7KC-induced caspase-3 activation
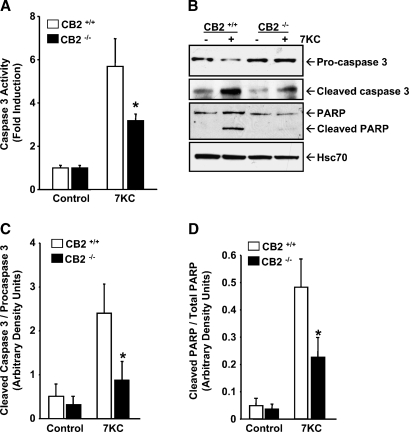
Proteolytic processing of the inactive proenzyme, procaspase-3, to its active form, caspase-3, represents a committed step in the execution phase of apoptosis (40). Activated caspase-3 then cleaves specific substrates, such as poly (ADP-ribose) polymerase (PARP), involved in downstream apoptotic events. Therefore, to validate the results obtained by TUNEL analysis, we performed caspase-3 activity assays and immunoblotting with caspase-3 and PARP-specific antibodies (Fig. 3). Induction of caspase-3 activity by 7KC treatment was apparent in MPMs from CB2−/− mice; however, the induction was significantly reduced in comparison to that observed in MPMs from wild-type mice (3.2 ± 0.31-fold vs. 5.7 ± 1.3, P < 0.05)(Fig. 3A). Immunoblots revealed a substantial decrease in procaspase-3 levels and increase in cleaved caspase-3 levels in CB2+/+ MPMs following 7KC treatment (Fig. 3B), a result that is consistent with activation of caspase-3 activity by proteolytic processing of procaspase-3. In comparison, immunoblots of CB2−/− MPMs treated with 7KC showed a much less significant decrease in procaspase-3 levels and increase in cleaved caspase-3 levels (Fig. 3B). Additionally, immunoblotting revealed that cleavage of PARP into its 89 kDa apoptotic fragment was also significantly greater in 7KC-treated CB2+/+ MPMs compared with similarly treated CB2−/− MPMs (Fig. 3B). Quantitation by scanning densitometric analysis of the immunoblots from three replicate experiments showed that the ratio of cleaved caspase-3 to procaspase-3 increased ∼4.7-fold (0.56 ± 0.28 to 2.4 ± 0.64, P < 0.05) in CB2+/+ MPMs treated with 7KC but only ∼2.6-fold (0.32 ± 0.19 to 0.86 ± 0.42, P < 0.05) in CB2−/− MPMs (Fig. 3C), and that the percentage of cleaved PARP in CB2+/+ macrophages increased ∼9.9-fold (4.9 ± 2.9% to 48.3 ± 10.5%, P < 0.05) following 7KC treatment, but only ∼6.1-fold (3.7 ± 1.8% to 22.6 ± 7.3%, P < 0.05) in CB2−/− macrophages (Fig. 3D).
Fig. 3.
Activation of caspase-3 by 7-ketocholesterol is reduced in CB2 deficient macrophages. Resident peritoneal macrophages, isolated and pooled from CB2+/+ and CB2−/− mice, were cultured for 16 h in the presence or absence of 10 μg/ml 7KC. A: Caspase-3 activity was determined as described in Materials and Methods, and the results are present as the mean fold induction ± SD for three independent experiments. B: Total cell lysates prepared from CB2+/+ and CB2−/− MPMs treated with and without 7KC for 16 h were subjected to immunoblotting using antibodies specific for procaspase-3, cleaved caspase-3 and poly-ADP ribose polymerase (PARP). The blots were striped and probed with an Hsc70 antibody to control for equal loading of the gels. The blots shown are representative of three independent experiments. C: The relative amounts of procaspase-3, cleaved caspase-3, full length PARP and cleaved PARP detected by the immunoblots from the three replicate experiments were quantified by densitometric analysis, normalized to Hsc70 levels, and expressed as the mean ratio of cleaved caspase-3 / procaspase-3 ± SD (C) and as the mean ratio of cleaved PARP / total PARP ± SD, respectively (D). * P < 0.05 between similarly treated CB2−/− and CB2+/+ macrophages.
Induction of arachidonate release and ACAT activity by 7KC is not affected by CB2 deficiency in macrophages
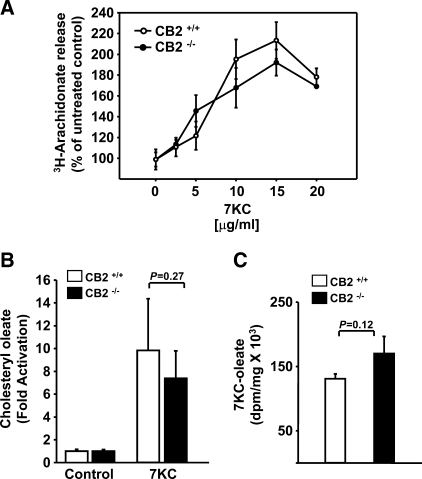
Release of AA, via activation of cPLA2, and ACAT activity are necessary upstream steps in the signaling pathway of oxysterol-induced apoptosis in macrophages (22, 24). We evaluated the ability of 7KC to stimulate AA release in CB2−/− macrophages by determining the amount of radioactivity released into the culture medium from [3H]arachidonate-labeled macrophages. 7KC-induced [3H]arachidonate release was detectable within 1 h and linear for up to 8 h (data not shown) and occurred in a concentration dependent manner, reaching a maximum at 15 μg/ml 7KC for CB2−/− and CB2+/+ macrophages (Fig. 4A). When treated with concentrations of 7KC below that required for induction of apoptosis (5 ⩽ μg/ml), CB2−/− MPMs released slightly more [3H]arachidonate on average than wild-type MPMs, and when treated with concentrations of 7KC (⩾10 μg/ml) that induce apoptosis, the CB2−/− MPMs released slightly less [3H]arachidonate on average than wild-type MPMs; however neither difference reached statistical significance (Fig. 4A).
Fig. 4.
CB2 deficiency does not impair arachidonate release or esterification of sterols in macrophages. Quantitation of the [3H]arachidonate released into the culture media from prelabled CB2+/+ and CB2−/− MPMs following a 6 h incubation in the presence of increasing concentrations of 7KC as indicated in A. MPMs pooled from groups (n ⩾ 6) of CB2+/+ and CB2−/− mice were cultured for 6 h in media containing 1 μCi/ml [3H]oleate and supplemented with and without 10 μg/ml 7KC. Neutral lipids were extracted, separated by TLC, and the amount of radioactivity incorporated into cholesteryl oleate and 7KC-oleate was determined by liquid scintillation counting as described in Materials and Methods. The graph in B shows the mean fold induction ± SD of cholesteryl oleate accumulation for three independent experiments performed in triplicate. The graph in C shows the mean dpm/mg ± SD of 7KC [3H]oleate accumulation for a representative experiment performed in triplicate.
ACAT activity in CB2−/− and CB2+/+ MPMs treated with and without an apoptosis-inducing concentration of 7KC (10 μg/ml) was compared by measuring the incorporation of [3H]oleate into cholesteryl [3H]oleate. The incorporation of [3H]oleate into 7-ketocholesteryl [3H]oleate was also determined in the MPMs treated with 7KC. In CB2+/+ and CB2−/− MPMs, 7KC stimulated cholesteryl 3H-oleate formation to approximately the same extent (9.8 ± 4.5-fold and 7.4 ± 2.4-fold, P = 0.27, respectively) (Fig. 4B). ACAT esterification of the exogenous 7KC, as determined by formation of 7-ketocholesteryl [3H]oleate, also was not statistically different between CB2+/+ and CB2−/− MPMs (133,142 ± 7,024 dpm/mg and 174,329 ± 26,331 dpm/mg, P = 0.12, respectively), (Fig. 4C).
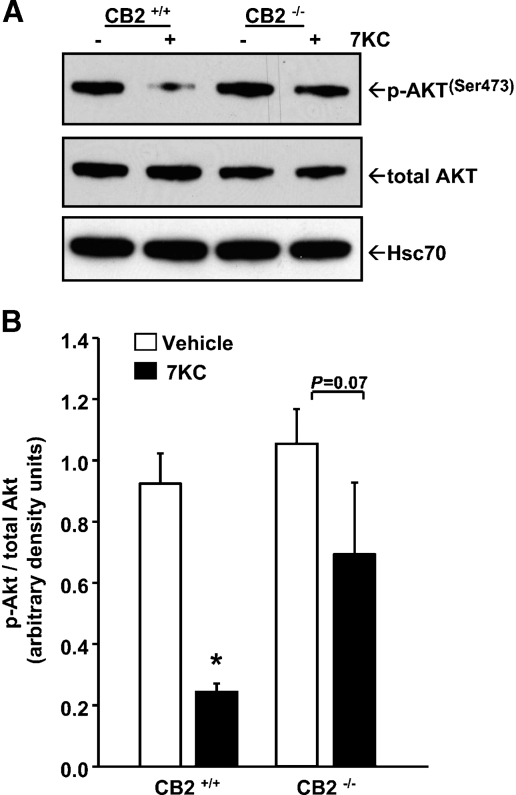
7KC-induced dephosphorylation of Akt is impaired in CB2−/− macrophages
Down-regulation of the prosurvival kinase Akt (protein kinase B), by degradation (21) or dephosphorylation (41), has been observed during oxysterol-induced apoptosis in macrophages. To gain insight into the mechanism by which CB2 deficiency in macrophages imparts resistance to 7KC-induced apoptosis, we used immunoblotting to compare the levels of total Akt and phosphorylated Akt in CB2−/− and CB2+/+ MPMs before and after treatment with an apoptosis-inducing concentration of 7KC (10 μg/ml). We used a phosho-AktSer473 antibody as phosphorylation of ser-473 is required for Akt kinase activity (42). Immunoblots showed that the level of total Akt detected in lysates from CB2−/− MPMs, in comparison to lysates from CB2+/+ MPMs, varied slightly from experiment to experiment. The immunoblot shown in Fig. 5A detected slightly lower levels (∼15% lower after normalizing to Hsc-70) of total Akt in lysates from untreated CB2−/− MPMs compared with lysates from untreated CB2+/+ MPMs. In other immunoblots, the levels of total Akt detected in CB2−/− MPMs were equal to or slightly elevated compared with CB2+/+ MPMs (data not shown). Despite the variability observed in total Akt levels, the immunoblots consistently showed no appreciable difference in active, p-AktSer473, levels between untreated CB2−/− and CB2+/+ MPMs (Fig. 5A). More importantly, 7KC treatment did not alter total Akt levels in either CB2−/− or CB2+/+ MPMs, but did reduce active, p-AktSer473, levels in both, with CB2+/+ MPMs showing a more substantial decrease than CB2 −/− MPMs. Quantitative densitometric analysis of the immunoblots revealed the ratio of p-AktSer473 to total Akt in untreated CB2 −/− MPMs was not statistically different from that in untreated CB2+/+ MPMs (1.1 ± 0.11 and 0.93 ± 0.1, respectively, P = 0.2) (Fig. 5B). After treatment with 7KC, the p-Akt/total Akt ratio in CB2+/+ and CB2 −/− MPMs decreased (∼3.9-fold and ∼1.6-fold, respectively); however, only in CB2+/+ MPMs did the decrease reach statistical significance (0.93 ± 0.1 and 0.24 ± 0.002, P < 0.05, for untreated and treated CB2+/+ MPMs compared with 1.05 ± 0.11 and 0.69 ± 0.23, P = 0.07, for untreated and treated CB2 −/− MPMs).
Fig. 5.
CB2 deficient macrophages have diminished capacity to dephosphorylate Akt in response to 7-ketocholesterol. A: CB2+/+ and CB2−/− MPMs were cultured for 16 h with and without 10 μg/ml 7KC, and the levels of phospho-AktSer473 and total Akt were determined by immunoblotting. The immunoblots were striped and probed with an Hsc70 antibody to evaluate equal loading. Representative blots from three independent experiments are shown. B: The levels of p-Akt and total Akt in the immunoblots were quantified by densitometric analysis, and the data presented as the mean ratio ± SD of p-Akt/total Akt. * P < 0.05 between similarly treated CB2−/− and CB2+/+ macrophages.
DISCUSSION
To determine if CB2 influences the apoptotic response of macrophages to OxLDL/oxysterols, we examined apoptosis in CB2−/− and CB2+/+ resident peritoneal macrophages following exposure to OxLDL or 7KC. By assaying several independent hallmarks of apoptosis, the current study revealed that the apoptotic response of CB2−/− macrophages to OxLDL and 7KC is significantly less than that of CB2+/+ macrophages. The ability to fully undergo apoptosis in response to staurosporine suggests that CB2−/− macrophages are not globally resistant to the induction of apoptosis and supports the conclusion that CB2 deficiency specifically affects OxLDL/oxysterol-induced macrophage apoptosis.
In a previous study, we demonstrated that receptor mediated uptake of OxLDL is a requirement for its cytotoxicity by showing that a cell line normally resistant to OxLDL-induced apoptosis, CHO cells, can be rendered sensitive by ectopic expression of CD36, a receptor for OxLDL (23). In data not shown we found that uptake of modified LDL was unaffected by CB2 deficiency, suggesting that the differential sensitivity of CB2−/− and CB2+/+ macrophages to OxLDL-induced apoptosis is not due to differences in expression of an OxLDL receptor. As oxysterol-induced cell death is independent of OxLDL receptor expression (23), the observation that CB2−/− and CB2+/+ macrophages are also differentially sensitivity to 7KC-induced apoptosis supports this conclusion.
The finding that CB2 deficiency affects both OxLDL- and 7KC-induced apoptosis is in agreement with studies that attribute a large portion of the cytotoxicity of OxLDL to its oxysterol constituents (16, 17). The ability of different oxysterols to induce apoptosis in vascular cells varies greatly and varying combinations of oxysterols can display differing toxicities (43). Therefore, the current study was limited to 7KC as 7KC is the predominant cytotoxic oxysterol formed upon copper-oxidation of LDL (16). Furthermore, the concentration of 7KC (10 μg/ml) used in the current study to induce apoptosis is within the reported physiological range (10–30 μM) of oxysterols present in human plasma following ingestion of a meal containing oxysterols (44) and in plasma of individuals with hypercholesterolemia (18, 19). We also have observed that CB2 null macrophages are resistant to apoptosis induced by another cytotoxic oxysterol, 25-hydroxycholesterol (data not shown).
CB2 deficiency results in only a partial resistance to OxLDL/oxysterol-induced apoptosis. This result is similar to our prior studies in which partial resistance to OxLDL/oxysterol-induced apoptosis was found in macrophages deficient in cPLA2 (22) or ACAT1 (24). This partial resistance likely indicates that multiple apoptotic pathways are initiated in macrophages in response to OxLDL/oxysterols. In support of this, Berthier et al. (41) observed that apoptosis in macrophages induced by very high levels of 7KC (40 μg/ml) involves the activation and interaction of several different Ca2+-dependent apoptotic pathways. Alternatively, CB2 null macrophages may have up regulated the expression of CB1, or another yet unidentified cannabinoid receptor, which partially compensates for the lack of CB2.
A particularly intriguing finding of the current study is that the differential sensitivity of CB2 null macrophages to OxLDL-induced apoptosis was only observed at higher concentrations (⩾ 10 μg/ml) of OxLDL (Fig. 1C). This suggests that an OxLDL threshold exists for the activation of a CB2-dependent apoptotic pathway. The observation that wild-type macrophages were much less sensitive to concentrations of 7KC below 10 μg/ml (Fig. 2A) suggest that this threshold for OxLDL may reflect the concentration of apoptotic oxysterols present in the OxLDL and that the apoptosis induced by lower concentrations of OxLDL is mediated by other (non-oxysterol) apoptotic inducers present in the OxLDL which function by CB2-independent mechanisms. If this were the case, in atherosclerosis one might expect CB2-dependent macrophage apoptosis to be most relevant in more advanced lesions which have accumulated larger amounts of OxLDL. As macrophage apoptosis in advanced lesions is considered a proatherogenic process, strategies for manipulating and inhibiting CB2-dependent apoptotic pathways might be useful to retard lesion progression and prevent lesion rupture, a suggestion given credence by the findings of Steffens et al. (31), demonstrating that oral administration of a cannabinoid reduces progression of established lesions in ApoE knockout mice via a presumably CB2-dependent mechanism.
In macrophages, one of the earliest events triggered by 7KC is an influx of external calcium (23). Our prior work has partially delineated a Ca2+-dependent signaling pathway initiated in macrophages after treatment with 7KC involving the Ca2+-dependent translocation and activation of cPLA2, which hydrolyzes membrane phospholipids releasing free fatty acids, primarily arachidonic acid, which are then esterified to the inducing oxysterol by ACAT to form a putative oxysterol ester signaling intermediate (22, 24). Activation of cPLA2 in CB2 null macrophages in response to 7KC treatment, as determined by release of AA, was equivalent to that in CB2+/+ macrophages (Fig. 4A). Furthermore, ACAT activity was also not significantly different between CB2 null and wild-type macrophages (Fig. 4B, 4C). These results suggest that CB2 deficiency imparts resistance to OxLDL/oxysterol-induced apoptosis in macrophages at a step downstream of cPLA2 and ACAT.
The observation that oxysterol-induced dephosphorylation of Akt is impaired in CB2−/− macrophages is in partial agreement with our previous study demonstrating that ectopic expression of constitutively active Akt transiently protects P388D1 monocyte/macrophages from oxysterol-induced apoptosis (21). In this prior report, oxysterol-induced inactivation of Akt was associated with enhanced proteasomal degradation of Akt. 7KC-induced degradation of Akt in either CB2+/+ or CB2−/− MPMs was not observed in the current study. Instead, 7KC treatment reduced the amount of active p-Akt, an effect that was significantly reduced in CB2 null macrophages (Fig. 5). This result is similar to those of Berthier et al. (41) who noted 7KC-induced reduction of p-Akt in THP-1 macrophages. These results may indicate that monocyte/macrophages of different lineages or from different species may utilize alternative mechanisms to down-regulate Akt in response to OxLDL/oxysterols.
THC-induced apoptosis in a human leukemia cell line, Jurkat cells, has been shown to involve a CB2-dependent stimulation of de novo ceramide synthesis (45). Macrophages treated with a minimally oxidized LDL (mOxLDL) produce ceramide (46) and ceramide has been implicated in mOxLDL-induced macrophage apoptosis (47). However, in another study, mOxLDL was found to protect isolated resident peritoneal macrophages from apoptosis induced by extensively oxidized LDL (OxLDL), as well as apoptosis induced by free cholesterol loading, by a mechanism involving upregulation of Akt phosphorylation (48). mOxLDL also protects bone marrow-derived macrophages from apoptosis induced by growth factor deprivation (49, 50). In this system, the apoptotic pathway is signaled through ceramide synthesis upstream of Akt inactivation and mOxLDL produces its antiapoptotic effect through inhibition of ceramide synthesis and, consequently, maintenance of Akt activation (50). The OxLDL used in the current study was produced by copper oxidation and is extensively oxidized. Whether or not modulation of ceramide levels by OxLDL or mOxLDL is defective in CB2 null macrophages is currently under investigation.
Activated Akt has been observed in atherosclerotic lesions (48), thus, the equilibrium between macrophage survival and death pathways within lesions may, in part, be the result of the degree of LDL oxidation and its differential effects on Akt. Cannabinoid receptor signaling has also been demonstrated to affect Akt pathways in a variety of tumor cell types (51, 52), and modulation of Akt signaling by cannabinoids reduces the susceptibility of some neuronal cell types to apoptosis induced by a variety of insults (53). These studies in other cell types, combined with the results of the current study, suggest that CB2 may play a role in modulating Akt survival pathways in lesional macrophages. Delineation of the exact role(s) of CB2-mediated Akt signaling in atherosclerosis awaits evaluation of the effect of CB2 deficiency on atherogenesis and lesional apoptosis.
In conclusion, elucidating the mechanism by which OxLDL/oxysterols induce macrophage apoptosis in vitro will identify steps in apoptotic pathways in vivo that are potentially amendable to pharmacological manipulation. Utilization of the CB2 receptor as target for the design of CB2-specific based therapies for atherosclerosis should also help avoid a prime limitation of the clinical application of cannabinoids, the psychoactive effects of these compounds mediated by CB1 receptors expressed in the brain. This study progresses toward this goal in that CB2 expression has been identified as a contributing factor in OxLDL-induced apoptosis in macrophages and suggests a functional role for CB2 as a modulator of the Akt survival pathway in macrophages.
Acknowledgments
The authors are grateful to Dr. Michael Sinensky for his many thoughtful discussions and wish to acknowledge his valuable assistance in revising the manuscript.
Abbreviations
ACAT, acyl-coenzyme A:cholesterol transferase
CB1, cannabinoid receptor 1
CB2, cannabinoid receptor 2
cPLA2, cytosolic phospholipase A2
mOxLDL, minimally oxidized LDL
MPMs, mouse peritoneal macrophages
OxLDL, oxidized low density lipoproteins
PARP, poly-ADP ribose polymerase
THC, Δ9-tetrahydrocannabinol
TUNEL, terminal transferase-mediated dUTP nick end labeling
7KC, 7-ketocholesterol
Published, JLR Papers in Press, July 9, 2008.
Footnotes
This work was supported by the National Institutes of Health (NIH) grant HL085137 (D.P.T).
References
- 1.Libby P. 2002. Inflammation in atherosclerosis. Nature. 420 868–874. [DOI] [PubMed] [Google Scholar]
- 2.Stary H. C., A. B. Chandler, R. E. Dinsmore, V. Fuster, S. Glagov, W. Insull, Jr., M. E. Rosenfeld, C. J. Schwartz, W. D. Wagner, and R. W. Wissler. 1995. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Arterioscler. Thromb. Vasc. Biol. 15 1512–1531. [DOI] [PubMed] [Google Scholar]
- 3.Liu J., D. P. Thewke, Y. R. Su, M. F. Linton, S. Fazio, and M. S. Sinensky. 2005. Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler. Thromb. Vasc. Biol. 25 174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arai S., J. M. Shelton, M. Chen, M. N. Bradley, A. Castrillo, A. L. Bookout, P. A. Mak, P. A. Edwards, D. J. Mangelsdorf, P. Tontonoz, et al. 2005. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 1 201–213. [DOI] [PubMed] [Google Scholar]
- 5.Libby P., Y. J. Geng, M. Aikawa, U. Schoenbeck, F. Mach, S. K. Clinton, G. K. Sukhova, and R. T. Lee. 1996. Macrophages and atherosclerotic plaque stability. Curr. Opin. Lipidol. 7 330–335. [DOI] [PubMed] [Google Scholar]
- 6.Kockx M. M., and A. G. Herman. 2000. Apoptosis in atherosclerosis: beneficial or detrimental? Cardiovasc. Res. 45 736–746. [DOI] [PubMed] [Google Scholar]
- 7.Yla-Herttuala S., W. Palinski, M. E. Rosenfeld, S. Parthasarathy, T. E. Carew, S. Butler, J. L. Witztum, and D. Steinberg. 1989. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J. Clin. Invest. 84 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palinski W., M. E. Rosenfeld, S. Yla-Herttuala, G. C. Gurtner, S. S. Socher, S. W. Butler, S. Parthasarathy, T. E. Carew, D. Steinberg, and J. L. Witztum. 1989. Low density lipoprotein undergoes oxidative modification in vivo. Proc. Natl. Acad. Sci. USA. 86 1372–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardwick S. J., L. Hegyi, K. Clare, N. S. Law, K. L. Carpenter, M. J. Mitchinson, and J. N. Skepper. 1996. Apoptosis in human monocyte-macrophages exposed to oxidized low density lipoprotein. J. Pathol. 179 294–302. [DOI] [PubMed] [Google Scholar]
- 10.Escargueil-Blanc I., O. Meilhac, M. T. Pieraggi, J. F. Arnal, R. Salvayre, and A. Negre-Salvayre. 1997. Oxidized LDLs induce massive apoptosis of cultured human endothelial cells through a calcium-dependent pathway. Prevention by aurintricarboxylic acid. Arterioscler. Thromb. Vasc. Biol. 17 331–339. [DOI] [PubMed] [Google Scholar]
- 11.Dimmeler S., J. Haendeler, J. Galle, and A. M. Zeiher. 1997. Oxidized low-density lipoprotein induces apoptosis of human endothelial cells by activation of CPP32-like proteases. A mechanistic clue to the ‘response to injury’ hypothesis. Circulation. 95 1760–1763. [DOI] [PubMed] [Google Scholar]
- 12.Yin J., X. Chaufour, C. McLachlan, M. McGuire, G. White, N. King, and B. Hambly. 2000. Apoptosis of vascular smooth muscle cells induced by cholesterol and its oxides in vitro and in vivo. Atherosclerosis. 148 365–374. [DOI] [PubMed] [Google Scholar]
- 13.Chisolm G. M., G. Ma, K. C. Irwin, L. L. Martin, K. G. Gunderson, L. F. Linberg, D. W. Morel, and P. E. DiCorleto. 1994. 7 beta-hydroperoxycholest-5-en-3 beta-ol, a component of human atherosclerotic lesions, is the primary cytotoxin of oxidized human low density lipoprotein. Proc. Natl. Acad. Sci. USA. 91 11452–11456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown A. J., and W. Jessup. 1999. Oxysterols and atherosclerosis. Atherosclerosis. 142 1–28. [DOI] [PubMed] [Google Scholar]
- 15.Sevanian A., H. N. Hodis, J. Hwang, L. L. McLeod, and H. Peterson. 1995. Characterization of endothelial cell injury by cholesterol oxidation products found in oxidized LDL. J. Lipid Res. 36 1971–1986. [PubMed] [Google Scholar]
- 16.Brown A. J., R. T. Dean, and W. Jessup. 1996. Free and esterified oxysterol: formation during copper-oxidation of low density lipoprotein and uptake by macrophages. J. Lipid Res. 37 320–335. [PubMed] [Google Scholar]
- 17.Lizard G., S. Monier, C. Cordelet, L. Gesquiere, V. Deckert, S. Gueldry, L. Lagrost, and P. Gambert. 1999. Characterization and comparison of the mode of cell death, apoptosis versus necrosis, induced by 7beta-hydroxycholesterol and 7-ketocholesterol in the cells of the vascular wall. Arterioscler. Thromb. Vasc. Biol. 19 1190–1200. [DOI] [PubMed] [Google Scholar]
- 18.Dzeletovic S., O. Breuer, E. Lund, and U. Diczfalusy. 1995. Determination of cholesterol oxidation products in human plasma by isotope dilution-mass spectrometry. Anal. Biochem. 225 73–80. [DOI] [PubMed] [Google Scholar]
- 19.Addis P. B., H. A. Emanuel, S. D. Bergmann, and J. H. Zavoral. 1989. Capillary GC quantification of cholesterol oxidation products in plasma lipoproteins of fasted humans. Free Radic. Biol. Med. 7 179–182. [DOI] [PubMed] [Google Scholar]
- 20.Yang L., and M. S. Sinensky. 2000. 25-Hydroxycholesterol activates a cytochrome c release-mediated caspase cascade. Biochem. Biophys. Res. Commun. 278 557–563. [DOI] [PubMed] [Google Scholar]
- 21.Rusinol A. E., D. Thewke, J. Liu, N. Freeman, S. R. Panini, and M. S. Sinensky. 2004. AKT/protein kinase B regulation of BCL family members during oxysterol-induced apoptosis. J. Biol. Chem. 279 1392–1399. [DOI] [PubMed] [Google Scholar]
- 22.Panini S. R., L. Yang, A. E. Rusinol, M. S. Sinensky, J. V. Bonventre, and C. C. Leslie. 2001. Arachidonate metabolism and the signaling pathway of induction of apoptosis by oxidized LDL/oxysterol. J. Lipid Res. 42 1678–1686. [PubMed] [Google Scholar]
- 23.Rusinol A. E., L. Yang, D. Thewke, S. R. Panini, M. F. Kramer, and M. S. Sinensky. 2000. Isolation of a somatic cell mutant resistant to the induction of apoptosis by oxidized low density lipoprotein. J. Biol. Chem. 275 7296–7303. [DOI] [PubMed] [Google Scholar]
- 24.Freeman N. E., A. E. Rusinol, M. Linton, D. L. Hachey, S. Fazio, M. S. Sinensky, and D. Thewke. 2005. Acyl-coenzyme A:cholesterol acyltransferase promotes oxidized LDL/oxysterol-induced apoptosis in macrophages. J. Lipid Res. 46 1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mechoulam R., S. Ben Shabat, L. Hanus, E. Fride, Z. Vogel, M. Bayewitch, and A. E. Sulcova. 1996. Endogenous cannabinoid ligands chemical and biological studies. J. Lipid Mediat. Cell Signal. 14 45–49. [DOI] [PubMed] [Google Scholar]
- 26.Matsuda L. A., S. J. Lolait, M. J. Brownstein, A. C. Young, and T. I. Bonner. 1990. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 346 561–564. [DOI] [PubMed] [Google Scholar]
- 27.Munro S., K. L. Thomas, and M. Abu-Shaar. 1993. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 365 61–65. [DOI] [PubMed] [Google Scholar]
- 28.Schlicker E., and M. Kathmann. 2001. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol. Sci. 22 565–572. [DOI] [PubMed] [Google Scholar]
- 29.Galiegue S., S. Mary, J. Marchand, D. Dussossoy, D. Carriere, P. Carayon, M. Bouaboula, D. Shire, G. Le Fur, and P. Casellas. 1995. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 232 54–61. [DOI] [PubMed] [Google Scholar]
- 30.Carlisle S. J., F. Marciano-Cabral, A. Staab, C. Ludwick, and G. A. Cabral. 2002. Differential expression of the CB2 cannabinoid receptor by rodent macrophages and macrophage-like cells in relation to cell activation. Int. Immunopharmacol. 2 69–82. [DOI] [PubMed] [Google Scholar]
- 31.Steffens S., N. R. Veillard, C. Arnaud, G. Pelli, F. Burger, C. Staub, M. Karsak, A. Zimmer, J. L. Frossard, and F. Mach. 2005. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 434 782–786. [DOI] [PubMed] [Google Scholar]
- 32.Maccarrone M., and A. Finazzi-Agro. 2003. The endocannabinoid system, anandamide and the regulation of mammalian cell apoptosis. Cell Death Differ. 10 946–955. [DOI] [PubMed] [Google Scholar]
- 33.Zhu W., H. Friedman, and T. W. Klein. 1998. Delta9-tetrahydrocannabinol induces apoptosis in macrophages and lymphocytes: involvement of Bcl-2 and caspase-1. J. Pharmacol. Exp. Ther. 286 1103–1109. [PubMed] [Google Scholar]
- 34.Buckley N. E., K. L. McCoy, E. Mezey, T. Bonner, A. Zimmer, C. C. Felder, M. Glass, and A. Zimmer. 2000. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. Eur. J. Pharmacol. 396 141–149. [DOI] [PubMed] [Google Scholar]
- 35.Nicholson D. W., A. Ali, N. A. Thornberry, J. P. Vaillancourt, C. K. Ding, M. Gallant, Y. Gareau, P. R. Griffin, M. Labelle, Y. A. Lazebnik, et al. 1995. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 376 37–43. [DOI] [PubMed] [Google Scholar]
- 36.Talanian R. V., C. Quinlan, S. Trautz, M. C. Hackett, J. A. Mankovich, D. Banach, T. Ghayur, K. D. Brady, and W. W. Wong. 1997. Substrate specificities of caspase family proteases. J. Biol. Chem. 272 9677–9682. [DOI] [PubMed] [Google Scholar]
- 37.Girish V., and A. Vijayalakshmi. 2004. Affordable image analysis using NIH Image/ImageJ. Indian J. Cancer. 41 47. [PubMed] [Google Scholar]
- 38.Kelly K. J., R. M. Sandoval, K. W. Dunn, B. A. Molitoris, and P. C. Dagher. 2003. A novel method to determine specificity and sensitivity of the TUNEL reaction in the quantitation of apoptosis. Am. J. Physiol. Cell Physiol. 284 C1309–C1318. [DOI] [PubMed] [Google Scholar]
- 39.Bertrand R., E. Solary, P. O'Connor, K. W. Kohn, and Y. Pommier. 1994. Induction of a common pathway of apoptosis by staurosporine. Exp. Cell Res. 211 314–321. [DOI] [PubMed] [Google Scholar]
- 40.Sun X. M., M. MacFarlane, J. Zhuang, B. B. Wolf, D. R. Green, and G. M. Cohen. 1999. Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J. Biol. Chem. 274 5053–5060. [DOI] [PubMed] [Google Scholar]
- 41.Berthier A., S. Lemaire-Ewing, C. Prunet, T. Montange, A. Vejux, J. P. Pais de Barros, S. Monier, P. Gambert, G. Lizard, and D. Neel. 2005. 7-Ketocholesterol-induced apoptosis. Involvement of several pro-apoptotic but also anti-apoptotic calcium-dependent transduction pathways. FEBS J. 272 3093–3104. [DOI] [PubMed] [Google Scholar]
- 42.Chan T. O., S. E. Rittenhouse, and P. N. Tsichlis. 1999. AKT/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu. Rev. Biochem. 68 965–1014. [DOI] [PubMed] [Google Scholar]
- 43.Biasi F., G. Leonarduzzi, B. Vizio, D. Zanetti, A. Sevanian, B. Sottero, V. Verde, B. Zingaro, E. Chiarpotto, and G. Poli. 2004. Oxysterol mixtures prevent proapoptotic effects of 7-ketocholesterol in macrophages: implications for proatherogenic gene modulation. FASEB J. 18 693–695. [DOI] [PubMed] [Google Scholar]
- 44.Emanuel H. A., C. A. Hassel, P. B. Addis, S. D. Bergamn, and J. H. Zavoral. 1991. Plasma cholesterol oxidation products (sterols) in human subjects fed a meal rich in oxysterols. J. Food Sci. 56 843–847. [Google Scholar]
- 45.Herrera B., A. Carracedo, M. Diez-Zaera, T. Gomez del Pulgar, M. Guzman, and G. Velasco. 2006. The CB2 cannabinoid receptor signals apoptosis via ceramide-dependent activation of the mitochondrial intrinsic pathway. Exp. Cell Res. 312 2121–2131. [DOI] [PubMed] [Google Scholar]
- 46.Kinscherf R., R. Claus, H. P. Deigner, O. Nauen, C. Gehrke, A. Hermetter, S. Russwurm, V. Daniel, V. Hack, and J. Metz. 1997. Modified low density lipoprotein delivers substrate for ceramide formation and stimulates the sphingomyelin-ceramide pathway in human macrophages. FEBS Lett. 405 55–59. [DOI] [PubMed] [Google Scholar]
- 47.Deigner H. P., R. Claus, G. A. Bonaterra, C. Gehrke, N. Bibak, M. Blaess, M. Cantz, J. Metz, and R. Kinscherf. 2001. Ceramide induces aSMase expression: implications for oxLDL-induced apoptosis. FASEB J. 15 807–814. [DOI] [PubMed] [Google Scholar]
- 48.Boullier A., Y. Li, O. Quehenberger, W. Palinski, I. Tabas, J. L. Witztum, and Y. I. Miller. 2006. Minimally oxidized LDL offsets the apoptotic effects of extensively oxidized LDL and free cholesterol in macrophages. Arterioscler. Thromb. Vasc. Biol. 26 1169–1176. [DOI] [PubMed] [Google Scholar]
- 49.Hundal R. S., B. S. Salh, J. W. Schrader, A. Gomez-Munoz, V. Duronio, and U. P. Steinbrecher. 2001. Oxidized low density lipoprotein inhibits macrophage apoptosis through activation of the PI 3-kinase/PKB pathway. J. Lipid Res. 42 1483–1491. [PubMed] [Google Scholar]
- 50.Hundal R. S., A. Gomez-Munoz, J. Y. Kong, B. S. Salh, A. Marotta, V. Duronio, and U. P. Steinbrecher. 2003. Oxidized low density lipoprotein inhibits macrophage apoptosis by blocking ceramide generation, thereby maintaining protein kinase B activation and Bcl-XL levels. J. Biol. Chem. 278 24399–24408. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez M. G., L. Ruiz-Llorente, A. M. Sanchez, and I. Diaz-Laviada. 2003. Activation of phosphoinositide 3-kinase/PKB pathway by CB(1) and CB(2) cannabinoid receptors expressed in prostate PC-3 cells. Involvement in Raf-1 stimulation and NGF induction. Cell. Signal. 15 851–859. [DOI] [PubMed] [Google Scholar]
- 52.Ozaita, A., E. Puighermanal, and R. Maldonado. 2007. Regulation of PI3K/Akt/GSK-3 pathway by cannabinoids in the brain. J Neurochem. [DOI] [PubMed]
- 53.Molina-Holgado E., J. M. Vela, A. Arevalo-Martin, G. Almazan, F. Molina-Holgado, J. Borrell, and C. Guaza. 2002. Cannabinoids promote oligodendrocyte progenitor survival: involvement of cannabinoid receptors and phosphatidylinositol-3 kinase/Akt signaling. J. Neurosci. 22 9742–9753. [DOI] [PMC free article] [PubMed] [Google Scholar]