Abstract
Background
Osteopetrosis, a genetic disease characterised by osteoclast failure, is classified into three forms: infantile malignant autosomal recessive osteopetrosis (ARO), intermediate autosomal recessive osteopetrosis (IRO), and autosomal dominant osteopetrosis (ADO).
Methods
We studied 49 patients, 21 with ARO, one with IRO, and 27 with type II ADO (ADO II).
Results
Most ARO patients bore known or novel (one case) ATP6i (TCIRG1) gene mutations. Six ADO II patients had no mutations in ClCN7, the only so far recognised gene implicated, suggesting involvement of yet unknown genes. Identical ClCN7 mutations produced differing phenotypes with variable degrees of severity. In ADO II, serum tartrate resistant acid phosphatase was always elevated. Bone alkaline phosphatase (BALP) was generally low, but osteocalcin was high, suggesting perturbed osteoblast differentiation or function. In contrast, BALP was high in ARO patients. Elevated osteoclast surface/bone surface was noted in biopsies from most ARO patients. Cases with high osteoclasts also showed increased osteoblast surface/bone surface. ARO osteoclasts were morphologically normal, with unaltered formation rates, intracellular pH handling, and response to acidification. Their resorption activity was greatly reduced, but not abolished. In control osteoclasts, all resorption activity was abolished by combined inhibition of proton pumping and sodium/proton antiport.
Conclusions
These findings provide a rationale for novel therapies targeting pH handling mechanisms in osteoclasts and their microenvironment.
Keywords: ATP6i/TCIRG1 gene, ClCN7 gene, osteoblast, osteoclast, osteopetrosis
Bone homeostasis is based on a strict balance between bone formation and bone resorption.1 Perturbation of this balance can lead to a reduction in bone mass, as seen in osteoporosis,2 or to abnormal accumulation of bone tissue, as observed in osteopetrosis. This latter disease is inherited, rare, and very heterogeneous.3 All forms of osteopetrosis are characterised by defective bone resorption, primarily due to altered function of the bone resorbing cells, the osteoclasts, which are motile multinuclear syncytia derived from precursors of the monocyte/macrophage lineage.4 Resorbing osteoclasts become polarised and seal an extracellular compartment (resorption lacuna) between their plasma membrane and the bone surface. Active acidification of the lacuna by osteoclasts plays a critical role in the resorptive process. The acid secretion process is initiated by the cytosolic enzyme carbonic anhydrase II (CA II), which favours CO2 hydration, with subsequent release of protons.5 Polarised secretion of protons occurs through the vacuolar H+ATPase (V‐H+ATPase), an ATP dependent proton pump.6 Chloride channel ClCN7 is essential for efficient proton pumping because of its role in maintaining electro‐neutrality.7
In humans, three clinically distinct types of osteopetrosis are recognised: infantile malignant autosomal recessive osteopetrosis (ARO), intermediate autosomal recessive osteopetrosis (IRO), and autosomal dominant osteopetrosis (ADO).3 ARO is a severe bone disease with early fatal outcome. Its symptoms consist of severe anaemia, hepatosplenomegaly, frontal bossing, nystagmus, growth retardation, and compression of cranial nerves with blindness and deafness.3 Approximately 50% of patients harbour mutations in the ATP6i (TCIRG1) gene,8,9 encoding the osteoclast specific a3 subunit of V‐H+ATPase; approximately 10% have mutations in the ClCN7 gene7; and two patients so far10,11,12 present mutations in the Gl gene, encoding a yet uncharacterised cytoplasmic protein involved in osteoclast activity.10
ADO is characterised by a variety of symptoms, including haematological and neural defects with diverse disease severity.3 It is generally seen in adults and has been divided into two subclasses, type I (ADO I) and type II (ADO II).13 The hallmark of ADO I is a generalised increase in bone mass; ADO I patients are unlikely to experience fractures.3 Mutations of the LDL receptor related protein 5 (LRP5) were found to affect two families with this form.14 ADO II is characterised by sclerosis of the skull base and pelvis and a typical “rugger jersey” or “sandwich” spine.13 Affected individuals may be asymptomatic, but more often experience clinical manifestations, including multiple fractures. ClCN7 gene mutations underlie ADO II.7
IRO can be associated with mutations in the CA7,15 II gene.16 Patients who harbour CA II gene mutations present with calcification of the brain and renal tubular acidosis; this disease has therefore been described as “marble brain” syndrome.17 IRO patients with no known mutations are also described.18
Before associated molecular abnormalities were found, the various forms of osteopetrosis were characterised by clinical description only. A comprehensive classification based on molecular findings would undoubtedly be more desirable. To date, however, not all genes causing osteopetrosis are known. Furthermore, the relationships between gene abnormalities and clinical features, such as specific serum data, remain elusive. In the present study, clinical, genetic, and cellular aspects of the disease have been investigated in a group of patients, with the aim of providing insights for diagnosis protocols and learning more about osteopetrosis pathophysiology, which may lead to novel effective therapeutic options.
Methods
Patients
We have collected material from 49 osteopetrotic patients of both sexes and of a wide age range, with the informed consent of the patients or of their parents. The study has been approved by the competent ethics committees of the institutions where the samples were collected. Samples were obtained from Italy, Greece, Spain, the United Kingdom, and the United States. Specimens include DNA, iliac crest bone biopsies, whole peripheral blood, and sera. Variable sets of clinical and radiological data were collected, together with laboratory data and serum bone marker evaluations. Asymptomatic patients with ADO II were siblings of affected individuals who were found to harbour a ClCN7 gene mutation.
Materials
Cell culture media, sera, and reagents were from Gibco (Uxbridge, UK). Sterile glassware was from Falcon Becton Dickinson (Meylan, France). DNA extraction kits and reagents for PCR were from Qiagen (Genenco, Florence, Italy). Dye Terminator Cycle‐Sequencing Ready Reaction Mix was from Perkin‐Elmer Applied Biosystems (Foster City, CA, USA). Reagents for histology were from Bio‐Optica (Milan, Italy). All other reagents were of the purest grade from Sigma Aldrich (St Louis, MO, USA).
Bone histology
Bone biopsies of the iliac crest were performed and processed for paraffin embedding according to standard procedures for EDTA decalcified samples. Sections were routinely stained with haematoxylin‐eosin. Alternatively, sections were stained, as detailed below, for histochemical detection of tartrate resistant acid phosphatase (TRAcP) activity.
Histomorphometric measurements were carried out with an interactive image analysis system (IAS 2000, Delta Sistemi, Rome, Italy) consisting of a colour video‐equipped computer linked to the microscope with a video camera. Nomenclature, symbols, and units of histomorphometric bone variables are those suggested by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research.19 The cellular variables measured were osteoclast surface/bone surface (Oc.S/BS, %) and osteoblast surface/bone surface (Ob.S/BS, %).
Mutation analysis
DNA was extracted from EDTA‐blood samples using the Qiagen DNA blood extraction kit #51104, according to the manufacturer's instructions. The entire coding region of the ATP6i (TCIRG1) and ClCN7 genes was amplified using intronic primers deduced from deposited gene sequences (GenBank accession numbers: AF033033 and AL031600) and National Biosciences OLIGO 4.1 Primer Analysis Software. PCR reactions were carried out using the Qiagen Master Mix Kit, including 1× PCR buffer, 1× Q‐solution, 200 µM dNTP, 0.5 µM of primer pair, and 2.5 U/reaction Taq DNA polymerase. PCR products were purified using the Qiagen PCR purification kit according to the manufacturer's instructions. A 10 ng sample of purified PCR products was used for 100 bp of DNA cycle sequencing which was performed using a Perkin‐Elmer Dye Terminator Cycle‐Sequencing Ready Reaction Mix and standard procedures. Reaction products were applied to a Perkin‐Elmer ABI 377 DNA sequencer, and obtained sequences were aligned using the NCBI BLAST 2 program.
Osteoclast preparation from peripheral blood monocytes
Blood mononuclear cells were prepared from human peripheral blood diluted 1:1 in Hank's balanced salt solution. Diluted blood was then layered over Histopaque 1077 solution centrifuged at 400 g for 30 min. “Buffy coat” cells thus isolated were collected and washed twice with Hank's solution, then centrifuged at the same speed for 15 min. Cells were resuspended in DMEM medium containing 4 mM l‐glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% FCS. Then 1×106 cells/cm2 were plated on cell culture dishes, containing glass or bone slices when required, and incubated at 37°C in a humidified atmosphere with 5% CO2. After 3 h, cell cultures were rinsed to remove non‐adherent cells and maintained in the same medium in the presence of 25 ng/ml recombinant human macrophage colony stimulating factor (M‐CSF), 30 ng/ml recombinant human receptor activator of NF‐κB ligand (RANKL), and 10 ng/ml parathyroid hormone (PTH) for 15 days. Medium and factors were replaced every 3–4 days.
Osteoclast acidification assay
Acridine orange [3,6‐bis(dimethylamine)acridine] at 10 mM was loaded into the cells for 10 min in serum‐less culture medium. The dye was then removed by washing cultures in the same medium and cells were observed by conventional fluorescence microscopy with a computer assisted Zeiss Axioplan microscope.
Tartrate resistant acid phosphatase (TRAcP) activity assays
Cells were fixed in 3% paraformaldehyde in 0.1 M cacodylate buffer for 15 min, then extensively washed with the same buffer. TRAcP activity was detected histochemically in cells or in paraffin embedded sections of bone biopsies, using Sigma‐Aldrich kit #386, according to the manufacturer's instruction.
Bone resorption assays
Cells were cultured in 96 well multiplates containing 4×4 mm bovine bone slices, and differentiated into osteoclasts as described above. Mature osteoclasts were then cultured for a week in the presence or absence of bafilomycin A1, 5‐(N‐ethyl‐N‐iso‐propyl)‐amiloride (EIPA), or both, at the indicated concentrations, in media at pH 7.25 or 6.90. These media were obtained by adding 5.8 or 13.6 µl of 1 M HCl to each 10 ml of normal medium. Cells were eventually mildly fixed in 3% paraformaldehyde in cacodylate buffer for 3 min, then slices were cleaned free of cells by prolonged sonication, stained with 1% toluidine blue, and observed by conventional light microscopy.
Quantitative analysis
Bone resorption was determined by the pit assay according to our previous report.20 Quantitative data were expressed as means±SEM. Statistical differences were identified using one way analysis of variance (ANOVA) followed by Student's t test; p<0.05 was considered significant.
Results
Clinical follow up
Patients with ARO
Clinical data were available for 17 of our ARO patients who were neonates and infants at the time of evaluation (table 1). Symptoms were typical of ARO. Most patients had hepatosplenomegaly (n = 15) and macrocephaly (n = 10); nine patients presented with growth retardation and four patients were hydrocephalic. Compromised vision due to optic nerve compression syndrome was observed in five patients. Laboratory findings revealed severe anaemia and altered white cell counts.
Table 1 Clinical findings of ARO patients.
| No. 15 | No. 2532 | No. 37 | No. 4032 | No. 4132 | No. 4232 | No. 46 | No. 62 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | F | F | M | M | F | M | F | M | ||
| Age (years) | 7 | 5 | 12 | 8 | 6 | 4 | 9 | 2 | ||
| Death | No | No | No | No | No | No | No | No | ||
| Hydrocephaly | No | √ | No | No | No | √ | √ | No | ||
| Hepatosplenomegaly | √ | √ | √ | √ | √ | No | No | √ | ||
| Macrocephaly | No | √ | No | √ | √ | √ | No | No | ||
| Growth retardation | No | √ | No | No | No | √ | No | No | ||
| Nystagmus | No | No | No | √ | √ | No | √ | √ | ||
| Hearing impairment | No | No | No | √ | No | No | No | √ | ||
| Others | Skull base osteosclerosis | Pale optic nerve papillae, dysmorphism, pale skin | Frontal eminences, pale skin, exophthalmoses | Severely reduced vision | Severely reduced vision, dwarfism | Pale optic nerve papillae, fever, rhinitis | No teeth, osteomyelitis | Skull base osteosclerosis, optic papillae sub‐atrophy | ||
| Prednisone treatment | √38 | √ | √ | No | √ | √ | No | √ | ||
| HSCT (age) | No | NA | √ NA | √ | √ | √ | No | √ | ||
| 2 months | 8 months | 6 months | 2 years | |||||||
| Current state | Alive and well | NA | Severely compromised | Alive and well | Alive and well | NA | Severely compromised | Alive and well |
| No. 91 | No. 95 | No. 96 | No. 97 | No. 99 | No. 100 | No. 106 | No. 114 | No. 117 | Frequency | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | F | F | M | M | F | M | M | M | F | |
| Age (years) | – | – | 2 | 15 | 4 | – | 24 | 3 | – | |
| Death | Yes | Yes | No | No | No | Yes | No | No | Yes | 4/17 |
| Age of death | 5 months | 2 years | 4 months | 14 months | ||||||
| Hydrocephaly | No | No | No | No | √ | No | No | No | No | 4/17 |
| Hepatosplenomegaly | √ | √ | √ | √ | √ | √ | √ | √ | √ | 15/17 |
| Macrocephaly | √ | √ | √ | √ | No | No | √ | √ | No | 10/17 |
| Growth retardation | √ | √ | No | √ | √ | No | √ | √ | √ | 9/17 |
| Nystagmus | No | √ | No | No | √ | √ | No | No | √ | 8/17 |
| Hearing impairment | No | No | No | No | √ | No | √ | No | No | 4/17 |
| Others | Exophthalmoses | Pale optic papillae | Multiple fractures, heart defects | Multiple infections, pancytopenia | ||||||
| Prednisone treatment | No | √ | √ | No | √ | No | No | √ | No | 10/17 |
| HSCT (age) | √ | No | √ | √ | √ | √ | √ | √ | √ | 13/16 |
| 5 months | 11 months | 4 years | 9 months | 2 months | 13 years | 6 months | 13 months | |||
| Current state | Dead TRM | Alive and well | Alive and well | Mildly reduced osteosclerosis | Dead TRM | Alive and well | Alive, engrafted, 3 months post‐HSCT no radiographic improvement, reduced osteoclasts |
HSCT, haematopoietic stem cell transplant; NA, not available; TRM, transplant related mortality.
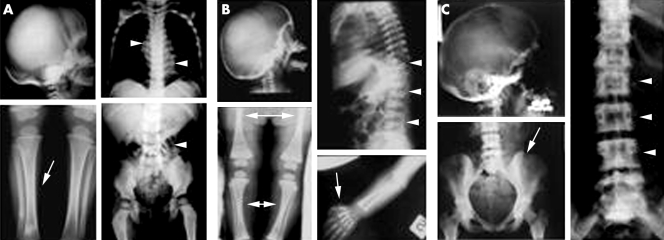
Radiographs showed a generalised increase in bone density and sclerosis of the basis of the skull with increased density of the orbits. Proximal metaphyses of femurs and tibias were irregularly shaped and, together with forearms, showed diffused osteosclerosis and absence of medullary cavities. Femurs, tibias, forearms, phalanges, and iliac crests showed “endobone” appearance (fig 1A). Four patients died during the early months of life. The oldest patient who had not received bone marrow transplantation was aged 9 years.
Figure 1 X ray analysis. (A) ARO patients. Sclerosis of skull base, obliterated tibia cavities (arrow), and endobone appearance (arrowheads). (B) IRO patient. High bone density, sclerosis of vertebrae (arrowheads), phalanges (arrow), “bone in bone” appearance (double arrows), and mild sclerosis of the skull. (C) ADO II patients. Osteosclerosis, especially at vertebral endplates (arrowheads), iliac wings (arrow), and skull base.
Patient with IRO
As previously described,18 the only patient affected by IRO presented with pneumonia and a generalised increase in bone density, sclerosis of vertebrae and of limb metaphyses with bone in bone appearance, and mild sclerosis of the skull (fig 1B). Dental eruption was delayed. At the age of 6 months, the patient developed erythematous patches accompanied by blistering and diffuse irregular hypo/hyperpigmentation which eventually evolved into poikiloderma‐like skin lesion (table 2).
Table 2 Clinical findings of IRO patient.
| No. 221 | |
|---|---|
| Sex | F |
| Age (years) | 15 |
| Number of fractures | 0 |
| Generalised osteosclerosis | √ |
| Rugger jersey spine | √ |
| Bone in bone appearance | √ |
| Haematological parameters alteration | √ |
| Others | Pneumonia, delayed dental eruption, and erythematous patches accompanied by blistering and diffuse irregular hypo/hyperpigmentation which eventually evolved into poikiloderma‐like skin lesion |
Patients with ADO II
Clinical follow up was available for 21 ADO II patients. As shown in table 3, although ADO II is generally accepted as a benign form of osteopetrosis, we examined patients presenting a wide phenotypic spectrum varying from virtually asymptomatic to severely affected conditions. In fact, three subjects showed no typical features of osteopetrosis (nos. 65, 81, and 89) and were genetically diagnosed because of a mutation present in the family, while another three patients were severely compromised: nos. 18, 51, and 57 had >10 fractures, and no. 51 eventually died at the age of 36. Fourteen patients showed typical radiological abnormalities, that is, generalised osteosclerosis, predominantly at the vertebral endplates (rugger jersey spine), iliac wings (bone in bone appearance), and skull base (fig 1C). The three asymptomatic patients were normal at radiological examination.
Table 3 Clinical findings of ADO II patients.
| No. 18 | No. 24 | No. 51 | No. 52 | No. 57 | No. 58 | No. 5922 | No. 60 | |
|---|---|---|---|---|---|---|---|---|
| Sex | M | F | M | M | F | F | M | F |
| Age (years) | 44 | 35 | 36 | 20 | 39 | 15 | 21 | 15 |
| Diffuse pain | No | No | No | No | No | √ | √ | No |
| Number of fractures | >10 | 0–3 | >10 | 0–3 | 4–10 | 0 | 0 | 0 |
| Generalised osteosclerosis | √ | NA | √ | No | √ | No | No | √ |
| Rugger jersey spine | √ | NA | √ | No | √ | √ | √ | √ |
| Bone in bone appearance | √ | NA | No | No | √ | No | √ | √ |
| Haematological parameters alteration | √ | No | √ | NA | √ | √ | √ | No |
| Others | Reduced hearing and vision | Blindness, dead | Arachnoid cyst, right ear deafness, recurrent infections |
| No. 6522 | No. 72 | No. 73 | No. 77 | No. 79 | No. 81 | No. 86 | No. 88 | |
|---|---|---|---|---|---|---|---|---|
| Sex | M | F | F | M | M | F | M | M |
| Age (years) | NA (no. 59's father) | 63 | 60 (no. 72's sister) | 34 | 38 | 37 (no. 60's mother) | 3 | 27 |
| Diffuse pain | No | √ | No | No | No | No | No | No |
| Number of fractures | 0 | 0–3 | NA | 0 | 4–10 | 0 | 0 | 4–10 |
| Generalised osteosclerosis | No | No | No | √ | NA | No | No | NA |
| Rugger jersey spine | No | √ | √ | √ | NA | No | √ | NA |
| Bone in bone appearance | No | No | No | √ | NA | No | √ | NA |
| Haematological parameters alteration | No | No | No | No | √ | No | √ | √ |
| Others | Upper jaw increased bone density | Mild mental retardation | Bilateral optic atrophy |
| No. 89 | No. 90 | No. 98 | No. 105 | No. 108 | Frequency | |||
|---|---|---|---|---|---|---|---|---|
| Sex | F | F | M | F | M | |||
| Age (years) | 61 (no. 88's mother) | 37 | 57 (no. 72 and 73's brother) | 42 | 12 | |||
| Diffuse pain | No | √ | No | √ | √ | 6/21 | ||
| Number of fractures | 0 | 0 | 0–3 | >10 | 0 | None: 10/20; 0–3: 4/20; 4–10: 3/20; >10: 3/20 | ||
| Generalised osteosclerosis | No | √ | √ | √ | √ | 8/18 | ||
| Rugger jersey spine | No | NA | No | √ | No | 10/17 | ||
| Bone in bone appearance | No | NA | No | √ | No | 6/17 | ||
| Haematological parameters alteration | No | √ | NA | √ | No | 10/19 | ||
| Others | Epilepsy, blindness, mental retardation, wheelchair. |
NA, not available.
Nine patients presented with a variable degree of blood dyscrasia, yet half of the patients were haematologically normal.
Serum bone markers
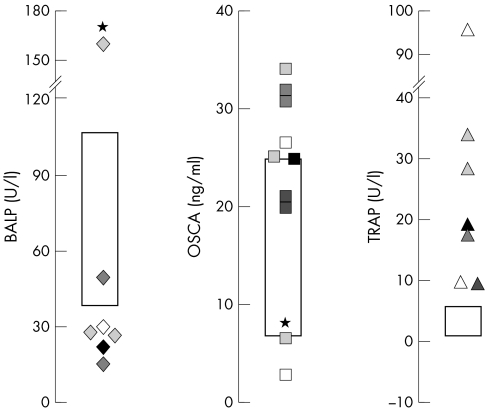
Evaluation of typical serum markers of bone activity was performed in subsets of ADO II patients, varying in number from six for bone alkaline phosphatase (BALP) to ten for osteocalcin (OSCA). Serum TRAcP values were measured in seven patients, and were elevated in all patients examined (fig 2),13 suggesting generally increased osteoclast formation and/or life span.21 Interestingly, the majority of ADO II patients also showed an unexpected imbalance in osteoblast serum markers, with low BALP and high OSCA (fig 2). These findings suggest generally altered osteoblast function and/or differentiation, resulting in decreased bone quality. In one patient (no. 51), presenting with the most severe conditions, the BALP/OSCA ratio was abnormally high, rather than low (fig 2, asterisks).
Figure 2 Serum bone markers. Boxes in graphs indicate conventional physiological ranges. All patients examined presented high circulating TRAcP, plus abnormally low BALP and high OSCA in most cases. *Patient with inversely unbalanced BALP and OSCA.
In contrast with ADO II, in all ARO patients examined serum BALP was slightly or greatly elevated (nine patients; data not shown).
Histomorphometry
Histomorphometric features
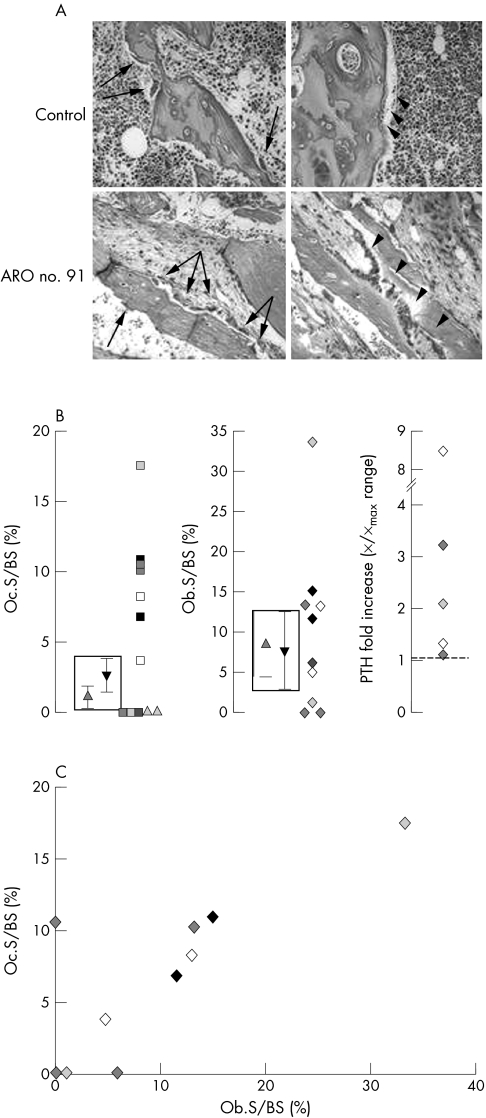
Iliac crest biopsies were obtained from 12 ARO patients and subjected to histomorphometric analysis, which showed obviously elevated Oc.S/BS values in six cases (fig 3A, left panels), whereas five patients (two of whom were from the same family) had no osteoclasts. One patient presented with a high but still normal osteoclast surface (fig 3B, left graph). Interestingly, Ob.S/BS was increased in all patients with a clearly elevated osteoclast surface (fig 3A), but not so in the patient with normal Oc.S/BS, and in those without osteoclasts (data available only for three patients; fig 3B, middle graph).
Figure 3 Histomorphometric analysis of iliac crest biopsies from a control and an ARO patient and serum PTH levels. (A) Haematoxylin‐eosin staining of biopsy sections. Arrows and arrowheads indicate osteoclasts and osteoblasts, respectively. Original magnification, 20×. (B) Histomorphometry data and serum PTH levels. Boxes indicate ranges of values in our controls (triangle down) and in those of Glorieux et al37 (triangle up). Triangles outside the box in the left panel identify two siblings for whom only the osteoclast parameter was available. (C) Correlation between Oc.S/BS and Ob.S/BS, r2 = 0.63.
Correlation of histomorphometric data
To explore the hypothesis of a PTH role in the observed alteration of bone cell parameters in patients, we measured serum PTH levels in five patients with very different Ob.S/BS values. Serum PTH was above normal values in all cases (fig 3B, right panel), suggesting no straightforward correlation with osteoblast parameters. In contrast, the correlation between Ob.S/BS and Oc.S/BS was found to be statistically significant (fig 3C; r2 = 0.63).
Genotypes
Mutation analysis was performed by sequencing all exons and intron junctions of the ClCN7 and ATP6i (TCIRG1) genes, after amplification from genomic DNA.
Patients with ADO II
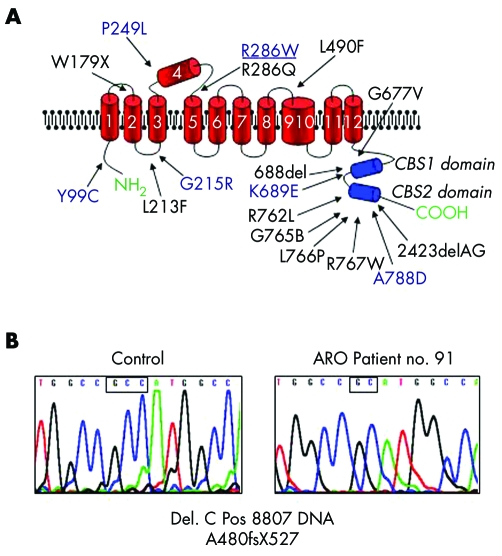
Fourteen out of 20 patients with ADO II were heterozygous for previously described point mutations in the ClCN7 gene. Three patients (nos. 51, 60, and 86) shared the same mutation in exon 10 (heterozygous mutation C894T RNA, R286W), but presented with very different phenotypes: patient no. 60 was asymptomatic, patient no. 86 had bilateral optic atrophy and radiological abnormalities but no fractures, while the severe symptoms of patient no. 51 (table 3) led to his death. We identified various CLCN7 gene polymorphisms in all three of these patients, but no straightforward genotype‐phenotype correlation could be established between ClCN7 variants and disease severity.16 Two previously described mutations mapped in sequences coding for the C‐terminal CBS domains of the protein, a region where approximately one half of ClCN7 gene mutations noted so far fall16,22,23,24,25,26 (fig 4A). Interestingly, six of our ADO II patients were found negative for ClCN7 gene mutations. This, along with phenotypic heterogeneity, indicates that some subtypes of the disease are due to the involvement of further genes.
Figure 4 (A) Organisation of polypeptide domains of chloride channel 7 and distribution of mutations.16,22,23,24,25,26 Mutations in our patients are in blue. Mutation associated with greatly divergent phenotypes is underscored. (B) Electropherograms of wild type ATP6i (TCIRG1) gene sequence in a control individual (left) and in an ARO patient with a novel mutation (right). Small boxes highlight the site of mutation, a single C deletion (right).
Patients with ARO
Mutational analysis of the ATP6i (TCIRG1) gene in our ARO cohort revealed five patients harbouring previously described mutations. Patient no. 91 was homozygous for a novel mutation in exon 12, a single cytosine deletion leading to a frameshift with truncation of the protein (A480fsX527) (fig 4B). No ATP6i (TCIRG1) gene mutation was found in three of the nine patients examined. The same three patients were also negative for mutations in the ClCN7 gene.
Patient with IRO
The only IRO patient with altered skin pigmentation and abnormal in vitro osteoclast morphology and adhesion to substrate, was examined for possible mutations in the MITF and alpha(V) integrin subunit genes. However, no mutations in these genes or in ATP6i (TCIRG1) or ClCN7 were found.
Osteoclast formation
As noted above, the osteopetrotic patients examined for serum markers showed increased serum TRAcP and, moreover, increased osteoclast numbers were observed in most biopsies from ARO patients. This prompted us to test the hypothesis of cell‐autonomously increased differentiation of osteoclast precursors in patients with high osteoclasts. To achieve this, mononuclear cells from peripheral blood were differentiated in vitro into TRAcP positive, multinucleated cells in the presence of M‐CSF and RANKL, but no evident alterations were noted relative to control cells (data not shown).
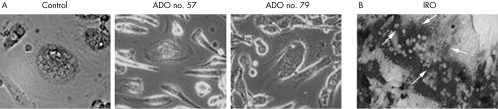
In most cases, no obvious morphological differences were observed between osteoclasts obtained from patients and those from controls. However, osteoclasts from three ADO II patients (nos. 57, 59, and 79) displayed a more motile phenotype, as shown by the appearance of lamellipodia and membrane ruffling (fig 5A). In patient no. 59, a motile pattern of podosome arrangement had already been evidenced.22 Moreover, osteoclasts obtained from the IRO patient displayed obvious cytological abnormalities, such as high numbers of nuclei (fig 5B) and altered adhesion to substrate and podosome formation.18 Notably, and consistently with our findings, such features point to altered resorption rather than abnormal osteoclast formation.
Figure 5 Blood derived in vitro osteoclasts. (A) Phase contrast microscopy evidencing motile phenotype in two ADO II (nos. 57 and 79) patients versus control. Original magnification, 40×. (B) TRAcP staining of an osteoclast from the IRO patient with a increased number of nuclei (arrows). Original magnification, 10×.
Intracellular pH handling
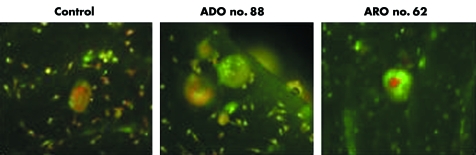
In bone resorbing osteoclasts, ClCN7 and V‐H+ATPase play pivotal roles in both acid secretion and intracellular pH handling. In view of this, we compared intracellular acidification in osteoclasts obtained from osteopetrotic patients and from healthy controls using the acridine orange technique. As shown in fig 6, no obvious differences were seen in the intracellular acidification of osteoclasts between the osteopetrotic patients and controls (fig 6), as also shown by Kornak et al7 but in contrast with the observations of Karsdal et al.27
Figure 6 Fluorescence microscopy of acridine orange stained osteoclast cultures from blood derived precursors, cultured on bone slices. Green and red indicate neutral and acidic pH, respectively. Original magnification, 20×.
In vitro bone resorption
Bone resorption defects vary greatly among the different forms of osteopetrosis. We tested whether osteoclasts from these different variants also showed analogously variable resorption function in vitro.
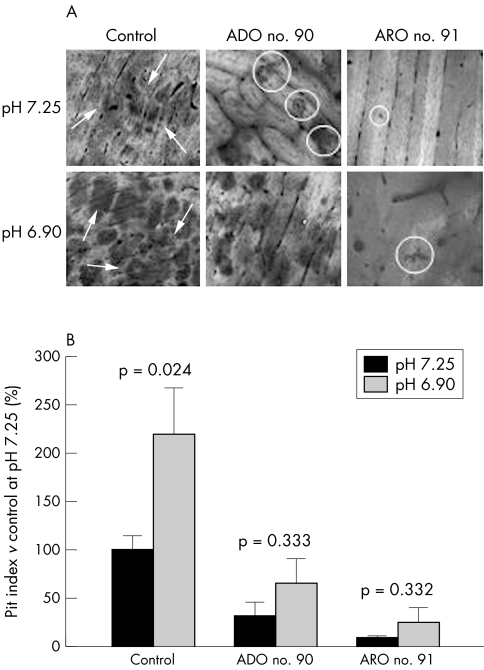
Notably, resorption pits were formed in all cultures. However, control osteoclasts created large pits with regular margins, intensely stainable with toluidine blue, while pits excavated by ADO II patients were smaller and those from ARO cells were even smaller and pale (fig 7A, upper panels). As expected, far fewer resorption pits were formed by patients' osteoclasts: pit index values were ∼30% for ADO II patients and ∼8% for ARO patients relative to controls (fig 7B, dark bars). Thus, it is evident that in the cell cultures of ARO patients, in vitro bone resorption is severely compromised but not completely abolished.
Figure 7 In vitro bone resorption assay. Blood derived differentiated osteoclasts from patients, or controls, were cultured on bone slices for a week in media at pH 7.25 or 6.90. Bone resorption was then quantified as pit indices. (A) Osteoclasts from patients excavated fewer and smaller pits (circles) than controls (arrows). Extracellular acidification (lower panels) enhanced pit areas and numbers. (B) Quantification of resorption.
Osteoclast response to extracellular acidification
Mouse osteoclasts were previously shown to be activated by extracellular acidification.28 Therefore, we asked whether blood derived osteoclasts from controls and osteopetrotic patients were also able to respond to this stimulus. To address this question, osteoclasts were exposed to media at a lower pH of 6.90. At the end of treatment, resorption activity was evaluated by the pit index method. Extracellular acidification enhanced pit formation by control osteoclasts more than twofold. Interestingly, ADO II osteoclasts also seemed to be stimulated to similar levels (approximately threefold), although the observed increases did not reach statistical significance. A trend towards an even higher approximately threefold induction was observed in ARO cell cultures (fig 7B). In this case, statistical significance was unsatisfactory due to the remarkable intrinsic variability of the parameter measured, that is, pit index.20 Nevertheless, both the area of resorption pits and their numbers were increased in most cultures (fig 7A). Thus, patients' osteoclasts appear to maintain, at least in vitro, the ability to respond to an acidification stimulus.
Role of Na+/H+ antiport in ARO residual resorption
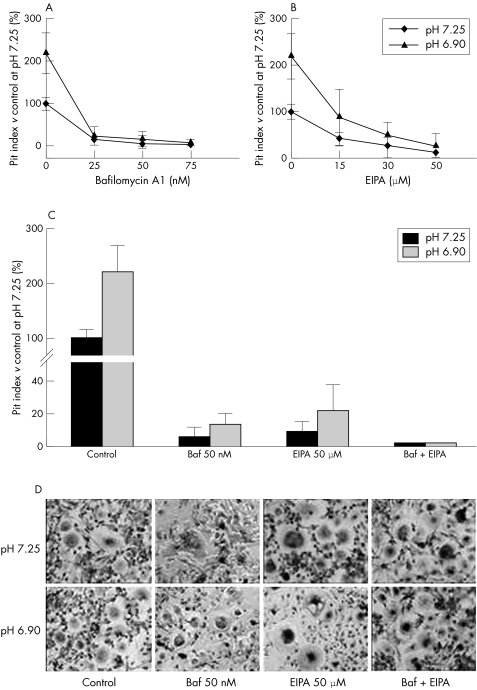
To further study the residual bone resorption activity of osteoclasts from ARO patients, we established an in vitro ARO model system of resorption mimicking ARO but utilising control osteoclasts. Blood derived osteoclast precursors were differentiated on bone slices and the osteoclast cultures thus obtained were then exposed to the proton pump inhibitor bafilomycin A1.29,30 As expected, in control cultures treated with the inhibitor, bone resorption was strongly reduced in a concentration dependent manner. At 50 nM bafilomycin, resorption activity was lowered to the same level as untreated ARO osteoclast cultures. Importantly, treated cells kept showing a trend of responsiveness to extracellular acidification (fig 8A).
Figure 8 Resorption inhibition in control osteoclasts. Blood derived differentiated osteoclasts were cultured on bone slices for a week in the presence of the indicated concentrations of bafilomycin A1, EIPA, or both, either in neutral (pH 7.25) or acidic (pH 6.90) media. Bone resorption was then quantified as pit indices. (A) Concentration dependent decrease of resorption in bafilomycin treated cultures. (B) Concentration dependent decrease of resorption in EIPA treated cultures. (C) Quantification of resorption at maximum doses used. No detectable resorption was noted in cultures treated with both inhibitors. (D) TRAcP staining of osteoclast cultures treated with vehicle or bafilomycin A1, EIPA, or both. No evident alterations in cell morphology were noted. Baf, bafilomycin A1.
Osteoclast bone resorption was previously shown to require functional Na+/H+ antiport.31 This prompted us to test whether ARO residual resorption could rely, to some extent, on this mechanism. To determine this, we combined bafilomycin with the Na+/H+ antiport inhibitor EIPA. When administered alone to control osteoclasts, EIPA caused a dose dependent decrease in bone resorption (fig 8B). In cultures where EIPA and bafilomycin were combined, pit formation was completely abrogated (fig 8C). Importantly, such an effect did not appear to be due to cell toxicity, as no obvious alterations of osteoclast morphology were observed in treated cultures (fig 8D).
Discussion
To date, clinical studies on osteopetrosis have been characterised by poor insight into the specific pathophysiology and cellular background of this extremely heterogeneous disease. As a result, osteopetrosis is still diagnosed almost exclusively on clinical and radiological evidence and recently with a variable input from genetic screening, since precise diagnostic criteria have not been sufficiently well established. Indeed, diagnoses of the mildest forms of the disease, as frequently in the case of ADO I/II, are often controversial because of the scarce radiological abnormalities.22 Genetic screening is necessary for unambiguous diagnosis in many such cases, but not all genes determining osteopetrosis are known.
In this study, some well known serum markers of bone cells have been highlighted as potentially relevant diagnostic tools in one form of the disease, namely ADO II. The most promising of such markers appears to be TRAcP, whose level was found to be clearly above normal range in all ADO II patients examined, as already observed by Bollerslev and Andersen Jr.13 In some cases, a serum concentration as high as ten times the maximum physiological value was recorded. Such findings may reflect either higher expression/activity of the enzyme per cell or a general increase in the number of mature osteoclasts, or both. An apparently higher TRAcP activity in ARO osteoclasts has already been noted.32 The role of TRAcP in osteoclast function is still controversial, so that up‐regulation of this enzyme in malfunctioning mature osteoclasts appears to be of interest.
There are substantial data supporting higher numbers of osteoclasts in ARO.33 Consistent with this, mild to much higher Oc.S/BS values were also observed in our ARO biopsies. To an extent, ADO II might share this characteristic, which would simply explain the observed increase in circulating TRAcP. Whatever its origin(s), it seems likely that high TRAcP may be considered a reliable hallmark of the disease. Incidentally, some evidence supports the idea that elevated osteoclast numbers in osteopetrosis may partly arise from a longer cell life span.27 Such a finding is consistent with the hypothesis, yet to be tested, that triggering of programmed cell death in osteoclasts is tethered to their resorption activity, so that inefficient resorption would delay apoptosis.
To date, osteoblast activity in osteopetrotic patients has been rarely investigated. In this study, circulating levels of two well known osteoblast markers, BALP and OSCA, have been evaluated in ADO II patients, and found to fall outside physiological ranges in almost all cases, with low BALP and high OSCA. Most interestingly, the observed abnormal levels of these two markers appeared to be correlated. In fact, only in one patient was elevated OSCA associated with a normal level of BALP. High circulating levels of OSCA have also been previously observed in animal models of osteopetrosis.34 It is a common notion that OSCA is a late osteoblast marker, and that BALP is somewhat down‐regulated in terminally differentiated osteoblasts (that is, mineralising cells and/or pre‐osteocytes). Thus, our data seem to point to a generally deregulated osteoblast function, possibly resulting from precocious differentiation.
Consistent with previous observations,32 abnormally elevated (rather than low) serum BALP was evidenced in ARO patients, implying that alterations of osteoblast differentiation/function may differ in ADO II and ARO. It must be noted that malfunctioning osteoblasts are likely to form low quality bone tissue, which might partly underlie the pathogenesis of fractures in some mild variants of ADO II.
In most of our ARO patients, high numbers of osteoblasts were recorded from bone biopsies, which may easily explain their high levels of circulating BALP. Extremely defective osteoclasts are considered the main, if not sole, determinant of ARO, which prompted us to ask whether the observed increases in osteoblast numbers were an osteoclast related effect. A statistically significant correlation was found between osteoclasts and osteoblasts, which were both above normal range in most patients. Consistently, patients not showing high osteoclasts in bone biopsies were also characterised by few osteoblasts and much lower serum BALB levels (∼2.3‐fold versus normal values) compared to patients with (high) osteoclasts (∼13.5‐fold). Circulating PTH, arising from deficient bone resorption, did not correlate with altered osteoblast formation in ARO, as the hormone was found to be always elevated, regardless of diverse osteoblast numbers in our biopsies. Thus, mature osteoclasts seem to be somehow favouring the formation of osteoblasts in their microenvironment, by means of mechanisms largely independent of their resorption activity.35,36
In ADO II patients, the ClCN7 gene was mutated in most cases, but, notably, ∼30% of cases were characterised by “normal” ClCN7 gene sequences. The relationship between ClCN7 gene mutations and disease severity was strikingly elusive. In particular, the same ClCN7 mutation (most frequent in ADO II: 18.4% of patients) was recorded in three patients presenting with extremely divergent disease severity. As analysis of ClCN7 gene polymorphisms in these patients showed no genotype‐phenotype correlation, the mechanisms underlying such low penetrance remain to be established. This set of data suggests that, in contrast with ARO, ADO II is probably caused by more than one mutated and/or variant gene in each affected individual.
Two out of the six different CLCN7 gene mutations found in our patients mapped to the CBS domains of the protein. About one half of the total known mutations in ClCN7 fall within these C‐terminal, cytoplasmic domains.16,22,23,24,25,26 Thus, this location could indicate a mutation hot spot, in which single amino acid changes can profoundly affect protein functions.
In vitro, blood derived osteoclasts from ADO II and ARO patients showed an apparently normal morphology. However, three cases of ADO II were associated with a motile phenotype. If confirmed with other patients, such a finding may be of considerable interest, in that it would indicate motility related genes as possible, previously unknown co‐players in ADO II determination. Among all cases examined, only cells from the IRO patient were grossly abnormal, but such a finding needs future statistical validation. Osteoclasts from our patients also did not show any abnormalities in intracellular pH handling. This is in disagreement with previous observations by Karsdal et al,27 but in agreement with a study by Kornak et al.7 The evaluation technique used, namely, acridine orange staining, is of limited sensitivity, so we also utilised other techniques, such as those based on the Lysosensor Y/B (yellow/blue) and Lysotracker Red reagents (Molecular Probes, Eugene, OR), again with unremarkable results. As expected, osteoclasts from patients were, on the other hand, very inefficient in in vitro bone resorption assays. However, even cells from ARO patients still retained some resorption activity and also responsiveness to extracellular acidification, although this latter finding needs statistical validation. Taken together, these data suggest that the intracellular mechanisms supporting osteoclast function are not (irreversibly) compromised in these cells. Conversely, combined pharmacological inhibition of proton pumping and the Na+/H+ antiport mechanism abolished all resorption activity in control osteoclasts, without any alterations in cell morphology. Such a finding suggests a very important role for Na+/H+ exchange in residual bone resorption in ARO, where osteoclast specific (that is, a3‐subunit dependent) proton pumping is impaired. Moreover, it points to this mechanism, and possibly other pH‐handling mechanisms, as a potential target(s) for early treatment of this severe form of osteopetrosis with osteoclasts. Pharmacological stimulation might prove efficient, for instance, at enhancing residual bone resorption in the early stages of ARO, with crucial consequences on irreversible events such as nerve compression syndromes.
Acknowledgements
The invaluable help of Dr Rita Di Massimo for editing this manuscript is gratefully acknowledged.
Abbreviations
ADO - autosomal dominant osteopetrosis
ARO - autosomal recessive osteopetrosis
BALP - bone alkaline phosphatase
CA II - carbonic anhydrase II
EIPA - 5‐(N‐ethyl‐N‐iso‐propyl)‐amiloride
IRO - intermediate autosomal recessive osteopetrosis
M‐CSF - macrophage colony stimulating factor
Ob.S/BS - osteoblast surface/bone surface
Oc.S/BS - osteoclast surface/bone surface
OSCA - osteocalcin
PTH - parathyroid hormone
RANKL - receptor activator of NF‐κB ligand
TRAcP - tartrate resistant acid phosphatase
V‐H+ATPase - vacuolar H+ATPase
Footnotes
This work was supported by Telethon grant #E.0831 and by FIRB (Fondo per gli Investimenti per la Ricerca di Base) grant #RBAUO1X3NH to AT. This work was also partially supported by grant “Ricerca Corrente” #2003/02/P001132 to the Division of Haematology, Ospedale Pedriatico Bambino Gesù, Rome, Italy
Competing interests: none declared
References
- 1.Pogoda P, Priemel M, Rueger J M, Amling M. Bone remodeling: new aspects of a key process that controls skeletal maintenance and repair. Osteoporos Int 200516(Suppl 2)S18–S24. [DOI] [PubMed] [Google Scholar]
- 2.Parfitt A M. The coupling of bone formation to bone resorption: a critical analysis of the concept and of its relevance to the pathogenesis of osteoporosis. Metab Bone Dis Relat Res 198241–6. [DOI] [PubMed] [Google Scholar]
- 3.Whyte M P. Osteopetrosis. In: Royce PM, Steinamann B, eds. Connective tissue and its heritable disorders: medical, genetic, and molecular aspects. 2nd ed. New York: Wiley‐Liss, 2002753–770.
- 4.Quinn J M, Gillespie M T. Modulation of osteoclast formation. Biochem Biophys Res Commun 200518739–745. [DOI] [PubMed] [Google Scholar]
- 5.Whyte M P. Carbonic anhydrase II deficiency. Clin Orthop Relat Res 199329452–63. [PubMed] [Google Scholar]
- 6.Nishi T, Forgac M. The vacuolar (H+)‐ATPase. Nature's most versatile proton pumps. Nat Rev 2002394–102. [DOI] [PubMed] [Google Scholar]
- 7.Kornak U, Kasper D, Bosl M R, Kaiser E, Schweizer M, Schulz A, Friedrich W, Delling G, Jentsch T J. Loss of the ClC‐7 chloride channel leads to osteopetrosis in mice and man. Cell 2001104205–215. [DOI] [PubMed] [Google Scholar]
- 8.Frattini A, Orchard P J, Sobacchi C, Giliani S, Abinun M, Mattsson J P, Keeling D J, Andersson A K, Wallbrandt P, Zecca L, Notarangelo L D, Vezzoni P, Villa A. Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet 200025343–346. [DOI] [PubMed] [Google Scholar]
- 9.Kornak U, Schulz A, Friedrich W, Uhlhaas S, Kremens B, Voit T, Hasan C, Bode U, Jentsch T J, Kubish C. Mutations in the a3 subunit of the vacuolar H+‐ATPase cause infantile malignant osteopetrosis. Hum Mol Genet 200092059–2063. [DOI] [PubMed] [Google Scholar]
- 10.Chalhoub N, Benachenhou N, Rajapurohitam V, Pata M, Ferron M, Frattini A, Villa A, Vacher J. Grey‐lethal mutation induces severe malignant autosomal recessive osteopetrosis in mouse and human. Nat Med 20039399–406. [DOI] [PubMed] [Google Scholar]
- 11.Quarello P, Forni M, Barberis L, Defilippi C, Campagnoli M F, Silvestro L, Frattini A, Chalhoub N, Vacher J, Remenghi U. Severe malignant osteopetrosis caused by a GL gene mutation. J Bone Miner Res 2004191194–1199. [DOI] [PubMed] [Google Scholar]
- 12.Ramirez A, Faupel J, Goebel I, Stiller A, Beyer S, Stockle C, Hasan C, Bode U, Kornak U, Kubisch C. Identification of a novel mutation in the coding region of the grey‐lethal gene OSTM1 in human malignant infantile osteopetrosis. Hum Mutat 200423471–476. [DOI] [PubMed] [Google Scholar]
- 13.Bollerslev J, Andersen P E., Jr Radiological, biochemical and hereditary evidence of two types of autosomal dominant osteopetrosis. Bone 198897–13. [DOI] [PubMed] [Google Scholar]
- 14.Van Wesenbeeck L, Cleiren E, Gram J, Beals R K, Benichou O, Scopelliti D, Key L, Renton T, Bartels C, Gong Y, Warman M L, De Vernejoul M C, Bollerslev J, Van Hul W. Six novel missense mutations in the LDL receptor‐related protein 5 (LRP5) gene in different conditions with an increased bone density. Am J Hum Genet 200372763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frattini A, Pangrazio A, Susani L, Sobacchi C, Mirolo M, Abinum M, Andolina M, Flanagan A, Horwitz E M, Mihci E, Notarangelo L D, Ramenghi U, Teti A, Van Hove J, Vujic D, Young T, Albertini A, Orchard P J, Vezzoni P, Villa A. Chloride channel ClCN7 mutations are responsible for severe recessive, dominant, and intermediate osteopetrosis. J Bone Miner Res 2003181740–1747. [DOI] [PubMed] [Google Scholar]
- 16.Ohlsson A, Cumming W A, Paul A, Sly W S. Carbonic anhydrase II deficiency syndrome: recessive osteopetrosis with renal tubular acidosis and cerebral calcification. Pediatrics 198677371–381. [PubMed] [Google Scholar]
- 17.Cotter M, Connell T, Colhoun E, Smith O P, McMahon C. Carbonic anhydrase II deficiency: a rare autosomal recessive disorder of osteopetrosis, renal tubular acidosis, and cerebral calcification. J Pediatr Hematol Oncol 200527115–117. [DOI] [PubMed] [Google Scholar]
- 18.Teti A, Migliaccio S, Taranta A, Bernardini S, DeRossi G, Lucani M, Iacobini M, De Felice L, Boldrini R, Bosman C, Corsi A, Bianco P. Mechanisms of osteoclast dysfunction in human osteopetrosis: abnormal osteoclastogenesis and lack of osteoclast‐specific adhesion structures. J Bone Miner Res 1999142107–2117. [DOI] [PubMed] [Google Scholar]
- 19.Parfitt A M, Drezner M K, Glorieux F H, Kanis J A, Malluche H, Meunier P J, Ott S M, Recker R R. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 19872595–610. [DOI] [PubMed] [Google Scholar]
- 20.Caselli G F, Mantovanini M, Gandolfi C A, Allegretti M, Fiorentino S, Pellegrini L, Melillo G, Bestini R, Sabbatici W, Anacardio R, Clavenna G, Sciortino G, Teti A. Tartronates: a new generation of drugs affecting bone metabolism. J Bone Miner Res 199712972–981. [DOI] [PubMed] [Google Scholar]
- 21.Migliaccio S, Luciani M, Taranta A, De Grossi G, Minisola S, El Hachem M, Bosman C, De Felice L, Boldrini R, Corsi A, Bianco P, Teti A. Association of intermediate osteopetrosis with poikiloderma. J Bone Miner Res 199914834–836. [DOI] [PubMed] [Google Scholar]
- 22.Letizia C, Taranta A, Migliaccio S, Caliumi C, Diacinti D, Delfini E, D'Erasmo E, Iacobini M, Roggini M, Albagha O M, Ralston S H, Teti A. Type II benign osteopetrosis (Albers‐Schönberg disease) caused by a novel mutation in ClCN7 presenting with unusual clinical manifestations. Calcif Tissue Int 20047442–46. [DOI] [PubMed] [Google Scholar]
- 23.Cleiren E, Benichou O, Van Hul E, Gram J, Bollerslev J, Singer F R, Beaverson K, Aledo A, Whyte M P, Yoneyama T, deVernejoul M C, Van Hul W. Albers‐Schonberg disease (autosomal dominant osteopetrosis, type II) results from mutations in the ClCN7 chloride channel gene. Hum Mol Genet 2001102861–2867. [DOI] [PubMed] [Google Scholar]
- 24.Waguespack S G, Koller D L, White K E, Fishburn T, Carn G, Buckwalter K A, Johnson M, Kocisko M, Evans W E, Foroud T, Econs M J. Chloride channel 7 (ClCN7) gene mutations and autosomal dominant osteopetrosis, type II. J Bone Miner Res 2003181513–1518. [DOI] [PubMed] [Google Scholar]
- 25.Henriksen K, Gram J, Schaller S, Dahl B H, Dziegiel M H, Bollerslev J, Karsdal M A. Characterization of osteoclasts from patients harboring a G215R mutation in ClC‐7 causing autosomal dominant osteopetrosis type II. Am J Pathol 20041641537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campos‐Xavier A B, Casanova J L, Doumaz Y, Feingold J, Munnich A, Cormier‐Daire V. Intrafamilial phenotypic variability of osteopetrosis due to chloride channel 7 (CLCN7) mutations. Am J Med Genet 2005133216–218. [DOI] [PubMed] [Google Scholar]
- 27.Karsdal M A, Henriksen K, Sorensen M G, Gram J, Schaller S, Dziegiel M H, Heegaard A M, Christophersen P, Martin T J, Christiansen C, Bollerslev J. Acidification of the osteoclastic resorption compartment provides insight into the coupling of bone formation to bone resorption. Am J Pathol 2005166467–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meghji S, Morrison M S, Henderson B, Arnett T R. pH dependence of bone resorption: mouse calvarial osteoclasts are activated by acidosis. Am J Physiol Endocrinol Metab 2001280E112–E119. [DOI] [PubMed] [Google Scholar]
- 29.Sundquist K, Lakkakorpi P, Wallmark B, Vaananen K. Inhibition of osteoclast proton transport by bafilomycin A1 abolishes bone resorption. Biochem Biophys Res Commun 1990168309–313. [DOI] [PubMed] [Google Scholar]
- 30.Sundquist K T, Marks S C., Jr Bafilomycin A1 inhibits bone resorption and tooth eruption in vivo. J Bone Miner Res 199491575–1582. [DOI] [PubMed] [Google Scholar]
- 31.Hall T J, Chambers T J. Na+/H+ antiporter is the primary proton transport system used by osteoclasts during bone resorption. J Cell Physiol 1990142420–424. [DOI] [PubMed] [Google Scholar]
- 32.Taranta A, Migliaccio S, Recchia I, Caniglia M, Luciani M, De Rossi G, Dionisi‐Vici C, Pinto R M, Francalanci P, Boldrini R, Lanino E, Dini G, Morreale G, Ralston S H, Villa A, Vezzoni P, Del Principe D, Cassini F, Palombo G, Teti A. Genotype‐phenotype relationship in human ATP6i‐dependent autosomal recessive osteopetrosis. Am J Pathol 200316257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tolar J, Teitelbaum S L, Orchard P J. Osteopetrosis. N Engl J Med 20043512839–2849. [DOI] [PubMed] [Google Scholar]
- 34.Lian J B, Marks S C., Jr Osteopetrosis in the rat: coexistence of reductions in osteocalcin and bone resorption. Endocrinology 1990126955–962. [DOI] [PubMed] [Google Scholar]
- 35.Martin T J, Sims N A. Osteoclast‐derived activity in the coupling of bone formation to resorption. Trends Mol Med 20051176–81. [DOI] [PubMed] [Google Scholar]
- 36.Lajeunesse D, Busque L, Menard P, Brunette M G, Bonny Y. Demonstration of an osteoblast defect in two cases of human malignant osteopetrosis. J Clin Invest 1996981835–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glorieux F H, Travers R, Taylor A, Bowen J R, Rauch F, Norman M, Parfitt A M. Normative data for iliac bone histomorphometry in growing children. Bone 200026103–109. [DOI] [PubMed] [Google Scholar]
- 38.Iacobini M, Migliaccio S, Roggini M, Taranta A, Werner B, Panero A, Teti A. Apparent cure of a newborn with malignant osteopetrosis using prednisone therapy. J Bone Miner Res 2001162356–2360. [DOI] [PubMed] [Google Scholar]