Abstract
Lithocholate (LC) (10–300 μM) in physiological solution is sensed by vascular myocyte large conductance, calcium- and voltage-gated potassium (BK) channel β1 accessory subunits, leading to channel activation and arterial dilation. However, the structural features in steroid and target that determine LC action are unknown. We tested LC and close analogs on BK channel (pore-forming cbv1+β1 subunits) activity using the product of the number of functional ion channels in the membrane patch (N) and the open channel probability (Po). LC (5β-cholanic acid-3α-ol), 5α-cholanic acid-3α-ol, and 5β-cholanic acid-3β-ol increased NPo (EC50 ∼45 μM). At maximal increase in NPo, LC increased NPo by 180%, whereas 5α-cholanic acid-3α-ol and 5β-cholanic acid-3β-ol raised NPo by 40%. Thus, the α-hydroxyl and the cis A-B ring junction are both required for robust channel potentiation. Lacking both features, 5α-cholanic acid-3β-ol and 5-cholenic acid-3β-ol were inactive. Three-dimensional structures show that only LC displays a bean shape with clear-cut convex and concave hemispheres; 5α-cholanic acid-3α-ol and 5β-cholanic acid-3β-ol partially matched LC shape, and 5α-cholanic acid-3β-ol and 5-cholenic acid-3β-ol did not. Increasing polarity in steroid rings (5β-cholanic acid-3α-sulfate) or reducing polarity in lateral chain (5β-cholanic acid 3α-ol methyl ester) rendered poorly active compounds, consistent with steroid insertion between β1 and bilayer lipids, with the steroid-charged tail near the aqueous phase. Molecular dynamics identified two regions in β1 transmembrane domain 2 that meet unique requirements for bonding with the LC concave hemisphere, where the steroid functional groups are located.
Keywords: steroids, protein-ligand interaction, computational modeling, maxiK channel, vascular smooth muscle, vasodilation
Upon activation, voltage- and Ca2+-gated K+ channels of large conductance (BKs) generate K+ outward currents, which tend to hyperpolarize the membrane potential and thus, negatively feed back on depolarization-induced Ca2+ influx. In vascular smooth muscle, this BK channel-mediated negative feedback mechanism serves to control myogenic tone and favors relaxation (1, 2). A wide variety of physiologically relevant steroids, including estrogens, androgens, mineralo- and glucocorticoids, and bile acids, increase BK channel activity (3–5), a mechanism that might contribute to nongenomic modulation of myogenic tone. In particular, lithocholate (LC) and other bile acids, at aqueous concentrations ranging from 10 to 300 μM, are highly effective activators of BK channels (4, 6). This action can explain or at least contribute to the well-known vasodilating properties and hyperkinetic circulation induced by bile acids in human pathophysiology (7). In a wide variety of human pathological conditions (8–12), a significant spillover of bile acids from the portal to the systemic circulation occurs, resulting in 10- to100-fold (even close to 1 mM) higher systemic bile acid concentrations (11, 13, 14). These levels are within and even above the concentrations of bile acids reported to enhance vascular myocyte BKCa channel activity (4).
In hepatobiliary disease, there may be not only an overall increase in systemic bile acid levels, but also a change in composition. In particular, free and conjugated LC levels, which normally constitute 5–10% of total bile acids, can dramatically increase in liver disease (11).
Among naturally occurring bile acids, the monohydroxylated LC is remarkably effective in increasing vascular myocyte BK channel activity (4), which is consistent with the powerful smooth muscle relaxant properties of hydrophobic bile acids (15). We have recently demonstrated that LC-induced vasodilation is largely endothelium-independent, and blunted after genetic ablation of KCNMB1 (6). This gene codes for the BK channel accessory subunit of the β1 type, which is particularly abundant in smooth muscle (16). Moreover, studies with recombinant BK channels in cell-free membrane patches demonstrate that coexpression of accessory β1 subunits is necessary for LC to increase the activity of BK channel complexes that include pore-forming subunits (cbv1; AY330293) cloned from arterial myocytes (6). Notably, channel accessory subunits of the β4 type, which prevail in nervous tissue, fail to render the BK channel complex sensitive to LC (6). Furthermore, recent data from BK channels that include β1-β4 chimeric subunits demonstrate that the β1 transmembrane domain 2 (TM2) distinctly behaves as a bile acid-sensing element (17). Whether β1 TM2 provides a docking area that allows selective interaction with LC, a structural hypothesis for this steroid effectiveness on channel function, remains unknown.
Structural specificity in protein binding sites requires defined structural determinants in the ligand molecule. Previous studies indicate that the overall hydrophobicity of the steroid nucleus and “planar polarity” of the molecule, i.e., the existence of distinct hydrophobic and hydrophilic sides or hemispheres in a rigid amphiphile (18, 19), favored BK channel activation by naturally occurring bile acids (4). However, the specific structural determinants that make LC such a highly effective activator of BK channels remain to be identified.
Using a combination of computational simulations and single-channel, patch-clamp electrophysiology on recombinant BK channels cloned from vascular smooth muscle (cbv1 pore-forming + accessory β1 subunits), the current study pinpoints the specific structural determinants in the steroid molecule that are necessary for the naturally occurring LC and related analogs to activate BK channels. In addition, we postulate a structural model(s) of LC insertion in the β1 TM2-bilayer interface and docking onto specific β1 TM2 regions, which explains the differential efficacy of bile acids on BK channel activity. This information provides critical insight for designing LC-based pharmacophores to select novel and effective vasodilators that target smooth muscle BK channels.
METHODS
cRNA preparation and injection into Xenopus oocytes
Full-length cDNA coding for cbv1-subunits (AY330293) was cloned from rat cerebral artery myocytes by PCR and ligated to the PCR-XL-TOPO cloning vector (Invitrogen, Carlsbad, CA). Cbv1 cDNA was then cleaved by BamHI (Invitrogen) and XhoI (Promega, Madison, WI), and directly inserted into the pOX vector for expression in Xenopus oocytes. pOX-cbv1 was linearized with NotI (Promega) and transcribed in vitro using T3 polymerase. BK β1 cDNA inserted into the EcoR I/Sal I sites of the pCI-neo expression vector was linearized with NotI and transcribed in vitro using T7 polymerase. The mMessage-mMachine kit (Ambion, Austin, TX) was used for transcription. The pOX vector and the BK β1 cDNA were generous gifts from Aguan Wei (Washington University, Saint Louis, MO) and Maria Garcia (Merck Research Laboratories, Whitehouse Station, NJ), respectively.
Oocytes were removed from Xenopus laevis (NASCO, Fort Atkinson, WI; Xenopus Xpress, Plant City, FL) and prepared as described elsewhere (20). cRNA was dissolved in diethyl polycarbonate-treated water at 5 (cbv1) and 15 (β1) ng/μl; 1 μl aliquots were stored at −70°C. Cbv1 cRNA (2.5 ng/μl) was coinjected with β1 (7.5 ng/μl) cRNA, giving molar ratios ≥6:1 (β:α). cRNA injection (23 nl/oocyte) was conducted using a modified micropipette (Drummond, Broomall, PA). The interval between injection and patch-clamp recordings was 48–72 h.
Electrophysiology data acquisition
Oocytes were prepared for patch-clamp recordings as described (20). Currents were recorded from inside-out (I/O) patches. Both bath and electrode solutions contained (mM): 135 K+ gluconate, 5 EGTA, 2.28 MgCl2, 5.22 CaCl2, 15 HEPES, 1.6 HEDTA, pH 7.35. In all experiments, the free Ca2+ in solution was adjusted to 10 μM by adding CaCl2. Free Ca2+ was calculated with MaxChelator Sliders (Stanford University, Palo Alto, CA) and validated experimentally using Ca2+-selective and reference electrodes (Corning Inc., Corning, NY).
Patch-recording electrodes were made as previously described (20). An agar bridge with gluconate as the main anion was used as the ground electrode. After excision from the oocyte, the membrane patch was exposed to a stream of bath solution containing each agent at final concentration. Solutions were applied onto the cytosolic side of the membrane patch using a pressurized, automated DAD12 system (ALA, New York, NY) via a micropipette tip with an internal diameter of 100 μm. Experiments were carried out at room temperature (21°C).
Currents were recorded using an EPC8 amplifier (HEKA, Lambrecht/Pfalz, Germany) at 1 kHz using a low-pass, eight-pole Bessel filter (Frequency Devices 902LPF, Haverhill, MA). Data were digitized at 5 kHz using a Digidata 1320A A/D converter and pCLAMP 8.0 (Molecular Devices, Haverhill, MA). As index of channel steady-state activity, we used the product of the number of functional ion channels in the membrane patch [N] and the open channel probability (Po). NPo was obtained from all-points amplitude histograms (21) from ≥30 s of continuous recording under each experimental condition.
Chemicals and compound perfusion
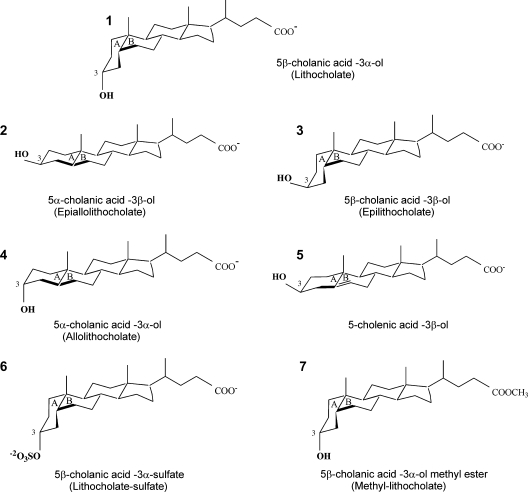
LC and its structural analogs (Fig. 1), with the exception of 5α-cholanic acid 3β-ol (epialloLC), were purchased from Steraloids (Newport, RI). EpialloLC was generously provided by Dr. Takashi Iida (Nihon University, Tokyo, Japan). All other chemicals were purchased from Sigma (St. Louis, MO). On the day of the experiment, a stock solution (333 mM) of each cholane derivative (LC or analog) was freshly made in DMSO by sonication for 5 min. For electrophysiological recordings, the cholane-containing stock solution was diluted 1/10 in 95% ethanol, and further diluted with bath solution to final cholane concentrations (3–1,000 μM). The DMSO-ethanol vehicle in bath solution (≤0.1/≤0.86% final concentrations) was used as control perfusion.
Fig. 1.
Molecular structures of lithocholate (LC) and its derivatives. In all structures (except number 7) the free carboxyl group (pKa = 5) at the end of the lateral chain is shown in its ionized form, which predominates in our experimental conditions (pH 7.4). In all panels, both chemical and trivial (when appropriate) names are provided.
For LC and analogs, both chemical and common names are given when first used (the latter in parenthesis). Then, common names (when available) are used throughout the manuscript.
Electrophysiology data analysis
Data are expressed as mean ± SEM; n = number of patches. NPo changes in response to perfusion with cholane-containing solution are shown as percentage of control, which was the NPo obtained under control perfusion (see above) immediately before applying cholane-containing solution. Data were analyzed with pCLAMP 8.0 (Molecular Devices). Further analysis, plotting, and fitting were conducted using Origin 7.0 (Originlab, Northampton, MA) and InStat 3.0 (GraphPad Software, San Diego, CA). Statistical analysis was conducted using one-way ANOVA and Bonferroni's multiple comparison test; significance was set at P < 0.05.
Computer modeling of steroid structures
Three-dimensional structures of LC and its structural analogs were modeled using Molecular Operating Environment (MOE) software (Chemical Computing Group, Montreal, Canada). Models were constructed in ionized states, which should be predominant at pH 7.4, and optimized with the MMFF94 forcefield (14) to a root mean square gradient (RMSG) of 0.1 kcal·mol−1·Å−1 (22). Conformations of LC were generated using the stochastic conformational search routine in MOE, with default dielectric settings equivalent to gas-phase. The conformational preferences in the low-dielectric lipid environment were expected to be similar. Structural analogs were flexibly aligned onto the lowest energy conformation of LC.
Computer modeling of steroid-BK hβ1 complexes
The polypeptide extending from residue Q155 to S179, which contains the BK hβ1 second transmembrane domain (TM2, residues A156-V178; 9) was modeled as an ideal α helix, and optimized with the AMBER99 forcefield to an RMSG of 0.1 kcal·mol−1·Å−1 (23). The dielectric constant for this model was set as 3, this value being appropriate for the lipid membrane interior (24). The lowest energy structure of LC was manually positioned with either its C3 hydroxyl near T165 and its C24 carboxyl facing the extracellular aqueous medium or its C3 hydroxyl near T169 and its C24 carboxyl facing the intracellular aqueous medium. Single bonds in the acyclic chain of LC were rotated as needed to position the functional groups as described. These starting complexes were optimized to an RMSG of 0.1 kcal·mol−1·Å−1. Molecular dynamics simulations (1 ns) using 2 fs time steps at 300°K were performed to assess the stability and structural changes of each complex over time.
RESULTS
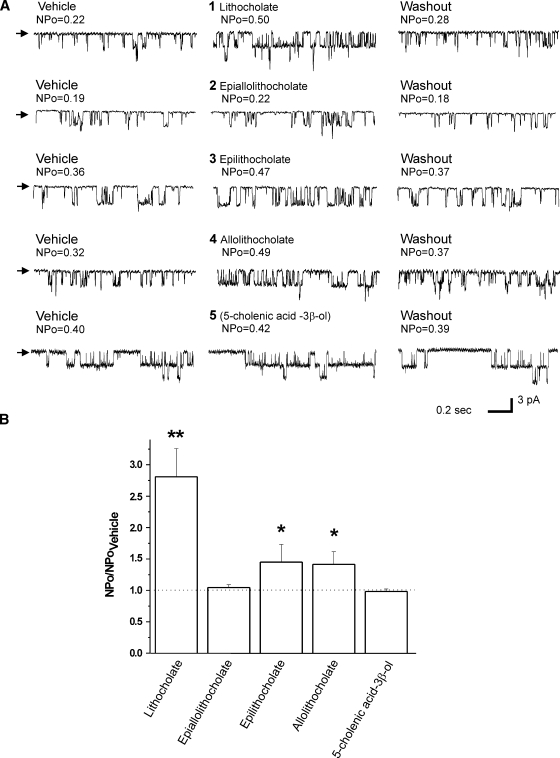
A cis junction between rings A and B and the α configuration of the C3 hydroxyl are both necessary for LC and analogs to robustly increase BK channel activity. The steroidal structure of LC (LC; compound 1 in Fig. 1) is characterized by: 1) a junction between rings A and B in cis configuration, which provides a bean shape to the steroidal ring structure, and 2) a single polar group, the hydroxyl located in C3 and in α configuration. This configuration ensures that the polar group has an axial orientation, that is, it points to the opposite side of the ring plane than the methyl groups (18) (Fig. 1). We first determined whether a cis junction between rings A and B and the α configuration of the C3 hydroxyl are, indeed, necessary for LC activation of BK channels. To do so, we compared LC action on BK channel NPo with that of 5α-cholanic acid-3β-ol, an LC analog with an A/B ring junction in trans configuration and its C3 hydroxyl in β configuration (epialloLC or compound 2 in Fig. 1). Each compound was perfused onto the intracellular side of I/O membrane patches (see Methods), with a free Ca2+i set to 10 μM and Vm = −20 mV. These conditions of Ca2+i and voltage approach those faced by native BK channels in cerebral artery myocytes during contraction (25, 26). At a concentration of 150 μM, which is the EC90 for LC to activate both native cerebral artery myocyte and recombinant (cbv1+β1) channels (6), epialloLC routinely failed to modify BK channel activity (Fig. 2A, B). The difference between LC and epialloLC persisted even when the two analogs were evaluated at concentrations as high as 300 μM (Fig. 3), that is, close to their critical micellar concentration (CMC) under our ionic recording conditions (≤1 mM) (4, 27). Application of epialloLC or any other monohydroxylated bile acid (see below) at ≥1 mM routinely resulted in loss of the gigaohm recording seal, probably due to nonspecific membrane disruption caused by bile acid micelles (4). These data indicate that a cis junction between rings A and B and/or the α configuration of the C3 hydroxyl are necessary for LC to increase BK channel activity.
Fig. 2.
Differential action of LC and derivatives on recombinant large conductance, calcium- and voltage-gated potassium (BK) (cbv1+β1) channels in inside-out (I/O) patches excised from Xenopus laevis oocytes. A: Representative single-channel records obtained at Vm = −20 mV in symmetric Ca2+ = 10 μM. Arrows indicate baseline. Each compound (150 μM) and vehicle was tested on the same patch; each compound-vehicle pair was tested on different patches excised from different oocytes. B: Averaged channel responses to LC and derivatives. * P < 0.05; ** P < 0.01 vs. vehicle; n = 6 (LC), n = 6 (epialloLC), n = 4 (epiLC), n = 3 (alloLC), and n = 4 (5-cholenic acid-3β-ol).
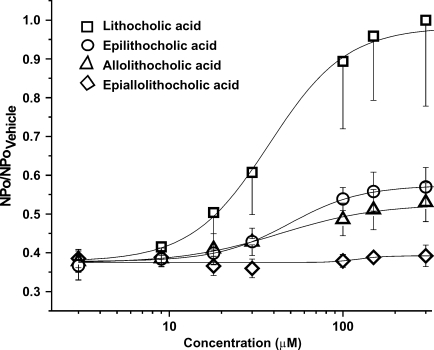
Fig. 3.
Concentration-response curves for cbv1+β1 channel activation by LC and structural analogs. Data were obtained under conditions identical to those described for Fig. 2. Each data point represents the average response from no fewer than three membrane patches. Each average value was normalized to the maximum activation of BK channels by LC. Error bars indicate SEM.
To determine whether the two structural features in the LC steroidal ring considered above are both critical for LC action, we used two other monohydroxylated bile acids, each differing from LC in only one structural feature. The two compounds examined are 5β-cholanic acid-3β-ol (epiLC or compound 3 in Fig. 1) and 5α-cholanic acid-3α-ol (alloLC or compound 4 in Fig. 1). These structures differ from LC in either the C3 hydroxyl configuration (epiLC) or ring junction (alloLC). Under recording conditions identical to those used to probe LC and epialloLC, 150 μM epiLC or alloLC applied to the cytosolic side of I/O patches also caused a reversible activation of recombinant cbv1+β1 channels (Fig. 2A). However, the maximal increase in NPo (Emax or “efficacy”) caused by epiLC (145% of control; Fig. 2B) or alloLC (141% of control; Fig. 2B) was considerably smaller (P < 0.05) than that evoked by LC (epiLC Emax/LC Emax = 0.54 and alloLC Emax/LC Emax = 0.52). The effects of epiLC and alloLC are statistically similar (P > 0.05). The results with epiLC on recombinant channels extend previous findings with vascular smooth muscle native BK channels showing that epiLC (100 and 333 μM) causes a reduced potentiation when compared with that evoked by LC (4). EpiLC and alloLC actions on recombinant cbv1+β1 channels were concentration-dependent, with potentiation of channel activity being smaller than evoked by LC at any given bile acid concentration, up to concentrations close to CMC (Fig. 3). These results indicate that the hydroxyl configuration and cis junction between rings A and B are both required to ensure a robust activation of BK channels by LC and analogs. The presence of one of these structural features in the steroid molecule, however, suffices to evoke some channel activation by bile acids.
Although epiLC and alloLC greatly differed from LC in Emax, the three monohydroxylated bile acids increased BK channel steady-state activity with similar apparent affinities (EC50 = 43.5 ± 5.9, 44.2 ± 6.3, and 48.6 ± 5.8 μM for LC, alloLC, and epiLC, respectively; P > 0.05) (Fig. 3; Table 1). This similarity appears to indicate that there are no significant differences in the access of these three analogs to a functional target(s) in the cell membrane.
TABLE 1.
Potency (EC50) and effectiveness (Emax) of different monohydroxylated bile acids for activating BK (cbv1+β1) channels
| Compound | EC50 | Maximum Effect |
|---|---|---|
| μM | % control | |
| Lithocholate | 43.53 ± 5.95 | 281 ± 45 |
| Epiallolithocholate | N/A | 109 ± 13 |
| Epilithocholate | 48.60 ± 5.76 | 145 ± 28 |
| Allolithocholate | 44.21 ± 6.28 | 141 ± 20 |
| 5-Cholenic acid-3β-ol | N/A | 98 ± 12 |
| Lithocholate-sulfate | 42.16 ± 7.12 | 153 ± 23 |
| Methyl-lithocholate | N/A | 119 ± 11 |
BK, large conductance, calcium- and voltage-gated potassium; N/A, non-applicable, because the analog was ineffective below critical micellar concentration. Half-maximal effective concentrations (EC50s) were obtained from linear interpolation from concentration-response curves (Fig. 3). Maximal effect (Emax) is shown as the increase in BK NPo as percent of control. For all compounds tested, Emax was obtained at 300 μM in aqueous solution; n = 4–6. Data are shown as mean ± SEM.
BK channel activation by monohydroxylated bile acids depends on the steroid molecular shape
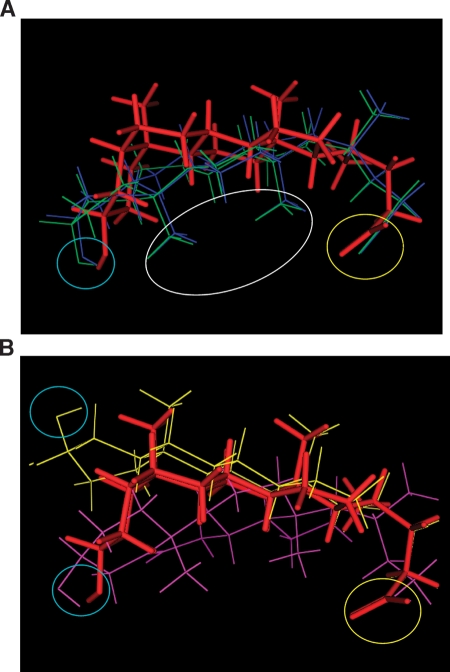
The results shown in Figs. 2 and 3 indicate that the cis junction between rings A and B and the α configuration of the C3 hydroxyl are both necessary for a robust activation of BK channels by LC. EpialloLC, lacking both structural constraints, failed to modify channel activity, whereas epiLC and alloLC, having just one, caused some activation. Moreover, the structural determinant that appears to enable epiLC and alloLC to activate the BK channel is different for each of these analogs. Conceivably, BK channel activation by monohydroxylated bile acids is favored by molecular resemblance to LC. Thus, to begin to relate the differences in monohydroxylated bile acid efficacy for activating BK channels with the overall shape of the bile acid molecule, we constructed three-dimensional structures of LC and its structural analogs in the ionized state. The carboxylic acid group of unconjugated bile acids in aqueous solution has been reported to have apparent pKas ranging from 4.98 (for cholate at concentrations <CMC) to 6.2 (for chenodeoxycholate in soluble micelles) (28, 29), which rise to ∼7 when bile acids are embedded within a proteolipid environment (29, 30). Our model of LC insertion in the membrane, however, proposes that the carboxylate in the steroid lateral chain is located in the aqueous face (pH 7.4) in close proximity to the membrane. Thus, although we do not dismiss a possible interaction between the channel receptor and bile acids in their nonionized carboxylic acid form (see Discussion), our steroid models were constructed considering that bile acids with ionized carboxylate groups predominate. Alignment of structural analogs to the lowest energy conformation of LC emphasizing the fit of polar functional groups during flexible superposition shows that only LC displays a molecular concave shape (including its movable side chain) with polar functional groups at the rim of the concave hemisphere. EpiLC and alloLC, which produce modest channel activation when compared with LC, can match the polar functional group positions of LC (Fig. 4A). However, an optimal match of the polar functional groups involved both conformational changes in the steroid A ring and reversed methyl group orientations relative to LC, which might compromise the ability of these compounds to induce the conformational change in their biomolecular target that gives maximal channel activation.
Fig. 4.
Three-dimensional superposition of LC and analogs. A: Molecular structures of alloLC (navy lines), and epiLC (green lines) superposed onto the lowest energy conformation of LC (red sticks). The white oval shows that when the fit of polar functional groups is prioritized during the flexible superposition, the methyl groups in steroid nucleus of epiLC and alloLC are in the reverse direction to those of LC. This superposition shows that only LC displays a concave shape with polar functional groups at the rim of the concavity, although another two structures can successfully place their polar functional groups in a similar location. B: Structures of epialloLC (yellow lines) and 5-cholenic acid-3β-ol (purple lines) cannot be fully superposed onto the LC molecule (red sticks). While epialloLC places its hydrogen bond acceptor away from the C3 hydroxyl of LC, 5-cholenic acid-3β-ol cannot adopt fully bean-shaped conformation due to the presence of a double bond in the B-ring and, therefore, rigidity of the A/B ring junction. For panels A and B, yellow and blue circles emphasize overlap of acidic groups and hydrogen bond acceptors, respectively.
EpialloLC, which failed to activate BK channels at all concentrations tested (Figs. 2, 3), is unable to simultaneously match the acid- and hydrogen bond-accepting groups to LC conformation and shape (Fig. 4B). Finally, we investigated 5-cholenic acid-3β-ol (compound 5 in Fig. 1), where the ring unsaturation provides extra rigidity to the A-B rings and a bend at the A-B ring junction that is intermediate between that formed by a cis A-B junction (found in LC and epiLC) and that formed by a trans A-B ring junction (found in alloLC and epialloLC). This analog could overlap with LC in both the carboxylate region and the hydrogen bond acceptors (Fig. 4B). However, its rigid A/B ring structure cannot overlap with the concavity of LC (Fig. 4B). Application of 5-cholenic acid-3β-ol (150 μM) barely modified BK channel activity (Fig. 2A, B), which buttresses the importance of the bean shape of the bile acid molecule for activating BK channels. In synthesis, the two compounds that fail to overlap with the LC molecule also failed to activate the BK channel. Indeed, the ability of a monohydroxylated bile acid to activate the BK channel is a direct function of the analog structural match with the LC structure (see supplementary Fig. I). Mismatch with LC, however, was primarily determined via different regions in the steroid molecule: the polar regions and the overall hydrophobic volume for epialloLC and 5-cholenic acid-3β-ol, respectively (Table 2). Collectively, these results indicate that small structural changes affecting the overall bean shape of the bile acid molecule drastically alter the efficacy of monohydroxylated bile acids in activating BK channels, and support the notion of a defined locus where the bile acid molecule should fit in order to stabilize a conformational change in their biomolecular target that results in increased channel activity.
TABLE 2.
RMSDs for heavy atoms and polar groups for different structural analogs when compared with the lithocholate molecule
| RMSD |
||
|---|---|---|
| Molecular Structure | Heavy Atoms | Polar Groups |
| Å | ||
| Lithocholate | 0 | 0 |
| Epilithocholate | 2.2 | 0.6 |
| Allolithocholate | 2.3 | 0.6 |
| Epiallolithocholate | 1.6 | 3 |
| 5-cholenic acid-3β-ol | 3.2 | 0.7 |
RMSD, root mean square deviation.
Putative docking model between LC and BK β1 subunits
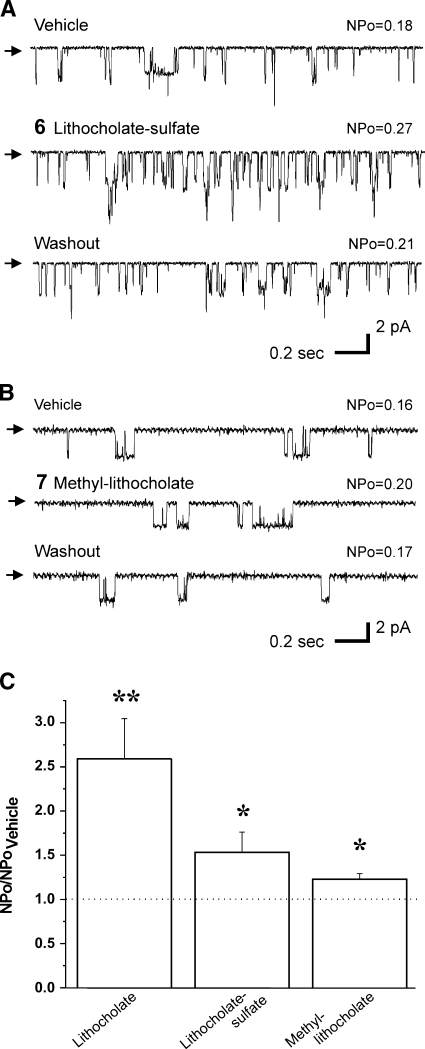
We recently demonstrated that the BK β1 subunit TM2 is necessary for LC to activate BK channels. Moreover, it is likely that this domain represents or at least contributes to the LC binding site (17). We proposed that the LC-target interaction required the steroid to insert normally to the bilayer plane: the hydrophobic hemisphere of the planar amphiphile faces the bilayer lipids while the hemisphere containing the polar hydroxyl faces polar amino acids provided by the β1-subunit, with the carboxylate/carboxylic acid of the lateral chain residing in or near the aqueous solution (6, 17). If this model is correct, substitution of highly ionized groups for the polar hydroxyl group should decrease steroid insertion in the bilayer and, thus, reduce steroid access to the putative target site. Indeed, application of 150 μM 5β-cholanic acid-3α-sulfate (LCsulfate or compound 6 in Fig. 1) to the cytosolic side of I/O patches caused an increase in BK NPo that was significantly smaller than that evoked by LC (LCsulfate Emax/LC Emax = 0.59), with NPo in LCsulfate reaching 153% of control (Fig. 5A, C; see supplementary Fig. II).
Fig. 5.
LCsulfate and methylLC barely modify cbv1+β1 channel activity. Representative single-channel records from I/O patches in the presence of 150 μM LCsulfate (A) or methylLC (B), and respective vehicle-containing controls. All records were obtained at Vm = −20 mV in symmetric Ca2+ = 10 μM. Arrows indicate baseline. C: Averaged channel responses to LC, LCsulfate, and methylLC. * P < 0.05 or ** P < 0.01 vs. vehicle-containing solution; n = 6 (LC), n = 6 (LCsulfate), and n = 5 (methylLC). Error bars indicate SEM.
Negatively charged lipids are very effective activators of BK channels, with sulfo derivatives even surpassing activation by analogs containing carboxyls in equivalent positions in the molecule (e.g., tetradecasulfonate vs. myristic acid) (31). In the case of negatively charged FAs, BK channel activation does not require accessory β subunits, with the channel-forming α being sufficient. Thus, to determine whether α(“cbv1”)+β1 channel response to LCsulfate could be explained by an action of this highly charged derivative on the channel α subunit, we probed LCsulfate on homomeric cbv1 channel function. Cbv1 channels, however, remained insensitive to concentrations of LCsulfate as high as 300 μM (see supplementary Fig. III). This result strongly suggests that LCsulfate effect on cbv1+β1 requires the presence of the accessory subunit, as LC does. Thus, the reduced efficacy of LCsulfate is probably determined by the reduced penetration of the bilayer core by LCsulfate molecules.
Finally, to further test our insertion model, we reasoned that suppression of charge in the carboxylate of the bile acid lateral chain should diminish the solubility and/or insertion of the ionized group in the aqueous phase and, thus, diminish channel activation by these steroids. Therefore, we probed 5β-cholanic acid 3α-ol methyl ester (methylLC or compound 7 in Fig. 1), which contains a polar yet nonionized group at the end of the lateral chain. As expected, 150 μM methylLC caused a very slight increase in BK channel NPo, not exceeding 123% of control (Fig. 5B, C).
Having explored the contribution of A/B cis junction bend, α conformation of the hydroxyl group, steroidal hydrophobic concavity, and polarity of the lateral chain to recombinant BK channel activation by monohydroxylated bile acids, and having advanced that the β1 TM2 contributes to the LC binding pocket (6, 17), we used molecular dynamics simulations to model the LC-β1 TM2 interaction.
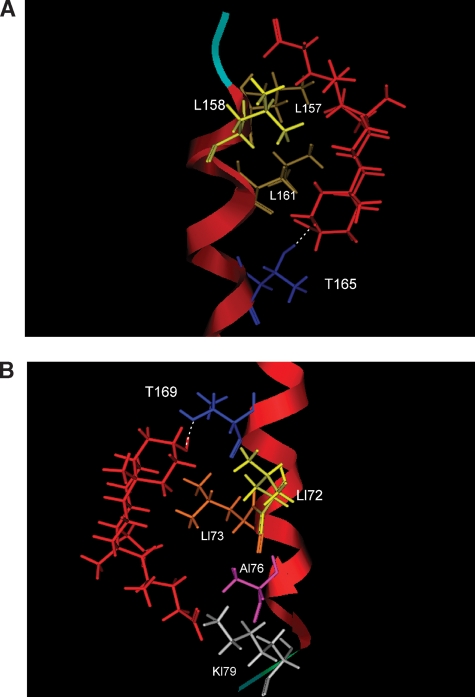
The primary amino acid sequence of the β1 TM2 domain was modeled three-dimensionally as an ideal α-helix. Our electrophysiological data indicate that the presence of the C3 hydroxyl group in an α-configuration is critical for LC to cause robust channel activation (Figs. 2, 3), and earlier electrophysiological results are consistent with the ionized lateral chain end of the bile acid molecule residing in the aqueous medium and/or at the aqueous-bilayer interface (6). Based on the minimum energy conformation of LC as a bean-shaped molecule (Fig. 4A), we computed that the distance between LC functional groups, located at C3 and C24, is ≈10.57 Å. Therefore, we inferred that the polar residues of TM2 β1, which could provide interacting sites for the LC hydroxyl and carboxylate, should be located no more than 10–12 Å from the bilayer-aqueous interface. In addition, we showed that β4 subunits could not render the BK channel complex sensitive to LC, as β1 subunits did (6). Primary alignment of β1 and β4 subunits reveals the existence of two polar residues unique to the β1 TM2: T165 and T169 (see supplementary Fig. IV). Notably, both are located at a distance of no more than 12 Å from the bilayer-aqueous interface, an ideal location for any of these residues to hydrogen bond with the LC C3 α hydroxyl, having the bile acid inserted in the membrane almost normally to the bilayer plane.
Whether T165, T169, or both residues are interacting with the LC C3 α hydroxyl, such interaction(s) requires TM2 to fill the hydrophobic cleft underneath the hydrophobic rings of the LC concave hemisphere (Fig. 4A). Thus, we hypothesized two nonmutually exclusive regions in the β1 TM2 that may serve as LC-docking areas: 1) if β1 T169 interacts with the LC C3 α hydroxyl, L172 and L173 are strategically suited to fill the LC hydrophobic cleft; 2) if β1 T165 interacts with the LC C3 α hydroxyl, L161 would fill the hydrophobic cleft. Molecular dynamics simulations (see Methods) confirmed that LC interaction with either of these two docking areas may, indeed, occur (Fig. 6): in the “165/161 model,” the carboxylate (predominantly ionized) in the side chain of LC resides in or near the extracellular aqueous compartment (Fig. 6, A); in the “169/172/173 model,” the ionized carboxylate in the side chain of LC resides in or near the cytosolic aqueous compartment (Fig. 6, B).
Fig. 6.
Putative models for LC docking on the BK β1 subunit transmembrane domain 2 (TM2). Molecular dynamics simulation (see Methods) indicates that LC interaction with one or two distinct regions in the channel β1 subunit TM2 may occur. In the “165/161 model” the ionized carboxylate in the side chain of LC resides in or near the extracellular aqueous compartment (A), whereas in the “169/172/173 model” the ionized carboxylate in the side chain of LC resides in or near the cytosolic aqueous compartment (B). The TM2 α helix is shown in red, whereas peptide backbone near the aqueous face is shown in pale blue (A) and green (B); selected β1 amino acids are shown in colors: T (dark blue), L (different types of yellow), A (purple), and K (light gray); LC is shown in red.
As a first step in testing the validity of our general model, we decided to introduce mutations into β1 TM2 that should affect the effective interactions between the docking area and LC functional groups. Thus, we constructed the mutant T169A,K179Aβ1, which was coexpressed with cbv1 to probe LC action. Considering the free movable nature of the LC lateral chain, the K179A mutation was included to prevent K179, located at the aqueous face near the lipid membrane, from stabilizing the LC carboxylate/carboxylic acid (see Fig. 6B and Discussion). An uncharged LC can still interact with K179 by hydrogen bonding rather than ion pairing (as the carboxylate does). Under recording conditions identical to those used in our LC studies on cbv1+β1 channels, LC potentiation of channel function was drastically blunted in channels containing β1 subunits with the double mutation (see supplementary Fig. VA, B), buttressing the idea that the α hydroxyl (with some possible contribution from the carboxylate) in LC interacts with the TM2 169/172/173 docking area. The channel with the mutated β1, however, was somewhat responsive to LC (≤25% increase in NPo over control values) (see supplementary Fig. VB). The simplest explanation is that the poor activation results from an impaired interaction between LC and the mutated 169/172/173 docking area. However, it may also reflect weak LC interactions via the 165/161 model (secondary or not to the mutations in 169/172/173), and/or interactions between nonionized bile acids with a site(s) anywhere else in the channel complex and its immediate lipidic microenvironment.
DISCUSSION
Vasodilation caused by an increase in bile acid levels in systemic circulation is a major determinant of the systemic hypotension associated with porto-systemic shunting that may occur in hepatobiliary disease (32). In hepatobiliary disease, not only overall increase in systemic bile acid levels but also changes in composition may occur, with drastic increase in LC levels (11). LC causes endothelium-independent relaxation of small arteries via activation of myocyte BK channels (6), a bile acid action that requires LC sensing by the channel β1 TM2 domain (17). Remarkably, among naturally occurring bile acids, LC is the most effective activator of vascular myocyte native BK channels (4). Our current study pinpoints the combination of a cis junction between steroidal rings A and B and the α configuration of the C3 hydroxyl in the LC molecule as the key features that lead to robust channel activation. This combination imprints a bean shape to the LC molecule, with two clear-cut hemispheres: a convex, hydrophobic (β) and a concave, hydrophilic (α) side, which stresses the distinct planar polarity of bile acids. Planar polarity ensures insertion of LC almost normally to the bilayer plane, making possible direct binding of the polar groups and the hydrophobic cleft that defines the concave side of LC with several polar and hydrophobic amino acids in β1 TM2.
Direct bile acid interactions with proteins and polypeptides require distinct chemical bonding between the steroid molecule and the peptide, with specificity and saturation in ligand action (as reviewed in Ref. 33; see also: 34–36). Indeed, all active bile acids that we tested fulfill these criteria (Figs. 2–4). Moreover, LC analogs that cannot meet LC steric requirements fail to modify BK channel activity (Figs. 2–4B), and analogs that can only meet these requirements partially, activate the BK channel with reduced effectiveness (Figs. 2–4A). Indeed, the relative efficacy of the various monohydroxylated bile acids in activating BK channels is related to the steroid capability to adopt the LC shape (Fig. 4; see supplementary Fig. I). These results, together with computer dynamics data of LC docking into defined β1 TM2 regions (Fig. 6), buttress the idea that BK channel activation is a result of direct binding of bile acid molecules to BK β1 TM2. While slo and BK β2–4 subunits are widely expressed, β1 subunits are highly abundant in smooth muscle and scarcely found in other tissues (16, 37). Therefore, pinpointing the structural features in LC and BK β1 TM2 leading to channel activation provides critical information for using LC as a template in designing BK channel modulators that selectively target the smooth muscle.
The simplest explanation for our data is that channel activation results from a direct interaction between bile acid monomers and β1 TM2, as advanced in our two docking models (Fig. 6). It is still possible, however, that LC monomers or small aggregates in the aqueous phase access the membrane and, once incorporated into the bilayer, form mixed micelles with lipids such as cholesterol. If so, sequestration of cholesterol from the channel environment could result in increased Po, because cholesterol is a well-known inhibitor of BK channels (38–40). Several pieces of evidence make this possibility unlikely. First, incubation with deoxycholate at concentrations that increase smooth muscle BK channel activity, i.e., 100 μM (4), fails to modify cholesterol or phospholipid content of rat aorta smooth muscle membranes (7). Second, LC itself does not decrease but actually increases the cholesterol/phospholipid ratio of red blood cell membranes (41). Finally, LC activation of cbv1+β1 channels remains when studied on channels reconstituted into cholesterol-free phospholipid bilayers (17).
Although we favor a bile acid monomer-β1 TM2 interaction(s), we cannot dismiss the possibility that LC could insert into the membrane as dimers or small aggregates and, as such, interact with the channel protein. A preferred form of bile acid insertion into membranes is as small aggregates consisting of two to four molecules of bile acids with their hydrophilic sides facing inwards, bound by hydrogen bonds between the hydroxyl groups (18, 42, 43). The pattern of bile acid effectiveness on BK channel function (cholate<deoxycholate<LC) argues against the possibility that channel activation results from channel protein interactions with this type of bile acid oligomer, as discussed in detail elsewhere (4). It is still possible, however, that the interaction with the channel protein involves small LC aggregates formed by a hydrophobic pairing between the convex planes (19, 28). If so, some LC molecules in the aggregate would be forced to expose their hydroxyl groups to the bilayer core, unless another domain of the BK channel (e.g., the channel α subunit, β1 TM1, or even TM2 from another β1 subunit within the channel complex), some yet-to-be-identified BK-asssociated membrane protein, and/or some endogenous polar lipid provide stabilization. It should be noted, however, that LC-induced activation of cbv1+β1 channels incorporated into artificial POPE-POPS (3:1; w/w) bilayers is similar to that observed in native cell membranes (≈3 times increase in channel Po) (17), strongly suggesting that a complex proteolipid environment is not necessary for LC-BK β1 interactions.
Whether in monomeric or small aggregate form, the planar and rigid nature of bile acids allows these steroids to insert into the membrane and reach the β1 TM2 domain with their steroidal nucleus deep in the bilayer while the lateral chain with its charged carboxyl remains at the water-lipid interface. Several results support this model of insertion. 1) Activation of smooth muscle BK channels by naturally occurring bile acids is a direct function of the steroid nucleus hydrophobicity (4), which is directly related to the bile acid capacity to partition in the bilayer core. Ursocholanic acid, however, being more hydrophobic than LC, routinely failed to activate BK channels when applied to the cytosolic side of I/O patches (4), probably because of its lack of C3 hydroxyl to interact with polar regions in the β1 TM2 surface. 2) LCsulfate fails to activate cbv1+β1 channels (Fig. 5A, C). The pKa of the sulfate is ∼1–2, making it highly unlikely that this analog interdigitates in the bilayer core and accesses the proper docking regions (Fig. 6). 3) Channel activation was reduced by neutralization of negative charge in the lateral chain (Fig. 5B, C), which decreases the solubility of the polar chain in the aqueous phase. This loss of activity is consistent with the reduced activation of native BK channels when the end group of the bile acid lateral chain is made less polar [CH2.OH or CO.OCH3 vs. CO.O− in a series of cholate derivatives (4)]. 4) BK channel activation is not dependent on a fixed length of the bile acid lateral chain (4), consistent with a freely movable chain in the aqueous phase.
Several steroids other than bile acids have been reported to increase BK NPo, including 17β-estradiol, xenoestrogens, androgens, and gluco- and mineralocorticoids. None of these steroids, however, appear to require the specific presence of β1 subunits for activating the channel (5, 44, 45), as LC does (6). Compared with other lipids and steroids, bile acids distinctly possess a unique bean shape and physicochemical properties (18). Our data indicate that BK channel activation directly relates to the capability of the steroid molecule to mimic the shape of LC (Table 2), supporting the contention that the molecular shape and planar polarity of bile acids are the essential features that allow these steroids to induce conformational changes in β1 TM2 that result in increased BK channel activity. Indeed, our molecular modeling data indicate that the LC molecule is energetically stable when it assumes the bean-shaped structure, with distinctive hydrophilic, concave and hydrophobic, convex hemispheres (Fig. 4A). This finding is in consonance with crystallographic data of sodium cholate monohydrate (46), a hydrophobic bile acid that shares with LC all basic structural features: steroidal nucleus with the C3 hydroxyl in the concave plane and an A/B cis junction (18, 19).
Based on bile acid monomer shape (Fig. 4) and molecular dynamics simulations of the β1 TM2, which acts as the BK channel LC sensor (17), we advance two docking models for LC. The first, which requires LC insertion from the extracellular side of the membrane, involves T165 forming a hydrogen bond with the LC C3 hydroxyl, a hydrophobic cleft that includes L161 stabilizing the LC hydrophobic steroidal nucleus, and L158 and L157 supporting the LC lateral chain (Fig. 6, top). This model agrees with a recently reported model of bile acid docking onto the farnesoid-X receptor, which also requires the presence of two hydrophobic amino acids (two phenylalanines) to interact with the bile acid steroid system (35). The second model, which requires LC insertion from the intracellular side of the membrane, involves T169 interacting via hydrogen bonding with the LC C3 hydroxyl, L172 and L173 providing the hydrophobic cleft for the LC nucleus, and A176 and K179 (Fig. 6, bottom) stabilizing the LC lateral chain, whether the latter includes a carboxylate or an unionized carboxylic acid. In the first model, however, the position of L158 and L157 will prevent interactions of LC derivatives with bulky lateral chains, and thus, it is more sterically restricted. In spite of small differences, both models provide complementary shapes and hydrogen bonding partners for LC, with epiLC and alloLC showing lower efficacy due to their different shape. Even when the C3 hydroxyls of these two analogs could be successfully positioned to hydrogen bond with T165 or T169, the position of methyl groups caused by the upside-down orientation adopted by epiLC and alloLC to match the LC shape (Fig. 4A) would alter their ability to stabilize the same conformation of the proposed binding areas, resulting in diminished channel activation (Figs. 2, 3).
The overall validity of our models is buttressed by the fact that LC action is drastically reduced when probed on channels including T169A,K179Aβ1 subunits, underscoring the requirement of an intact 169/172/173/179 docking site for full LC effectiveness. Alanine scanning mutagenesis followed by substitution by amino acid residues varying in physicochemical properties such as molecular volume, length, and polarity, will help to determine the separate or concerted contribution of each docking area to bile acid action on BK channels. Notably, tauroLC, a compound that can effectively flip-flop across membranes only after several minutes (47), is equally effective to readily (<1 s) activate vascular myocyte native BK channels when applied either from the extracellular or intracellular leaflet of the membrane (4). These data suggest that the models for bile acid docking into and activation of BK channels are not mutually exclusive. However, considering the poor LC action on channels that contain T169A,K179Aβ1 subunits, it is likely that extracellularly applied tauroLC, after being built up in the extracellular leaflet of the membrane (42), modulates BK channel function via a different, “fatty acid” site. This yet-to-be-identified site was reported for native pulmonary artery smooth muscle channels (made of α+β1 subunits), being accessible solely from the extracellular medium (31, 48).
Supplementary Material
Acknowledgments
The authors thank Dr. Daniel Baker (University of Memphis, Memphis, TN) for probing the purity of 5α-cholanic acid 3β-ol by mass spectrometry, Dr. Alan Hofmann (University of California, La Jolla, CA) for valuable advice concerning handling of cholane derivatives, and Maria Asuncion-Chin (The University of Tennessee Health Science Center) for excellent technical assistance.
Abbreviations
BK, large conductance, calcium- and voltage-gated potassium
CMC, critical micellar concentration
LC, lithocholate
N, number of functional ion channels in the membrane patch
Po, open channel probability
RMSG, root mean square gradient
TM2, transmembrane domain 2
Published, JLR Papers in Press, June 23, 2008.
Footnotes
This work was supported by National Institutes of Health Grant HL-77424 (A.M.D.). A.L.P. gratefully acknowledges the Chemical Computing Group for the MOE software license.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of five figures.
References
- 1.Brayden J. E., and M. T. Nelson. 1992. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 256 532–535. [DOI] [PubMed] [Google Scholar]
- 2.Jaggar J. H., G. C. Wellman, T. J. Heppner, V. A. Porter, G. J. Perez, M. Gollasch, T. Kleppisch, M. Rubart, A. S. Stevenson, W. J. Lederer, et al. 1998. Ca2+ channels, ryanodine receptors and Ca(2+)-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol. Scand. 164 577–587. [DOI] [PubMed] [Google Scholar]
- 3.Valverde M. A., P. Rojas, J. Amigo, D. Cosmelli, P. Orio, M. I. Bahamonde, G. E. Mann, C. Vergara, and R. Latorre. 1999. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science. 285 1859–1860. [DOI] [PubMed] [Google Scholar]
- 4.Dopico A. M., J. V. Walsh, Jr., and J. J. Singer. 2002. Natural bile acids and synthetic analogues modulate large conductance Ca2+-activated K+ (BKCa) channel activity in smooth muscle cells. J. Gen. Physiol. 119 251–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King J. T., P. V. Lovell, M. Rishniw, M. I. Kotlikoff, M. L. Zeeman, and D. P. McCobb. 2006. Beta2 and beta4 subunits of BK channels confer differential sensitivity to acute modulation by steroid hormones. J. Neurophysiol. 95 2878–2888. [DOI] [PubMed] [Google Scholar]
- 6.Bukiya A. N., J. Liu, L. Toro, and A. M. Dopico. 2007. Beta1 (KCNMB1) subunits mediate lithocholate activation of large-conductance Ca2+-activated K+ channels and dilation in small, resistance-size arteries. Mol. Pharmacol. 72 359–369. [DOI] [PubMed] [Google Scholar]
- 7.Bomzon A., and P. Ljubuncic. 1995. Bile acids as endogenous vasodilators? Biochem. Pharmacol. 49 581–589. [DOI] [PubMed] [Google Scholar]
- 8.Lewis B., D. Panveliwalla, S. Tabaqchali, and I. D. Wootton. 1969. Serum bile acids in intestinal disorders. J. Physiol. 202 46P. [PubMed] [Google Scholar]
- 9.Bomzon A., J. P. Finberg, D. Tovbin, S. G. Naidu, and O. S. Better. 1984. Bile salts, hypotension and obstructive jaundice. Clin. Sci. (Lond.). 67 177–183. [DOI] [PubMed] [Google Scholar]
- 10.Tarantino G., S. Cambri, A. Ferrara, M. Marzano, A. Liberti, G. Vellone, and A. F. Ciccarelli. 1989. Serum concentration of bile acids and portal hypertension in cirrhotic patients. Possible correlations. Riv. Eur. Sci. Med. Farmacol. 11 195–205. [PubMed] [Google Scholar]
- 11.Greco A. V., and G. Mingrone. 1993. Serum bile acid concentrations in mild liver cirrhosis. Clin. Chim. Acta. 221 183–189. [DOI] [PubMed] [Google Scholar]
- 12.Hamdan H., and N. H. Stacey. 1993. Mechanism of trichloroethylene-induced elevation of individual serum bile acids. I. Correlation of trichloroethylene concentrations to bile acids in rat serum. Toxicol. Appl. Pharmacol. 121 291–295. [DOI] [PubMed] [Google Scholar]
- 13.Ohkubo H., K. Okuda, S. Iida, K. Ohnishi, S. Ikawa, and I. Makino. 1984. Role of portal and splenic vein shunts and impaired hepatic extraction in the elevated serum bile acids in liver cirrhosis. Gastroenterology. 86 514–520. [PubMed] [Google Scholar]
- 14.Ceryak S., B. Bouscarel, and H. Fromm. 1993. Comparative binding of bile acids to serum lipoproteins and albumin. J. Lipid Res. 34 1661–1674. [PubMed] [Google Scholar]
- 15.Ljubuncic P., O. Said, Y. Ehrlich, J. Meddings, E. Shaffer, and A. Bomzon. 2000. On the in vitro vasoactivity of bile acids. Br. J. Pharmacol. 131 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenner R., T. J. Jegla, A. Wickenden, Y. Liu, and R. W. Aldrich. 2000. Cloning and functional characterization of novel large conductance calcium-activated potassium channel beta subunits, hKCNMB3 and hKCNMB4. J. Biol. Chem. 275 6453–6461. [DOI] [PubMed] [Google Scholar]
- 17.Bukiya A., T. Vaithianathan, L. Toro, and A. Dopico. 2008. The second transmembrane domain of the large conductance, voltage- and calcium-gated potassium channel β1 subunit is a lithocholate sensor. FEBS Lett. 582 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey, M. 1985. Physical-chemical properties of bile acids and their salts. In Sterols and Bile Acids. H. Danielsson and J. Sjovall, editors. Elsevier Science Publishers, Amsterdam, Netherlands. 345–403.
- 19.Miyajima K., K. Machida, T. Taga, H. Komatsu, and M. Nakagaki. 1988. Correlation between the hydrophobic nature of monosaccharides and cholotes and their hydrophobic indices. J. Chem. Soc., Faraday Trans. 84 2537–2544. [Google Scholar]
- 20.Dopico A., V. Anantharam, and S. Treistman. 1998. Ethanol increases the activity of Ca2+-dependent K+ (mslo) channels: functional interaction with cytosolic Ca2+. J. Pharmacol. Exp. Ther. 284 258–268. [PubMed] [Google Scholar]
- 21.Liu J., T. Vaithianathan, K. Manivannan, A. Parrill, and A. M. Dopico. 2008. Ethanol modulates BKCa channels by acting as an adjuvant of calcium. Mol. Pharmacol. 74 628–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halgren T. A. 1996. Merck Molecular Force Field. J. Comput. Chem. 17 490–641. [Google Scholar]
- 23.Wang J., P. Cieplak, and P. A. Kollman. 2000. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J. Comput. Chem. 21 1049–1074. [Google Scholar]
- 24.Cornell B. A., G. Krishna, P. D. Osman, R. D. Pace, and L. Wieczorek. 2001. Tethered-bilayer lipid membranes as a support for membrane-active peptides. Biochem. Soc. Trans. 29 613–617. [DOI] [PubMed] [Google Scholar]
- 25.Knot H. J., and M. Nelson. 1998. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J. Physiol. 508 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez G. J., A. D. Bonev, and M. T. Nelson. 2001. Micromolar Ca(2+) from sparks activates Ca(2+)-sensitive K(+) channels in rat cerebral artery smooth muscle. Am. J. Physiol. Cell Physiol. 281 C1769–C1775. [DOI] [PubMed] [Google Scholar]
- 27.Roda A., A. F. Hoffman, and K. J. Mysels. 1983. The influence of bile salt structure on self-association in aqueous solutions. J. Biol. Chem. 258 6362–6370. [PubMed] [Google Scholar]
- 28.Carey M. C., and D. M. Small. 1972. Micelle formation by bile salts. Physical-chemical and thermodynamic considerations. Arch. Intern. Med. 130 506–527. [PubMed] [Google Scholar]
- 29.Ko J., J. A. Hamilton, H. T. Ton-Nu, C. D. Schteingart, A. F. Hofmann, and D. M. Small. 1994. Effects of side chain length on ionization behavior and transbilayer transport of unconjugated dihydroxy bile acids: a comparison of nor-chenodeoxycholic acid and chenodeoxycholic acid. J. Lipid Res. 35 883–892. [PubMed] [Google Scholar]
- 30.Cabral D. J., J. A. Hamilton, and D. M. Small. 1986. The ionization behavior of bile acids in different aqueous environments. J. Lipid Res. 27 334–343. [PubMed] [Google Scholar]
- 31.Clarke A. L., S. Petrou, J. V. Walsh, Jr., and J. J. Singer. 2002. Modulation of BK(Ca) channel activity by fatty acids: structural requirements and mechanism of action. Am. J. Physiol. Cell Physiol. 283 C1441–C1453. [DOI] [PubMed] [Google Scholar]
- 32.Pak J. M., and S. S. Lee. 1993. Vasoactive effects of bile salts in cirrhotic rats: in vivo and in vitro studies. Hepatology. 18 1175–1181. [PubMed] [Google Scholar]
- 33.Stolz A. H., M. Takikawa, M. Ookhtens, and N. Kaplowitz. 1989. The role of cytoplasmic proteins in hepatic bile acid transport. Annu. Rev. Physiol. 51 161–176. [DOI] [PubMed] [Google Scholar]
- 34.Mizushina Y., N. Kasai, K. Miura, S. Hanashima, M. Takemura, H. Yoshida, F. Sugawara, and K. Sakaguchi. 2004. Structural relationship of lithocholic acid derivatives binding to the N-terminal 8-kDa domain of DNA polymerase beta. Biochemistry. 43 10669–10677. [DOI] [PubMed] [Google Scholar]
- 35.Katona B. W., C. L. Cummins, A. D. Ferguson, T. Li, D. R. Schmidt, D. J. Mangelsdorf, and D. F. Covey. 2007. Synthesis, characterization, and receptor interaction profiles of enantiomeric bile acids. J. Med. Chem. 50 6048–6058. [DOI] [PubMed] [Google Scholar]
- 36.Ishizawa M., M. Matsunawa, R. Adachi, S. Uno, K. Ikeda, H. Masuno, M. Shimizu, K. I. Iwasaki, S. Yamada, and M. Makishima. 2008. Lithocholic acid derivatives act as selective vitamin D receptor modulators without inducing hypercalcemia. J. Lipid Res. 49 763–772. [DOI] [PubMed] [Google Scholar]
- 37.Orio P., P. Rojas, G. Ferreira, and R. Latorre. 2002. New disguises for an old channel: MaxiK channel beta-subunits. News Physiol. Sci. 17 156–161. [DOI] [PubMed] [Google Scholar]
- 38.Bolotina V., V. Omelyanenko, B. Heyes, U. Ryan, and P. Bregestovski. 1989. Variations of membrane cholesterol alter the kinetics of Ca2(+)-dependent K+ channels and membrane fluidity in vascular smooth muscle cells. Pflugers Arch. 415 262–268. [DOI] [PubMed] [Google Scholar]
- 39.Chang H. M., R. Reitstetter, R. P. Mason, and R. Gruener. 1995. Attenuation of channel kinetics and conductance by cholesterol: an interpretation using structural stress as a unifying concept. J. Membr. Biol. 143 51–63. [DOI] [PubMed] [Google Scholar]
- 40.Crowley J. J., S. N. Treistman, and A. M. Dopico. 2005. Distinct structural features of phospholipids differentially determine ethanol sensitivity and basal function of BK channels. Mol. Pharmacol. 68 4–10. [DOI] [PubMed] [Google Scholar]
- 41.Balistreri W. F., M. H. Leslie, and R. A. Cooper. 1981. Increased cholesterol and decreased fluidity of red blood cell membranes (spur cell anemia) in progressive intrahepatic cholestasis. Pediatrics. 67 461–466. [PubMed] [Google Scholar]
- 42.Schubert R., K. Beyer, H. Wolburg, and K-H. Schmidt. 1986. Structural changes in membranes of large unilamellar vesicles after binding of sodium cholate. Biochemistry. 25 5263–5269. [DOI] [PubMed] [Google Scholar]
- 43.Schubert R., and K. H. Schmidt. 1988. Structural changes in vesicle membranes and mixed micelles of various lipid compositions after binding of different bile salts. Biochemistry. 27 8787–8794. [DOI] [PubMed] [Google Scholar]
- 44.Korovkina V. P., A. M. Brainard, P. Ismail, T. Schmidt, and S. England. 2004. Estradiol binding to maxi-K channels induces their down-regulation via proteasomal degradation. J. Biol. Chem. 279 1217–1223. [DOI] [PubMed] [Google Scholar]
- 45.Duncan R. K. 2005. Tamoxifen alters gating of the BK alpha subunit and mediates enhanced interactions with the avian beta subunit. Biochem. Pharmacol. 70 47–58. [DOI] [PubMed] [Google Scholar]
- 46.Cobbledick R. L., and F. W. Einstein. 1980. The structure of sodium 3α, 7α, 12α-trihydroxy-5β-cholan-24-oate monohydrate (sodium cholate monohydrate). Acta Crystallogr. B. 36 287–292. [Google Scholar]
- 47.Kamp F., J. A. Hamilton, F. Kamp, H. V. Westerhoff, and J. A. Hamilton. 1993. Movement of fatty acids, fatty acid analogues, and bile acids across phospholipid bilayers. Biochemistry. 32 11074–11086. [DOI] [PubMed] [Google Scholar]
- 48.Clarke A. L., S. Petrou, J. V. Walsh, Jr., and J. J. Singer. 2003. Site of action of fatty acids and other charged lipids on BKCa channels from arterial smooth muscle cells. Am. J. Physiol. Cell Physiol. 284 C607–C619. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.