Abstract
Background
Nijmegen breakage syndrome (NBS) is an autosomal recessive chromosomal instability disorder with hypersensitivity to ionising radiation. The clinical phenotype is characterised by congenital microcephaly, mild dysmorphic facial appearance, growth retardation, immunodeficiency, and greatly increased risk for lymphoreticular malignancy. Most NBS patients are of Slavic origin and homozygous for the founder mutation 657del5. The frequency of 657del5 heterozygotes in the Czech population is 1:150. Recently, another NBS1 mutation, 643C>T(R215W), with uncertain pathogenicity was found to have higher frequency among tumour patients of Slavic origin than in controls. This alteration results in the substitution of the basic amino acid arginine with the non‐polar tryptophan and thus could potentially interfere with the function of the NBS1 protein, nibrin.
Methods and Results
Children with congenital microcephaly are routinely tested for the 657del5 mutation in the Czech and Slovak Republics. Here, we describe for the first time a severe form of NBS without chromosomal instability in monozygotic twin brothers with profound congenital microcephaly and developmental delay who are compound heterozygotes for the 657del5 and 643C>T(R215W) NBS1 mutations. Both children showed reduced expression of full length nibrin when compared with a control and a heterozygote for the 657del5 mutation. Radiation response processes such as phosphorylation of ATM and phosphorylation/stabilisation of p53, which are promoted by NBS1, are strongly reduced in cells from these patients.
Conclusions
Interestingly, the patients are more severely affected than classical NBS patients. Consequently, we postulate that homozygosity for the 643C>T(R215W) mutation will also lead to a, possibly very, severe disease phenotype.
Keywords: 657del5/643C>T(R215W) mutations, chromosomal instability syndromes, congenital microcephaly, nibrin‐Trp215 , Nijmegen breakage syndrome
Nijmegen breakage syndrome (NBS) is an autosomal recessive chromosomal instability disorder characterised by congenital microcephaly, growth retardation with pre‐ or postnatal onset, immunodeficiency, hyperradiosensitivity, and cancer predisposition.1,2 Most NBS patients are of Slavic origin and are homozygous for the founder NBS1 mutation 657del5.2,3,4 Congenital microcephaly is a hallmark of NBS2 and could be used, together with the identification of the responsible NBS1 mutations, as signal criteria for an early diagnosis of NBS in the Czech and Slovak Republics. Recently, we showed that the proportion of NBS patients among Czech infants with congenital microcephaly was 13%. In the Slovak Republic, this incidence was found to be as high as 50% among infants with both microcephaly and increased chromosomal instability.5,6 Among infants with primary microcephaly, screened for the 657del5 mutation, we diagnosed monozygotic twin brothers at the age of 2 months to be heterozygous for this mutation. The severe congenital microcephaly and hypotrophy were indications for further analysis of the NBS1 gene. Another NBS1 mutation, namely 643C>T(R215W), was detected on the second allele of the twin brothers. This mutation has been previously described in patients with acute lymphoblastic leukaemia,7 non‐Hodgkin's lymphoma,8 and at a high frequency among tumour patients of Slavic origin.9 Further clinical investigations of the children revealed partial lissencephaly and epileptic seizures, and respiratory and feeding problems were also noted. Based on the clinical and molecular findings, we propose that the compound heterozygosity, 657del5/643C>T(R215W), of the twin brothers is the primary cause of their clinical phenotype which seems to be more severe than the classical form of NBS.
Clinical report
The propositi, monozygotic twin boys (JR and PR), were born to a 27 year old primigravid mother and a 29 year old father. The couple is healthy, non‐consanguineous, non‐endogameous, and had no contributory family history.
The pregnancy was uncomplicated until the 31st+1 week of gestation when fetal ultrasound examination showed an abnormal head shape and growth retardation of both fetuses. Two weeks later oligohydramnion occurred in one of the twins after amniotic fluid disruption; the next day both children were spontaneously delivered head first.
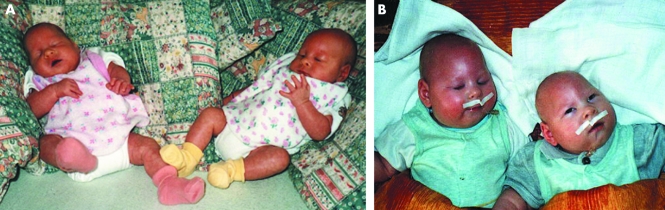
Twin A, a boy (PR; fig 1A), was born after 33+1 weeks gestation with an Apgar score of 7‐9‐9. His birth weight was 1520 g, length was 40 cm, and OFC was 26 cm. Immediately after birth, an abnormal skull shape with a small anterior fontanel (0.5×0.5 cm), without a small fontanel, and with synostosis of coronal and sagittal sutures, was noted. The sutura metopica was prominent, occiput was flat, and the skull developed a microturricephalic shape. Ultrasound investigation after birth showed enlarged, mild asymmetric lateral ventriculus, enlarged subarachnoidal areas (4 mm), and poor gyrification of the brain. No signs of intraventricular bleeding were found. Phototherapy was applied at 5 days because of icterus. Enteral feeding was administered for the first 5 days, but after that the child was breast fed and could be discharged home at the age of 33 days with 2095 g weight, 43 cm length, and OFC 29.8 cm (all parameters below the third percentile, OFC very low, corresponding to 3 σ). PR showed mild muscle hypertonia and brisk reflexes particularly involving the legs. A first attack of myoclonic seizures was documented at the age of 3 months and progression to status epilepticus at 5 months was rapid. During his stay in the neurology clinic, he received medication for the status epilepticus and improved, but some 15 seizures per day were common and feeding per sonda was necessary. Feeding and respiratory problems were progredient and therefore gastrostomy was necessary at the age of 7 months. Psychomotor development was severely retarded and correlated with development in infants in the first trimenon. OFC at the age of 1 year was 37.6 cm, weight was 8750 g, and length was 69 cm (third percentile except for OFC, which corresponded to −5 SD). The child was last seen at the age of 15 months and his somatic development due to feeding by gastrostomy and despite rare respiratory problems was satisfactory (9500 g weight, 74 cm length, OFC 39 cm). His microturricephaly with prominent sutura metopica was severely pronounced. The frequency of seizures was 20–30 per day.
Figure 1 Twin brothers, PR and JR. The twin boys are shown at the age of 5 months (A) and 7 months (B). Note microturricephaly, micrognathia, and large auricles. (Photographs are reproduced with permission.)
Twin B, a boy (JR), was born with a birth weight of 1600 g, length of 40 cm, and OFC of 26 cm. His Apgar score was 8‐9‐9 and the clinical findings of microcephaly were identical to those of his twin brother, PR. He was discharged home with a weight of 2380 g, length of 45 cm, and OFC of 30.5 cm. Testes were descended as they were in his brother, but he had also bilateral hydrocele and diastasis m. recti abdominis. Development was identical to that of his brother although his weight was always somewhat heavier than that of PR (fig 1B).
Family history
The father is a policemen and the mother is a nurse in a paediatric clinic. Both parents have increased contact with infections (homeless people, drug addicts, respiratory infections). Both were born the second child of three healthy sibs (brothers on the father's side and sister's on the mothers side). The paternal line comes from Moravia and the maternal from Bohemia, both in the Czech Republic.
Methods
Mutation analysis
DNA was isolated from peripheral blood and lymphoblastoid cell lines (LCLs) were established according to standard procedures. Informed consent was obtained from the parents of the children analysed in this study. Because of their congenital microcephaly and Slavic origin, both children were tested for the NBS1 mutation 657del5 at the age of 2 months. The mutation analysis was carried out as previously described5 and it was found that both children were heterozygous for the Slavic mutation. In an attempt to find a second mutation, further analysis of the NBS1 gene was carried out. The DNA samples were analysed by PCR amplification and sequencing of NBS1 exons and flanking intronic sequences on an ABI 3100 DNA Analyzer (Applied Biosystems, Foster City, CA).
Western blot analysis
Proteins were isolated from LCLs of both patients according to standard procedures and examined in comparison with control cells and cells heterozygous for the 657del5 or 643C>T(R215W) mutation. Blots were probed with a rabbit polyclonal antibody (Novus Biologicals, Littleton, CO) directed against the C‐terminal portion of nibrin. Antibodies against MRE11 (Novus Biologicals) and actin (Amersham, Freiburg, Germany) were used as controls.
Phosphorylation assays
For examination of nibrin phosphorylation, LCLs were irradiated with 12 Gy and proteins extracted after 1 h of further cultivation at 37°C. A portion of each extract was treated with λ‐phosphatase (New England Biolabs, Frankfurt am Main, Germany) before separation of all probes on polyacrylamide gels, blotting, and immunodetection using anti‐nibrin antibody. Phosphorylated nibrin runs more slowly during gel electrophoresis and is visible as an upwardly shifted band. Loss of the mobility shift after treatment with λ‐phosphatase indicated that this shift was indeed due to phosphorylation.
Phosphorylation of (ataxia telangiectasia mutated) ATM was measured at 30 min after 0, 0.5, 1, and 2 Gy irradiation. Proteins were separated on polyacrylamide gels and blots were probed with an anti‐ATM serine‐1981p antibody (Abcam, Cambridge, UK), stripped, and reprobed with an anti‐ATM antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Phosphorylation of p53 after ionising radiation (IR) was detected using the anti‐p53 serine‐15p antibody (Cell Signalling Technology, Beverly, MA) and, after stripping, an anti‐tubulin antibody as a loading control (Abcam, Cambridge, UK).
Zygosity testing
To determine whether the twin brothers were mono‐ or dizygotic, we performed microsatellite analyses using markers on chromosomes 1 and 14 (D1S80, D14S63, D14S68, D14S70, and D14S72). The analysis was done by means of PCR amplification with fluorescently labelled primers and fragment length analysis on an ABI 3100 DNA Analyzer.
Chromosome analysis
Chromosome analyses from peripheral blood lymphocytes and LCLs of the twins were performed using standard techniques. Irradiation of cells was carried out using the Muller MG 150 x ray apparatus (UA, 100 kV; I, 10 mA; filter, 0.3 mm Ni; dose rate, 2.1 Gy/min; Seifert, Hamburg, Germany) with doses of 0.5, 1.0, and 2.0 Gy. The radiosensitivity was determined by analysing the number of breaks per cell in 75 metaphases per Irradiation dose. We also performed spectral karyotyping (SKY)10 as an additional and highly sensitive method for identifying translocations in patient cells. The results were compared with a control LCL and LCLs derived from a patient with Fanconi anaemia.
FISH analysis of the LIS1 gene was performed using a commercially available probe (Vysis, Downers Grove, IL).
Results
The severe congenital microcephaly and developmental delay of the twin brothers reported here were indications for analysing DNA samples for the major NBS1 mutation, 657del5 in exon 6. Figure 2A shows the domain structure of NBS1 and its protein product, nibrin. The mutation 657del5 leads to premature termination of translation and reinitiation at a cryptic start codon. Partially functional proteins of 26 and 70 kDa are the products of the hypomorphic 657del5 NBS1 mutation. The sequence analysis showed that both children were heterozygous for this mutation (fig 2B). Further mutation screening of the NBS1 gene, illustrated in fig 2B, revealed that the twins carry on their second allele another NBS1 exon 6 mutation, namely 643C>T(R215W). This confirmed the suspected diagnosis of NBS in both children. Analysis of parents' DNA samples showed that the mother carries the 657del5 and the father the 643C>T(R215W) mutation. Further investigation of this family showed that the two grandfathers were carriers of the respective NBS1 mutations. Microsatellite analysis confirmed that the twins were monozygotic (data not shown).
Figure 2 NBS1 mutation analysis. (A) The domain structure of NBS1 and its protein product, nibrin. The intron/exon structure and the 11 NBS1 mutations are indicated on the cDNA with the 657del5 and 643C>T mutations underlined. The full length nibrin protein and the two protein fragments arising from the 657del5 mutation are shown. The serines phosphorylated by ATM in response to irradiation are indicated as is the site of interaction with MRE11. BRCT, breast cancer 1 carboxy terminal domain; FHA, fork head associated domain. (B) Sequence analysis of NBS1. A segment of genomic sequence from exon 6 in a control DNA and the patient, JR, is illustrated here. The region contains the 657del5 and the 643C>T (R215W) mutations.
Chromosome analysis indicated normal karyotypes in both patients. Because of their partial lissencephaly, a microdeletion analysis of the LIS1 gene in 17p13.3 was performed using FISH with the LSI LIS1 probe. FISH showed regular signals on both chromosomes 17 (data not shown). Thus, a Miller‐Dieker lissencephaly syndrome could be excluded with a probability of 90%.
The number of chromosome breaks was not increased in the lymphocytes of PR; in particular, the translocations or inversions involving chromosomes 7 and 14, which are characteristic of ataxia telangiectasia and NBS, were not observed. In comparison, generally 10–45% of NBS patient lymphocytes are found to be chromosomally aberrant, and of these aberrations, 66% involve chromosome 7 and/or chromosome 14.2 In addition to standard cytogenetics, the particularly sensitive SKY technique for the detection of translocations was used to examine the lymphoblastoid metaphases. In 60 metaphases, no evidence for an increased translocation rate for either twin was found in comparison to control cells (data not shown).
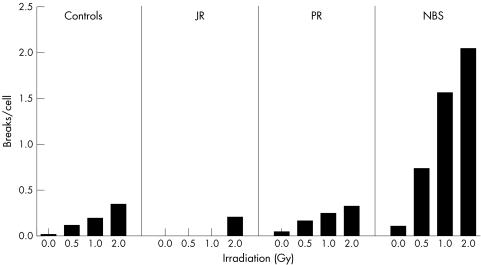
Radiosensitivity was assessed by counting chromosome breakage in LCLs of both twins in response to 0.5, 1.0, and 2.0 Gy radiation (fig 3). The increase in chromosome damage following radiation of the LCLs from the twins was comparable to that of a control cell line. NBS cells generally show high levels of damage after IR with 64–71% of cells noted as aberrant after 2 Gy.11 The NBS LCL examined here in parallel shows a comparably high level of chromosome damage after IR (fig 3). In conclusion, chromosomal instability is not a feature of the patients presented here.
Figure 3 Analysis of chromosomal instability after irradiation. Lymphoblastoid cells of the two probands, JR and PR, were irradiated with the indicated dose and chromosomal damage was scored in preparations of mitotic cells. Data are shown as breaks per cell and in comparison to the mean values of 18 normal control subjects (controls) and those of four NBS patients homozygous for the 657del5 mutation. A total of 75 metaphases were counted for each preparation.
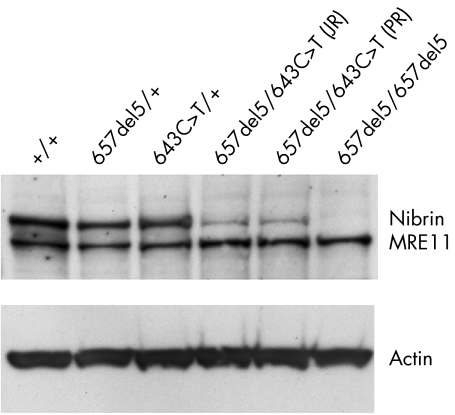
We performed Western blot analysis to compare the amount of nibrin in lymphoblastoid cell extracts from both patients with control cell lines and heterozygotes for the 657del5 or 643C>T(R215W) mutation. This analysis showed a striking difference in the amount of nibrin in both patients when compared with a control, a heterozygote for the 657del5 mutation, or a heterozygote for the 643C>T(R215W) mutation (fig 4). Nibrin‐Trp215 is clearly much less abundant than the wildtype nibrin‐Arg215.
Figure 4 Immunoblot analysis of nibrin expression. The Western blot shows the expression levels of nibrin and MRE11 in lysates of LCLs with the given NBS1 genotypes. The same blot was reprobed with an antibody directed towards actin as a loading control. The protein bands are labelled on the right of the blot.
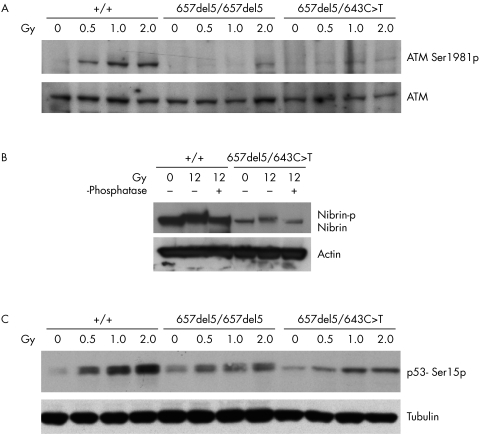
We next examined three essential functional aspects of nibrin: firstly, its role in the activation of ATM, measured as ATM phosphorylation after IR; secondly, nibrin's own IR induced phosphorylation by ATM; and, finally, the phosphorylation of p53 by ATM after IR, which is promoted by nibrin. For examination of ATM phosphorylation, we used a specific antibody for ATM phosphorylated on serine‐1981. As shown in fig 5A, phosphorylation of ATM is seen in wildtype cells 30 min after irradiation with just 0.5 Gy and with a dose dependent increase at 1 and 2 Gy. In contrast, in 657del5 homozygous cells, ATM phosphorylation is first detected at 2 Gy, indicating a fourfold reduction in ATM activation in these cells. In cells compound heterozygous for 657del5 and 643C>T(R215W), ATM phosphorylation is barely detectable at 2 Gy. The nibrin‐Trp215 protein is apparently even less able to sustain IR induced ATM activation than the 70 kDa carboxy terminal protein fragment.
Figure 5 Cellular response to ionising irradiation in 657del5/643C>T compound heterozygous cells. (A) ATM activation after ionising irradiation. An immunoblot of cell lysates with the indicated genotypes 30 min after 0, 0.5, 1.0, and 2 Gy ionising IR is shown. The blot was probed first with an antibody directed against ATM‐serine‐1981p and then striped and reprobed with an antibody directed against ATM. The protein bands are labelled on the right of the blot. (B) Nibrin phosphorylation after ionising irradiation was examined using a gel shift assay. An immunoblot of cell lysates from unirradiated cells and from cells 1 h after 12 Gy, probed for nibrin is shown. Genotypes of the cells are given above the lanes. A portion of the irradiated lysates was treated with λ‐phosphatase as indicated to demonstrate that the shift in nibrin mobility after irradiation is due to phosphorylation. The same blot was reprobed with an antibody directed towards actin as a loading control. The protein bands are labelled on the right of the blot. (C) p53 phosphorylation after ionising irradiation. An immunoblot of cell lysates with the indicated genotypes 30 min after 0, 0.5, 1.0, and 2 Gy ionising IR is shown. The blot was probed with an antibody directed against p53‐serine‐15p and then reprobed with an antibody directed towards tubulin as a loading control. The protein bands are labelled on the right of the blot.
For evaluation of nibrin phosphorylation, a specific gel mobility assay was employed.12 As shown in fig 5B, both wildtype nibrin‐Arg215 and nibrin‐Trp215 show slower electrophoretic mobility after irradiation. This mobility shift is lost after phosphatase treatment of proteins from irradiated cells indicating that it is due to phosphorylation. The extremely reduced level of nibrin‐Trp215 in patient's cells is again clearly shown in fig 5B. Anti‐actin was used as a loading control.
Finally, we examined the phosphorylation and stabilisation of p53 in response to IR using immunoblots and an antibody directed against p53 phosphorylated on serine‐15. As can be seen in fig 5C, the phosphorylation of p53 is attenuated in cells homozygous for 657del5 and also in the cells compound heterozygous for 657del5 and 643C>T(R215W). Anti‐tubulin antibody was used as a loading control.
Discussion
The frequency of the common NBS1 mutation 657del5 is relatively high (0.5–1%) in the Slavic population3 and in homozygotes leads to the classical manifestation of NBS. The twin brothers described here are compound heterozygotes for two NBS1 mutations – the major truncating NBS1 mutation, 657del5, and the missense mutation, 643C>T(R215W). Originally, the 657del5 mutation was regarded as a null mutation, however, Maser et al showed that the NBS1 657del5 mutation is actually a hypomorphic mutation and a truncated protein is produced, at least in lymphoblastoid patient cells.13 Similar alternative translation products have been shown for two further truncating NBS1 mutations.13,14 The relatively mild clinical course of patients with NBS contrasts strongly with the lethality of null mutation of the murine NBS1 homologue.15,16 We have recently been able to confirm the hypomorphic nature of the major human NBS1 mutation by complementation of an inducible murine null mutation.17
Unlike the 657del5 mutation, the pathogenicity of the 643C>T(R215W) mutation has been uncertain for some time. Arginine is conserved at position 215 of the mouse protein and the substitution of a basic amino acid, arginine, with a non‐polar amino acid, tryptophan, can obviously be postulated to have an effect on function. The 643C>T(R215W) mutation has been shown to be more common among tumour patients of Slavic origin than in the general Slavic population,9 a further finding pointing to a possible involvement of this mutation in cellular metabolism and cancerogenesis.
We find nibrin‐Trp215, the product of the 643C>T(R215W) allele, to be much less abundant than wildtype nibrin‐Arg215 as shown by Western blot analysis of lymphoblastoid cell extracts (compare lanes 3, 4, and 5 with lane 1 in fig 4, and control and patient lanes in fig 5C). This difference may indicate a lower expression level of the 643C>T(R215W) allele, a shorter half life of the mRNA or, more likely, reduced stability of the nibrin‐Trp215 protein. This might in turn reflect an inability of the mutant protein to associate correctly with its cellular partners, Mre11 and Rad50, and subsequent degradation of non‐bound nibrin monomer. In cells with only a truncated nibrin as an alternative, the 643C>T(R215W) mutation might therefore lead to a reduction in active trimeric complex below a critical level.
It has been hypothesised that missense ATM mutations might act by dominantly interfering with the function of the second allele, thus resulting in additional phenotypic effects beyond what might be expected from an absence or reduction of the protein, as normally occurs with truncating mutations.18 A similar argument could also be made here, with a missense NBS1 mutation interfering with the residual activity of the truncated protein from the 657del5 allele. This hypothesis, however, awaits experimental verification.
It is becoming clear that NBS1 plays a role not only in the active repair of DNA double strand breaks but also within the cellular signal cascades responding to changes in the status of the genome. Thus, NBS1 is required not only for the activation of ATM after DNA damage19,20 but also for the efficient phosphorylation by ATM of downstream targets, most of which are concerned with the assembly of cell cycle checkpoints rather than DNA repair per se.21,22 Nibrin‐Trp215 is strongly affected in its capacity to activate ATM as measured by extremely reduced phosphorylation of ATM in patient cells after IR. Indeed, phosphorylation is even poorer than in 657del5 homozygous cells. Interestingly, a similar finding was reported for AT‐like disorder (AT‐LD) in which different MRE11 mutations led to more or less severe abrogation of ATM phosphorylation.19 In the case of MRE11, ATM activation correlated with disease severity with the poorly activating mutations associated with a more severe phenotype.19
In contrast to its inability to mediate ATM activation, nibrin‐Trp215 is itself efficiently phosphorylated by ATM and, indeed, chromosomal breakage after IR is not increased in cells from the patients described here. Phosphorylation of a critical downstream target of ATM, p53, is promoted by nibrin.23 As in cells from patients with the 657del5 mutation, the compound heterozygous cells also phosphorylate p53 poorly (fig 5C) and this is accompanied by a failure to stabilise p53 after irradiation.
We recently described the effects of targeted Nbs1 null mutation in mice neuronal precursors. In these animals, the cerebellum was extremely diminished in size and foliation was also greatly reduced.24 We were able to demonstrate that this cerebellar phenotype was due to a p53 dependent reduction in proliferation of neuronal stem cells and increased p53 dependent apoptosis in post‐mitotic neurons.24 Since these mice had a tissue specific null mutation, the neuronal precursors were devoid of nibrin. The partial lissencephaly in the patients described here might perhaps arise from a reduced cellular level of full length nibrin, due to the destabilising 643C>T(R215W) mutation. In patients homozygous for the 657del5 mutation, the truncated 70 kDa protein may maintain aspects of nibrin function sufficient to prevent this neurological anomaly. Alternatively, a dominant‐negative function of the 643C>T(R215W) mutation might be responsible for the phenotypic differences between the patients described here and 657del5 homozygotes.
The clinical picture of our patients is similar to the classical NBS phenotype due to homozygosity for the Slavic mutation with respect to the severe congenital microcephaly and growth retardation with prenatal onset. However, there are substantial differences in several other aspects. Firstly, cranial investigations on over 16 NBS patients from 1.75 to 19 years of age has indicated decreased size of the frontal lobes and narrowing of the frontal horns of the lateral ventricles.25,26 The only NBS patient described without microcephaly even has these neurological features.26 This clinical picture differs from that of enlarged lateral ventricles, enlarged subarachnoidal areas, and poor gyrification found in the patients described here.
Secondly, mental development is severely retarded in these patients but is nearly normal in classical NBS. Thirdly, with respect to sexual development, ovarian dysgenesis occurs in females and frequent cryptorchism in males with the classical type of NBS. Finally, immunodeficiency is generally more pronounced in classical NBS than in our patients, although clearly this may change with age. Severe developmental delay, unusual for NBS, has been reported in patients diagnosed initially as having Fanconi anaemia but later found to have a homozygous truncating mutation (1089C>A/Y363X) in NBS1.27,28
Compound heterozygosity in patients with NBS is not a rare event, especially in non‐Slavic populations.4,29 The only difference compared to our patients is, however, that most compound heterozygotes carry two truncating mutations. Despite the monozygosity of the twins described here, their symptoms with respect to developmental delay, respiratory, and feeding problems are not absolutely identical. Twin B (JR) is progressing better than his brother and his mental development is also more satisfactory.
Patients with classical NBS have an elevated cancer risk as a result of chromosomal instability and radiosensitivity. Considering their age, evaluation of increased risk for malignancy in the twin boys described here is obviously premature. However, we propose that the two NBS1 mutations found in our patients are responsible for their clinical phenotype which deviates from classical NBS and seems to be even more severe. Apparently, the combination of a truncated and a missense NBS1 mutation leads to a partially different form of the disease. The presence of congenital microcephaly and the absence of chromosomal instability and radiosensitivity found in our patients suggests that the development of microcephaly might even be independent of IR sensitivity and is perhaps more related to other factors, for example, low cell proliferation.
In conclusion, with this study we provide the first data on a novel variant of NBS, characterised by severe congenital microcephaly and neurological features unusual for the classical form and due to compound heterozygosity for the truncating 657del5 and the missense 643C>T(R215W) NBS1 mutations. The clinical phenotype as a result of homozygosity for the 643C>T(R215W) mutation is so far unknown, despite the moderate frequency (1:234) of 643C>T(R215W) heterozygotes among Czech newborns.30 Therefore, we postulate that homozygosity for the 643C>T(R215W) mutation may lead to a disease phenotype which might be lethal early in development.
Acknowledgements
We are indebted to family R for their participation in this study. We thank Mrs Susanne Rothe and Mrs Christina Steglich for excellent technical assistance.
Abbreviations
IR - irradiation
LCL - lymphoblastoid cell line
NBS - Nijmegen breakage syndrome
SKY - spectral karyotyping
Footnotes
This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB577) to MD and KS and from the Sander Stiftung to KS. This work was also supported by Grants IGA: NR 7916‐/2004 and NE 6912‐4/2003
Competing interests: none declared
References
- 1.Weemaes C M, Hustinx T W, Scheres J M, van Munster P J, Bakkeren J A, Taalman R D. A new chromosomal instability disorder: the Nijmegen breakage syndrome. Acta Paediatr Scand 198170557–564. [DOI] [PubMed] [Google Scholar]
- 2.The International Nijmegen Breakage Syndrome Study Group Nijmegen breakage syndrome. Arch Dis Child 200082400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varon R, Seemanova E, Chrzanowska K, Hnateyko O, Piekutowska‐Abramczuk D, Krajewska‐Walasek M, Sykut‐Cegielska J, Sperling K, Reis A. Clinical ascertainment of Nijmegen breakage syndrome (NBS) and prevalence of the major mutation, 657del5, in three Slav populations. Eur J Hum Genet 20008900–902. [DOI] [PubMed] [Google Scholar]
- 4.Varon R, Vissinga C, Platzer M, Cerosaletti K M, Chrzanowska K H, Saar K, Beckmann G, Seemanova E, Cooper P R, Nowak N J, Stumm M, Weemaes C M, Gatti R A, Wilson R K, Digweed M, Rosenthal A, Sperling K, Concannon P, Reis A. Nibrin, a novel DNA double‐strand break repair protein, is mutated in Nijmegen breakage syndrome. Cell 199893467–476. [DOI] [PubMed] [Google Scholar]
- 5.Seeman P, Gebertova K, Paderova K, Sperling K, Seemanova E. Nijmegen breakage syndrome in 13% of age‐matched Czech children with primary microcephaly. Pediatr Neurol 200430195–200. [DOI] [PubMed] [Google Scholar]
- 6.Seemanova E, Pohanka V, Seeman P, Misovicova N, Behunova J, Kvasnicova M, Dlholucky S, Valachova A, Cisarik F, Veghova E, Varon R, Sperling K. [Nijmegen breakage syndrome in Slovakia]. Cas Lek Cesk 2004143538–41 discussion, 542. [PubMed] [Google Scholar]
- 7.Varon R, Reis A, Henze G, von Einsiedel H G, Sperling K, Seeger K. Mutations in the Nijmegen Breakage Syndrome gene (NBS1) in childhood acute lymphoblastic leukemia (ALL). Cancer Res 2001613570–3572. [PubMed] [Google Scholar]
- 8.Taylor G M, O'Brien H P, Greaves M F, Ravetto P F, Eden O B. Correspondence re: R. Varon et al., Mutations in the Nijmegen breakage syndrome gene (NBS1) in childhood acute lymphoblastic leukemia. Cancer Res. 61: 3570–3572, 2001, Cancer Res. 2003;63: 6563–4; author reply 6565 [PubMed]
- 9.Steffen J, Varon R, Mosor M, Maneva G, Maurer M, Stumm M, Nowakowska D, Rubach M, Kosakowska E, Ruka W, Nowecki Z, Rutkowski P, Demkow T, Sadowska M, Bidzinski M, Gawrychowski K, Sperling K. Increased cancer risk of heterozygotes with NBS1 germline mutations in Poland. Int J Cancer 200411167–71. [DOI] [PubMed] [Google Scholar]
- 10.Schrock E, Veldman T, Padilla‐Nash H, Ning Y, Spurbeck J, Jalal S, Shaffer L G, Papenhausen P, Kozma C, Phelan M C, Kjeldsen E, Schonberg S A, O'Brien P, Biesecker L, du Manoir S, Ried T. Spectral karyotyping refines cytogenetic diagnostics of constitutional chromosomal abnormalities. Hum Genet 1997101255–262. [DOI] [PubMed] [Google Scholar]
- 11.Antoccia A, Stumm M, Saar K, Ricordy R, Maraschio P, Tanzarella C. Impaired p53‐mediated DNA damage response, cell‐cycle disturbance and chromosome aberrations in Nijmegen breakage syndrome lymphoblastoid cell lines. Int J Radiat Biol 199975583–591. [DOI] [PubMed] [Google Scholar]
- 12.Gatei M, Young D, Cerosaletti K M, Desai‐Mehta A, Spring K, Kozlov S, Lavin M F, Gatti R A, Concannon P, Khanna K. ATM‐dependent phosphorylation of nibrin in response to radiation exposure. Nat Genet 200025115–119. [DOI] [PubMed] [Google Scholar]
- 13.Maser R S, Zinkel R, Petrini J H. An alternative mode of translation permits production of a variant NBS1 protein from the common Nijmegen breakage syndrome allele. Nat Genet 200127417–421. [DOI] [PubMed] [Google Scholar]
- 14.Tanzanella C, Antoccia A, Spadoni E, di Masi A, Pecile V, Demori E, Varon R, Marseglia G L, Tiepolo L, Maraschio P. Chromosome instability and nibrin protein variants in NBS heterozygotes. Eur J Hum Genet 200311297–303. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Petersen S, Tessarollo L, Nussenzweig A. Targeted disruption of the Nijmegen breakage syndrome gene NBS1 leads to early embryonic lethality in mice. Curr Biol 200111105–109. [DOI] [PubMed] [Google Scholar]
- 16.Dumon‐Jones V, Frappart P O, Tong W M, Sajithlal G, Hulla W, Schmid G, Herceg Z, Digweed M, Wang Z Q. Nbn heterozygosity renders mice susceptible to tumor formation and ionizing radiation‐induced tumorigenesis. Cancer Res 2003637263–7269. [PubMed] [Google Scholar]
- 17.Demuth I, Frappart P O, Hildebrand G, Melchers A, Lobitz S, Stockl L, Varon R, Herceg Z, Sperling K, Wang Z Q, Digweed M. An inducible null mutant murine model of Nijmegen breakage syndrome proves the essential function of NBS1 in chromosomal stability and cell viability. Hum Mol Genet 2004132385–2397. [DOI] [PubMed] [Google Scholar]
- 18.Gatti R A, Tward A, Concannon P. Cancer risk in ATM heterozygotes: a model of phenotypic and mechanistic differences between missense and truncating mutations. Mol Genet Metab 199968419–423. [DOI] [PubMed] [Google Scholar]
- 19.Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, Shiloh Y. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J 2003225612–5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J H, Paull T T. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science 200430493–96. [DOI] [PubMed] [Google Scholar]
- 21.Kitagawa R, Bakkenist C J, McKinnon P J, Kastan M B. Phosphorylation of SMC1 is a critical downstream event in the ATM‐NBS1‐BRCA1 pathway. Genes Dev 2004181423–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buscemi G, Savio C, Zannini L, Micciche F, Masnada D, Nakanishi M, Tauchi H, Komatsu K, Mizutani S, Khanna K, Chen P, Concannon P, Chessa L, Delia D. Chk2 activation dependence on Nbs1 after DNA damage. Mol Cell Biol 2001215214–5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girard P M, Riballo E, Begg A C, Waugh A, Jeggo P A. Nbs1 promotes ATM dependent phosphorylation events including those required for G1/S arrest. Oncogene 2002214191–4199. [DOI] [PubMed] [Google Scholar]
- 24.Frappart P O, Tong W M, Demuth I, Radovanovic I, Herceg Z, Aguzzi A, Digweed M, Wang Z Q. An essential function for NBS1 in the prevention of ataxia and cerebellar defects. Nat Med 200511538–544. [DOI] [PubMed] [Google Scholar]
- 25.Bekiesinska‐Figatowska M, Chrzanowska K H, Sikorska J, Walecki J, Krajewska‐Walasek M, Jozwiak S, Kleijer W J. Cranial MRI in the Nijmegen breakage syndrome. Neuroradiology 20004243–47. [DOI] [PubMed] [Google Scholar]
- 26.Chrzanowska K H, Stumm M, Bekiesiska‐Figatowska M, Varon R, Biaecka M, Gregorek H, Michakiewicz J, Krajewska‐Walasek M, Jowiak S, Reis A. Atypical clinical picture of the Nijmegen breakage syndrome associated with developmental abnormalities of the brain. J Med Genet 200138E3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakanishi K, Taniguchi T, Ranganathan V, New H V, Moreau L A, Stotsky M, Mathew C G, Kastan M B, Weaver D T, D'Andrea A D. Interaction of FANCD2 and NBS1 in the DNA damage response. Nat Cell Biol 20024913–920. [DOI] [PubMed] [Google Scholar]
- 28.Gennery A R, Slatter M A, Bhattacharya A, Barge D, Haigh S, O'Driscoll M, Coleman R, Abinun M, Flood T J, Cant A J, Jeggo P A. The clinical and biological overlap between Nijmegen Breakage Syndrome and Fanconi anemia. Clin Immunol 2004113214–219. [DOI] [PubMed] [Google Scholar]
- 29.Resnick I B, Kondratenko I, Togoev O, Vasserman N, Shagina I, Evgrafov O, Tverskaya S, Cerosaletti K M, Gatti R A, Concannon P. Nijmegen breakage syndrome: clinical characteristics and mutation analysis in eight unrelated Russian families. J Pediatr 2002140355–361. [DOI] [PubMed] [Google Scholar]
- 30.Seemanová E, Koutecky J, Radvanská J, Starý J, Seeman P, Gebertová K, Varon R, Sperling K. Nositelé mutací NBS1 genu mezi pacienty detské onkologie. Csl Pediat 200459242–245. [Google Scholar]