Abstract
Background
Oxidative stresses including cigarette smoking are implicated in the pathogenesis of cerebrovascular diseases, which are associated with pneumonia because of frequent aspiration. Haem oxygenase‐1 (HO‐1) acts in cytoprotection against oxidants, provides anti‐inflammatory effects, and inhibits atherogenesis. A (GT)n dinucleotide repeat in the human HO‐1 promoter modulates HO‐1 gene expression and shows length polymorphism, which is grouped into three classes: class S (<27 repeats), class M (⩾27, <33 repeats), and class L (⩾33 repeats) alleles.
Objective
To investigate the correlation between the HO‐1 gene polymorphism and development of pneumonia in elderly Japanese.
Methods
The length of the (GT)n repeats was analysed in 200 elderly patients with pneumonia and 200 control subjects. The association of the HO‐1 gene polymorphism with risk of pneumonia was estimated by logistic regression.
Results
The proportion of allele frequencies in class L, and the proportion of genotypic frequencies in the L‐allele carriers (L/L, L/M, and L/S), was significantly higher in patients with pneumonia than in controls (20% v 10% in class L, and 34% v 18% in L‐allele carriers). After adjustment for potentially confounding factors, both cerebrovascular disorders and HO‐1 gene L‐allele carriers were significant and independent risk factors for pneumonia. The adjusted odds ratio for L‐allele carriers v non‐L‐allele carrier was 2.1 (95% confidence interval, 1.2 to 3.6).
Conclusions
The large size of a (GT)n repeat in the HO‐1 gene promoter may be associated with susceptibility to pneumonia in the older Japanese population.
Keywords: pneumonia, haem oxygenase, gene polymorphism, cerebrovascular disease
Pneumonia is not only a common infection in older people, it is also the most common cause of death from nosocomial infection in the Japanese population.1 Disorders of the central nervous system are more likely to develop in the elderly, and pneumonia has been estimated to occur in about one third of patients with stroke.2 Cerebrovascular disease is associated with a high incidence of pneumonia owing to frequent aspiration.3 As well as factors including diabetes mellitus, hyperlipidaemia, and hypertension, oxidative stresses such as cigarette smoking are also associated with the pathogenesis of cerebrovascular disease.4 Genetic factors affecting antioxidants may be involved in the susceptibility to atherosclerosis of the cerebral arteries and the subsequent development of pneumonia in the elderly. Although the antioxidant enzymes inhibit the formation of atherosclerosis,5 the roles of reduced expression of these enzymes on the development of pneumonia in elderly people are still uncertain.
Haem oxygenase (HO) oxidatively degrades haem to biliverdin, which is subsequently reduced to bilirubin, an efficient scavenger of reactive oxygen species (ROS), by biliverdin reductase.6 HO‐1, an inducible form of HO—and also a constitutive form of HO, including HO‐2—provides cellular protection against haem mediated and non‐haem‐mediated oxidant injury.6 HO‐1 is thought to be an essential component in protection against various ROS.
A (GT)n repeat in the 5′ flanking region of the human HO‐1 gene is polymorphic,7 and modulates human HO‐1 gene transcription by thermal stress8 and hydrogen peroxide.7 The size of the (GT)n repeat in the HO‐1 gene is associated with the antiapoptotic effects of HO‐1 in lymphoblastoid cell lines.9 We have shown that the size of the (GT)n repeat in the HO‐1 gene is associated with susceptibility to chronic pulmonary emphysema (CPE)7 and lung adenocarcinoma,10 and with longevity11 in Japanese populations. This HO‐1 gene polymorphism is also associated with coronary artery disease, one of vascular diseases related to ROS.12 However, the association between the size of the (GT)n repeat in the HO‐1 gene and the development of pneumonia in older populations is still uncertain.
In the present study, we screened allelic frequencies of the (GT)n repeats in the HO‐1 gene promoter in elderly people with and without pneumonia, and examined the association between the risk of senile pneumonia and length of the (GT)n repeats.
Methods
Clinical protocol and patient characteristics
We studied 200 elderly patients with pneumonia and 200 elderly control subjects without pneumonia, attending the departments of internal medicine in six hospitals in Miyagi prefecture. The hospitals were a university hospital, a Red Cross hospital, three public general hospitals, and a municipal hospital. All participants were Japanese and aged 65 and older. To evaluate whether HO‐1 genotypes are associated with the development of pneumonia in elderly Japanese people, we selected the subjects with a performance status of 2 or better13 and in a stable state as potential participants, because those with too low a performance status ran a greater risk of infectious disease, which might mask the preventive effect of any genetic factors. Patients were given a score of 0 if they were fully active and asymptomatic, 1 if they were symptomatic but fully ambulatory, 2 if they were symptomatic and confined to bed or chair for less than 50% of their waking hour, 3 if they were symptomatic and confined to bed or chair for more than 50% of their waking hours, and 4 if they were completely bedridden. The study was approved by the Tohoku University ethics committee, and informed consent was obtained from each subject. This study was carried out between April 2002 and December 2004.
During the study period, 264 elderly patients with pneumonia were identified. Pneumonia was defined as pulmonary infiltrate on chest radiograph, cough, and a temperature higher than 38.0°C.3 All patients with pneumonia had the features of pulmonary infiltrate on chest radiographs, cough, and a temperature above 38.0°C. The patients were enrolled consecutively. Among them, we selected for the case group those with a performance status of 2 or better and in a stable state. We excluded patients who were immunocompromised—for example, those with active malignant disease, on renal dialysis, receiving corticosteroid treatment, or with HIV‐1 infection. Patients were also excluded if they had obvious swallowing dysfunction, chronic sepsis in pressure sores, venous ulcers, or an indwelling urinary catheter. After these selections and exclusions were applied, 200 elderly patients with pneumonia were enrolled in the case group.
Potential control subjects were 439 elderly patients who continued attending the departments of hospitals over the study period and who had never had pneumonia at any time in their life including the study period. Control subjects were excluded if their past history relating to pneumonia were unclear. After the same selection and exclusion criteria as in the case group were applied, 383 control subjects were available for frequency matching. To carry out a case–control study, we randomly selected 200 control subjects in a frequency matched manner from the control cohort. They were frequency matched on age (±5 years), sex, smoking history, and performance status with the patients with pneumonia. Physical characteristics, smoking history, and complications in patients with pneumonia and control subjects are shown in table 1.
Table 1 Characteristics of the study subjects.
| Characteristics | Control subjects | Patients with | p Value |
|---|---|---|---|
| (n = 200) | pneumonia (n = 200) | ||
| Age (years)* | 73.8 (0.7) | 75.4 (1.0) | NS |
| Sex | |||
| Male | 99 (50%) | 101 (50%) | NS |
| Female | 101 (50%) | 99 (50%) | |
| Performance status | |||
| 0–1 | 114 (57%) | 108 (54%) | NS |
| 2 | 86 (43%) | 92 (46%) | |
| Smoking history (pack‐year)* | 18.2 (2.6) | 19.3 (2.8) | NS |
| Cerebrovascular disease | |||
| Yes | 14 (7%) | 101 (50%) | <0.0001 |
| No | 186 (93%) | 99 (50%) | |
| COPD | |||
| Yes | 35 (18%) | 38 (19%) | NS |
| No | 165 (82%) | 162 (81%) | |
| Congestive heart failure | |||
| Yes | 17 (9%) | 28 (14%) | NS |
| No | 183 (91%) | 172 (86%) | |
| Hypertension | |||
| Yes | 43 (22%) | 59 (30%) | NS |
| No | 157 (78%) | 141 (70%) | |
| Diabetes mellitus | |||
| Yes | 21 (10%) | 34 (17%) | NS |
| No | 179 (90%) | 166 (83%) | |
| Hyperlipidaemia | |||
| Yes | 9 (5%) | 10 (5%) | NS |
| No | 191 (95%) | 190 (95%) |
Values are n (%) or *mean (SD).
COPD, chronic obstructive pulmonary disease.
Analysis of length variability of (GT)n repeats in HO‐1 gene promoter
Genomic DNAs were extracted from leucocytes in peripheral venous blood by conventional procedures. The 5′‐flanking region containing a poly (GT)n repeat of the HO‐1 gene was amplified by polymerase chain reaction (PCR)7,11 with a fluorescently labelled primer p1‐s (5′‐AGAGCCTGCAGCTTCTCAGA‐3′) and an unlabeled antisense primer p1‐as (5′‐ACAAAGTCTGGCCATAGGAC‐3′), which were designed according to the published sequence.7,14 The PCR was carried out over 30 cycles of 20 seconds at 94°C, 10 seconds at 60°C, and 20 seconds at 72°C. The PCR products were analysed in a DNA sequencer (ALF express II DNA sequencer version 2.2, Amersham Pharmacia Biotech, Piscataway, New Jersey, USA). Each size of (GT)n repeat in the PCR product was calculated with ALFwin fragment analysis version 1.03 (Amersham Pharmacia Biotech) using four cloned alleles as size markers, which were already sequenced with the ABI prism dye terminator sequencing kit (Perkin‐Elmer Applied Biosystems, Foster City, California, USA).7 The repeat numbers of these size markers were 16, 23, 29, and 38, respectively. The investigators of genetic analysis were blinded with respect to the status of the subjects.
Carboxyhaemoglobin concentrations in patients with pneumonia
Blood samples were taken from the radial artery in patients with pneumonia on the first day of hospital admission. The patients for the carboxyhaemoglobin analysis were all non‐smokers and consisted of five L‐allele carriers and five non‐L‐allele carriers (L/L genotype and S/S genotype, respectively), who showed a similar C reactive protein concentration (15.0 to 20.0 mg/dl) and white blood cell (WBC) count (9500 to 12 500 cells/μl) at the time of analysis. The carboxyhaemoglobin concentrations were measured with a spectrophotometer (ASL System, Radiometer, Copenhagen, Denmark).15
Statistical analysis
In the analysis of HO‐1 gene polymorphism in this study, the patient and control groups were frequency matched by age, sex, performance status, and smoking history. For statistical analysis, age and smoking history (pack‐year) between the two groups were compared using Student's t test, and sex, performance status, and the frequency of the complications between the two groups were compared using χ2 tests (table 1), as described previously in coronary artery disease.12 The proportion of allelic frequencies and genotypic frequencies between the two groups were also compared using the χ2 test (table 2). Factors associated with the presence of senile pneumonia such as age, sex, performance status, smoking status, complications, and HO‐1 gene polymorphism (L‐allele carrier) were examined with multivariate analysis by logistic regression analysis (table 3). Odds ratios (OR) and their 95% confidence intervals (CI) were calculated to assess the relative risk conferred by a particular genotype (L‐allele carrier), and adjusted for age, sex, performance status, smoking history, and complications using logistic regression as described previously (table 3).12 All the statistical analyses were undertaken using SYSTAT (version 10.2; SYSTAT Software, Richmond, California, USA). The values for age and smoking history (pack‐year) are reported as means (SD). The HO‐1 genotype distributions were in Hardy–Weinberg equilibrium. Significance was accepted at p<0.05.
Table 2 Allele and genotypic frequencies of HO‐1at polymorphic locus.
| Control subjects (n = 200) | Patients with pneumonia (n = 200) | OR (95% CI) v all other classes or subjects | p Value | |
|---|---|---|---|---|
| Allele class | ||||
| L | 38 (10%) | 79 (20%) | 2.3 (1.5 to 3.5) | <0.0001 |
| M | 189 (47%) | 159 (40%) | 0.7 (0.5 to 0.9) | <0.05 |
| S | 173 (43%) | 162 (40%) | 0.9 (0.7 to 1.2) | NS |
| Genotype group | ||||
| L‐allele carrier | 36 (18%) | 68 (34%) | 2.3 (1.5 to 3.7) | <0.001 |
| Non‐L‐allele carrier | 164 (82%) | 132 (66%) |
CI, confidence interval; OR, odds ratio.
Table 3 Multivariate analysis of risk factors related to pneumonia in older adults.
| Variable | OR (95% CI) | p Value | ||
|---|---|---|---|---|
| Haem oxygenase‐1 genotype subgroup | ||||
| L‐allele carriers v non‐L‐allele carriers | 2.1 (1.2 to 3.6)* | <0.01 | ||
| Cerebrovascular disease | ||||
| Yes v no | 28.0 (13.3 to 58.6)† | <0.0001 |
*OR was calculated with the non‐L‐allele carriers as the reference group, and adjusted for age, gender, performance status, smoking history, and complications.
†OR was calculated with the patients without cerebrovascular disease as the reference group, and adjusted for age, gender, performance status, smoking history, HO‐1 genotype, and complications other than cerebrovascular disease.
CI, confidence interval; OR, odds ratio.
For statistical analysis in the study on the correlation between carboxyhaemoglobin level and HO‐1 genotype in the patients with pneumonia, the mean values for age (year), smoking history (pack‐year), WBC number (cells/μl), C reactive protein (mg/dl), and carboxyhaemoglobin concentration (%) between the five L‐allele carriers and the five non‐L‐allele carriers were compared using Student's t test and sex using the χ2 test (table 4).
Table 4 Arterial blood carboxyhaemoglobin in patients with pneumonia.
| Patient | HO‐1 | Age | Sex† | Smoking history | WBC | CRP | Arterial blood |
|---|---|---|---|---|---|---|---|
| genotype | (years)* | (pack‐year)* | (cells/μl)* | (mg/dl)* | Hb‐CO (%)‡ | ||
| L‐allele carrier 1 | LL | 71 | M | 0 | 12 300 | 18.3 | 0.57 |
| L‐allele carrier 2 | LL | 65 | F | 0 | 10 500 | 15.2 | 0.20 |
| L‐allele carrier 3 | LL | 79 | F | 0 | 9 700 | 15.7 | 0.80 |
| L‐allele carrier 4 | LL | 73 | F | 0 | 9 600 | 19.0 | 0.21 |
| L‐allele carrier 5 | LL | 76 | F | 0 | 10 020 | 19.4 | 1.20 |
| Non‐L‐allele carrier 1 | SS | 65 | M | 0 | 12 400 | 18.5 | 1.50 |
| Non‐L‐allele carrier 2 | SS | 77 | M | 0 | 11 000 | 16.3 | 1.20 |
| Non‐L‐allele carrier 3 | SS | 79 | F | 0 | 10 500 | 19.2 | 1.02 |
| Non‐L‐allele carrier 4 | SS | 65 | F | 0 | 9 900 | 15.6 | 1.10 |
| Non‐L‐allele carrier 5 | SS | 75 | F | 0 | 9 600 | 19.5 | 0.90 |
*There was no significant difference in the mean value between L‐allele carrier and non‐L‐allele carrier (p>0.7).
†There was no significant difference in the ratio between L‐allele carrier and non‐L‐allele carrier (p>0.5).
‡There was a significant difference in the mean value between L‐allele carrier and non‐L‐allele carrier (p<0.04).
CRP, C reactive protein; F, female; Hb‐CO, carboxyhaemoglobin; M, male; WBC, white blood cell count.
Results
Allele frequencies of HO‐1 gene in control and patients with pneumonia in older adults
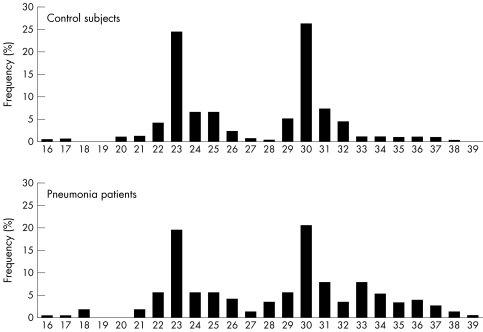
There were between 16 and 39 (GT)n repeats in the human HO‐1 gene in the study subjects (fig 1). The distribution of the number of (GT)n repeats was trimodal, as previously reported, with two main peaks located at 23 and 30 GT repeats and another peak located at 33 GT repeats.7,10,11 We therefore divided the alleles into three subclasses, as previously reported7: class S (<27 repeats), class M (⩾27 and <33 repeats), and class L (⩾33 repeats) alleles.
Figure 1 Frequency distribution of the number of (GT)n repeats in control subjects (n = 400 alleles) and patients with pneumonia (n = 400 alleles).
In the control subjects, the distributions of the 400 alleles were 173 (43%) class S, 189 (45%) class M, and 38 (10%) class L (table 2); in the patients with pneumonia, the distributions were 162 (40%) class S, 159 (40%) class M, and 79 (20%) class L. The proportion of allelic frequencies in class L was significantly higher in all patients with pneumonia (n = 79, 20%) than that in all control subjects (n = 38, 10%) (p<0.0001). The odds ratio for pneumonia with L alleles v non‐L alleles (class M allele + class S allele) was 2.3 (95% CI, 1.5 to 3.5) (table 2).
Genotypic frequencies of HO‐1 gene in control and patients with pneumonia
Six genotypes (L/L, L/M, L/S, M/M, M/S, and S/S) of (GT)n repeats in the human HO‐1 gene promoter were divided into two subgroups according to allelic subclasses: L‐allele carriers with a class L allele (L/L, L/M, L/S) and non‐L‐allele carriers without a class L allele (M/M, M/S and S/S).7 The proportion of genotypic frequencies in L‐allele carriers was significantly higher in all patients with pneumonia (n = 68, 34%) than that in all control subjects (n = 36, 18%) (p<0.0001). The odds ratio for patients with pneumonia with L‐allele carriers v non‐L‐allele carriers was 2.3 (95% CI, 1.4 to 3.7) (table 2).
Risk factors for pneumonia
On multivariate analysis, cerebrovascular disease (p<0.0001) and HO‐1 genotype (p<0.01) were significantly and independently associated with the development of pneumonia (table 3), when the variables were adjusted by age, sex, performance status, smoking history, and complications including congestive heart failure, COPD, hypertension, diabetes mellitus, and hyperlipidaemia. The adjusted odds ratio (95% CI) was 2.1 (1.2 to 3.6) for HO‐1 genotype and 28.0 (18.3 to 58.6) for cerebrovascular disease (table 3).
Carboxyhaemoglobin concentrations in patients with pneumonia
To show the correlation between HO‐1 genotype and HO‐1 activity caused by the inflammation of pneumonia, we examined the carboxyhaemoglobin concentration in several patients with pneumonia on their first day of hospital admission. The subjects for carboxyhaemoglobin analysis were five L‐allele carriers and five non‐L‐allele carriers (L/L genotype and S/S genotype, respectively). There were no significant differences in age, sex, smoking history, WBC count, and C reactive protein concentration level between these two groups. However, the patients without the L‐allele showed significantly higher carboxyhaemoglobin levels than those with the L‐allele (1.14 (0.23)% v 0.5 (0.42)%, respectively; p<0.04) (table 4).
Discussion
In this study we analysed HO‐1 gene polymorphism and showed that the proportion of allele frequencies in class L and the proportion of genotypic frequencies in the L‐allele carriers (L/L, L/M, and L/S) were significantly higher in elderly people with pneumonia than in control subjects. The proportion of subjects with cerebrovascular disease in the pneumonia group was significantly higher than in the control group. With multivariate analysis, HO‐1 genotype and the presence of cerebrovascular disease were significant and independent risk factors for pneumonia. These findings suggest that the large size of a (GT)n repeat in the HO‐1 gene promoter may be associated with the development of pneumonia in older Japanese people with cerebral infarction.
Disorders of the central nervous system are more likely to develop in the elderly, and pneumonia has been estimated to occur in about one third of patients with stroke.2 Basal ganglia infarction is associated with a high incidence of pneumonia owing to frequent aspiration3 resulting from the reduction in the cough and swallowing reflexes.16 In fact, in the present study, half these older patients with pneumonia also had cerebrovascular disease.
Oxidative stress such as cigarette smoking4 is one of the important risk factors for cerebrovascular diseases, including basal ganglia infarction. Various ROS including superoxide and hydrogen peroxide induce lipid peroxide formation, which is a key process in atherosclerotic plaques in hypercholesterolaemia.17 ROS are also involved in the brain tissue damage in stroke.18 On the other hand, antioxidant systems such as glutathione, superoxide dismutase, and HO are suggested to protect the vascular disease caused by ROS.19 The initial degradation of haem by microsomal HO involves the liberation of iron and CO and the formation of biliverdin, which is subsequently reduced to bilirubin by cytosolic biliverdin reductase.6 Higher intracellular HO‐1 activity may increase the content of bilirubin, which is an efficient scavenger of ROS,6 and a natural inhibitor of intimal hyperplasia after balloon injury.20 In fact, Ishikawa et al. reported inhibitory effects of HO‐1 on the atherogenesis in hyperlipidaemic rabbits.21 Enhanced endothelial cell injury caused by oxidative stress was observed in a human case of HO‐1 deficiency.22 Reduced expression of HO‐1 might be partly associated with the development of stroke and subsequent pneumonia.
A (GT)n dinucleotide repeat in the 5′‐flanking region of human HO‐1 gene shows length polymorphism.7 We previously reported the influence of the number of the (GT)n repeats on the inducibility of the HO‐1 gene promoter under oxidative stimulus by transient transfection assay in human cell lines. The promoter activity of HO‐1 is modulated by the length variability of the (GT)n repeats, and large (GT)n repeats have a potent inhibitory activity on H2O2 induced gene expression of HO‐1.7 Furthermore, Epstein‐Barr virus transformed lymphoblastoid cell lines were established from smokers with class L alleles (L/L) and with class S (S/S). When treated with H2O2, lymphoblastoid cells with the L/L genotype showed lower viability than those with the S/S genotype.9 The GT dinucleotide repeat polymorphism has emerged as a potent genetic risk factor in various diseases, including vascular diseases such as coronary arteriosclerosis12 and restenosis after balloon angioplasty.23 These findings are consistent with the view that tissues of the non‐L allele carrier could employ the antioxidant activity of HO‐1 to a greater extent than that of the L‐allele carrier when exposed to reactive oxygen species.10 Large (GT)n repeats may affect the protective function against oxidant induced vascular endothelial injury and arteriosclerosis through the inhibition of HO‐1 expression.
The results of our study suggest that the HO‐1 genotype is associated with susceptibility to pneumonia independently of cerebrovascular disease. Senile pneumonia is characterised by a high likelihood of aspiration pneumonia.16 The severity of aspiration pneumonia is associated with the lung inflammation mediated by cytokines such as tumour necrosis factor α (TNFα).24 On the other hand, it was reported that overexpression of the HO‐1 gene attenuated inflammation and decreased apoptosis of bronchial epithelial cells in a murine model of lung inflammation induced by Pseudomonas aeruginosa.25 Furthermore, overexpression of the HO‐1 gene could reduce TNFα mediated apoptotic cell death in human endothelial cells.26 These findings suggest that HO‐1 gene expression could be associated with the progress of aspiration pneumonia, and that reduced expression of the HO‐1 gene in elderly L‐allele carriers might allow the development of pneumonia independently of cerebrovascular disease.
To examine the association between HO‐1 genotype and HO‐1 activity in the pneumonia, we evaluated the carboxyhaemoglobin level in L‐allele carriers and non‐L‐allele carriers with pneumonia. As a result, even after adjustment for the peripheral WBC count and C reactive protein level, patients without the L‐allele showed higher carboxyhaemoglobin levels than those with the L‐allele. Carbon monoxide (CO) is produced endogenously by HO and combines haemoglobin to form carboxyhaemoglobin complex. Therefore, the carboxyhaemoglobin concentration in the subject is a good marker of endogenous HO activity.27 Furthermore, it has been reported that HO‐1 is strongly induced in patients with bacterial infection.28 We have already shown that arterial carboxyhaemoglobin increases at the onset of pneumonia in untreated patients returns to baseline on recovery after treatments.15 We also showed that an increase in arterial carboxyhaemoglobin in pneumonia would be caused by carbon monoxide production in pulmonary inflammation, and that the arterial carboxyhaemoglobin is significantly correlated with disease severity in patients with bacterial pneumonia.29 A study of lymphoblastoid cell lines by Hirai et al showed that mRNA level and activity of HO‐1 were significantly higher in lymphoblastoid cells with the S/S genotype than in those with the L/L genotype after oxidant stimulation.9 Therefore, analysis of the carboxyhaemoglobin level in pneumonia according to HO‐1 genotype would clarify the association between the HO‐1 genotype and HO‐1 activity—that is, the HO‐1 protein level, resulting from pneumonia. These findings suggest that HO‐1 induction might be associated with the HO‐1 genotype (S>M>L).
In contrast to arterial blood carboxyhaemoglobin concentrations, we did not measure HO‐1 activity in patients with pneumonia at the onset. However, we obtained new blood samples from eight people in the control group and seven in the pneumonia group after recovery from pneumonia, and analysed the serum HO‐1 protein levels using enzyme linked immunosorbent assay methods as previously described.30 There was no significant difference between these two groups when they were in good physical condition (2.6 (1.2) v 2.4 (1.0) ng/ml, p>0.2). These values were compatible with the results from a previous report.30 Because the HO‐1 gene is inducible by inflammation or oxidative stress, the baseline expression of the this gene should be low regardless of the HO‐1 genotype, which was demonstrated in lymphoblastoid cell by Hirai et al.9 Further studies are needed to clarify the relation between HO‐1 activity and the HO‐1 genotype at the onset of pneumonia.
Conclusions
This is the first study to show that the 5′‐flanking polymorphism in the HO‐1 gene is associated with the development of pneumonia in an older Japanese population with basal ganglia infarction. Increased susceptibility to developing pneumonia may be associated with sclerosis in the cerebral arteries.
Acknowledgements
We thank Drs Daisuke Inoue, Hisao Hirai, and Satoru Ebihara for samples, and Mr G Crittenden for English language editing. This study was supported by a grant‐in‐aid for scientific research from the Ministry of Education, Science and Culture (17790524, 16590732, and 17590591) of the Japanese government to HY, MY, and TO, respectively, and also supported in part by grants from the Japanese Foundation for Aging and Health to KN.
Abbreviations
COPD - chronic obstructive pulmonary disease
CPE - chronic pulmonary emphysema
HO - haem oxygenase
HO‐1 - inducible haem oxygenase
ROS - reactive oxygen species
TNF - tumour necrosis factor
Footnotes
Conflicts of interest: none declared.
References
- 1.Niederman M S. Nosocomial pneumonia in the elderly patient: chronic care facility and hospital considerations. Clin Chest Med 199314479–490. [PubMed] [Google Scholar]
- 2.Walker A E, Robins M, Weinfeld F D. Clinical findings: the National Survey of Stroke. Stroke 198112(suppl 1)I13–I44. [PubMed] [Google Scholar]
- 3.Nakagawa T, Sekizawa K, Arai H, Kikuchi R, Manabe K, Sasaki H. High incidence of pneumonia in elderly patients with basal ganglia infarction. Arch Intern Med 1997157321–324. [PubMed] [Google Scholar]
- 4.Sacco R L. Newer risk factors for stroke. Neurology 200157(suppl 2)S31–S34. [DOI] [PubMed] [Google Scholar]
- 5.Fukai T, Folz R J, Landmesser U, Harrison D G. Extracellular superoxide dismutase and cardiovascular disease. Cardiovasc Res 200255239–249. [DOI] [PubMed] [Google Scholar]
- 6.Maines M D. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol 199737517–554. [DOI] [PubMed] [Google Scholar]
- 7.Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, Sasaki H. Microsatellite polymorphism in the heme oxygenase‐1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet. 2000;66: 187–95, [Erratum, Am J Hum Genet 2001;68: 1542. ] [DOI] [PMC free article] [PubMed]
- 8.Okinaga S, Takahashi K, Takeda K, Yoshizawa M, Fujita H, Sasaki H, Shibahara S. Regulation of human heme oxygenase‐1 gene expression under thermal stress. Blood 1996875074–5084. [PubMed] [Google Scholar]
- 9.Hirai H, Kubo H, Yamaya M, Nakayama K, Numasaki M, Kobayashi S, Suzuki S, Shibahara S, Sasaki H. Microsatellite polymorphism in heme oxygenase‐1 gene promoter is associated with susceptibility to oxidant‐induced apoptosis in lymphoblastoid cell lines. Blood 20031021619–1621. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi A, Yamaya M, Suzuki S, Yasuda H, Kubo H, Nakayama K, Handa M, Sasaki T, Shibahara S, Sekizawa K, Sasaki H. Association of susceptibility to the development of lung adenocarcinoma with the heme oxygenase‐1 gene promoter susceptibility. Hum Genet 2005116354–360. [DOI] [PubMed] [Google Scholar]
- 11.Yamaya M, Nakayama K, Ebihara S, Hirai H, Higuchi S, Sasaki H. Relationship between microsatellite polymorphism in the haem oxygenase‐1 gene promoter and longevity of the normal Japanese population. J Med Genet 200340146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y H, Lin S J, Lin M W, Tsai H L, Kuo S S, Chen J W, Charng M J, Wu T C, Chen L C, Ding P Y A, Pan W H, Jou Y S, Chau L Y. Microsatellite polymorphism in promoter of heme oxygenase‐1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Gen 20021111–8. [DOI] [PubMed] [Google Scholar]
- 13.Oken M M, Creech R H, Tormey D C, Horton J, Davis T E, McFadden E T, Carbone P P. Toxicity and response criteria of the eastern cooperative oncology group. Am J Clin Oncol 19825649–655. [PubMed] [Google Scholar]
- 14.Shibahara S, Sato M, Muller R M, Yoshida T. Structural organization of the human heme oxygenase gene and the function of its promoter. Eur J Biochem 1989179557–563. [DOI] [PubMed] [Google Scholar]
- 15.Yasuda H, Yamaya M, Yanai M, Ohrui T, Sasaki H. Increased blood carboxyhemoglobin concentrations in inflammatory pulmonary diseases. Thorax 200257779–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamaya M, Yanai M, Ohrui T, Arai H, Sasaki H. Interventions to prevent pneumonia among older adults. J Am Geriatr Soc 20014985–90. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson J, Regnstrom J, Frostegard J, Stiko A. Lipid oxidation and atherosclerosis. Herz 199217263–269. [PubMed] [Google Scholar]
- 18. Braughler JM, Hall , eds. Central nervous system trauma and stroke. I. Biochemical considerations for oxygen radical formation and lipid peroxidation. Free Radic Biol Med 19896289–301. [DOI] [PubMed] [Google Scholar]
- 19.Maytin M, Leopold J, Loscalzo J. Oxidant stress in the vasculature. Curr Atherosclerosis Rep 19991156–164. [DOI] [PubMed] [Google Scholar]
- 20.Öllinger R, Bilban M, Erat A, Froio A, McDaid J, Tyagi S, Csizmadia E, Graça‐Souza A V, Liloia A, Soares M P, Otterbein L E, Usheva A, Yamashita K, Bach F H. Bilirubin: a natural inhibitor of vascular smooth muscle cell proliferation. Circulation 20051121030–1039. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa K, Sugawara D, Goto J, Watanabe Y, Kawamura K, Shiomi M, Itabe H, Maruyama Y. Heme oxygenase‐1 inhibits atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Circulation 20011041831–1836. [DOI] [PubMed] [Google Scholar]
- 22.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase‐1 deficiency. J Clin Invest 1999103129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Exner M, Schillinger M, Minar E, Mlekusch W, Schlerka G, Haumer M, Mannhalter C, Wagner O. Heme oxygenase‐1 gene promoter microsatellite polymorphism is associated with restenosis after percutaneous transluminal angioplasty. J Endovasc Ther 20018433–440. [DOI] [PubMed] [Google Scholar]
- 24.Davidson B A, Knight P R, Helinski J D, Nader N D, Shanley T P, Johnson K J. The role of tumor necrosis factor‐alpha in the pathogenesis of aspiration pneumonitis in rats. Anesthesiology 199991486–499. [DOI] [PubMed] [Google Scholar]
- 25.Tsuburai T, Kaneko T, Nagashima Y, Ueda A, Tagawa A, Shinohara T, Ishigatsubo Y. Pseudomonas aeruginosa‐induced neutrophilic lung inflammation is attenuated by adenovirus‐mediated transfer of the heme oxygenase 1 cDNA in mice. Hum Gene Ther 200415273–285. [DOI] [PubMed] [Google Scholar]
- 26.Kushida T, Li Volti G, Quan S, Goodman A, Abraham N G. Role of human heme oxygenase‐1 in attenuating TNF‐alpha‐mediated inflammation injury in endothelial cells. J Cell Biochem 200287377–385. [DOI] [PubMed] [Google Scholar]
- 27.Marks G S, Vreman H J, McLaughlin B E, Brien J F, Nakatsu K. Measurement of endogenous carbon monoxide formation in biological systems. Antioxid Redox Signal 20024271–277. [DOI] [PubMed] [Google Scholar]
- 28.Yachie A, Toma T, Mizuno K, Okamato H, Shimura S, Ohta K, Kasahara Y, Koizumi S. Heme oxygenase‐1 production by peripheral blood monocytes during acute inflammatory illnesses of children. Exp Biol Med 2003228550–556. [DOI] [PubMed] [Google Scholar]
- 29.Yasuda H, Sasaki T, Yamaya M, Ebihara S, Maruyama M, Kanda A, Sasaki H. Increased arteriovenous carboxyhemoglobin differences in patients with inflammatory pulmonary diseases. Chest 20041252160–2168. [DOI] [PubMed] [Google Scholar]
- 30.Kirino Y, Takeno M, Iwasaki M, Ueda A, Ohno S, Shirai A, Kanamori H, Tanaka K, Ishigatsubo Y. Increased serum HO‐1 in hemophagocytic syndrome and adult‐onset Still's disease: use in the differential diagnosis of hyperferritinemia. Arthritis Res Ther 20057R616–R624. [DOI] [PMC free article] [PubMed] [Google Scholar]