Abstract
Mental retardation is more common in males than females in the population, assumed to be due to mutations on the X chromosome. The prevalence of the 24 genes identified to date is low and less common than expansions in FMR1, which cause Fragile X syndrome. Systematic screening of all other X linked genes in X linked families with mental retardation is currently not feasible in a clinical setting. The phenotypes of genes causing syndromic and non‐syndromic mental retardation (NLGN3, NLGN4, RPS6KA3(RSK2), OPHN1, ATRX, SLC6A8, ARX, SYN1, AGTR2, MECP2, PQBP1, SMCX, and SLC16A2) are first discussed, as these may be the focus of more targeted mutation analysis. Secondly, the relative prevalence of genes causing only non‐syndromic mental retardation (IL1RAPL1, TM4SF2, ZNF41, FTSJ1, DLG3, FACL4, PAK3, ARHGEF6, FMR2, and GDI) is summarised. Thirdly, the problem of recurrence risk where a molecular genetics diagnosis has not been made and what proportion of the male excess of mental retardation is due to monogenic disorders of the X chromosome are discussed.
Keywords: mental retardation, recurrence risks, X chromosome, X linked, X linked mental retardation, XLMR
In 1938 Lionel Penrose first observed that more males than females in the population are mentally retarded in a survey and classification of those in institutional care and their relatives.1 The ratio of males to females was 1.25:1. This figure has been substantiated by numerous subsequent studies in the USA, Canada, Australia, and Europe and all agree with the observation of an approximately 30% excess of males being affected with mental retardation.2,3,4,5,6
The definition of mental retardation requires there to be significant sub‐average general intellectual functioning (criterion A) that is accompanied by limitations in adaptive functioning in at least two of the following skill areas: communication, self care, home living, social/interpersonal skills, use of community resources, self‐direction, functional academic skills, work, leisure, health, and safety (criterion B). The onset must also occur before 18 years of age (criterion C). General intellectual functioning is defined by the intelligence quotient (IQ). Adaptive functioning refers to how effectively individuals cope with common life demands. Although these observations are less objective measures and rely on information gathered from independent sources, for example, teacher evaluation and educational, developmental, and medical history, they are extremely useful in assessing children. In the UK, the ICD‐10 Classification of Mental and Behavioural Disorders7 is used, while in the USA the DSM‐IV diagnostic classification, which is similar to the WHO classification, is used.8
IQ across the population is normally distributed with the mean set at 100 and an IQ of <70 classified as mental retardation. Mild mental retardation is defined as an IQ of 50–70, moderate as an IQ of 35–49, severe as an IQ of 20–34, and profound as an IQ of <20. Approximately 2–3% of the population have mild to moderate intellectual disability and 0.5–1% of the population have moderate to severe mental retardation.
The publication in 1943 of the study by Martin and Bell9 subsequently led to the identification of the first single gene defect where the phenotype was predominantly mental retardation, Fragile X syndrome.10
In 1991 Kerr et al identified families where no clinical features other than mental retardation were observed and where Fragile X syndrome was not the cause of disease. This phenomenon was termed non‐specific X linked mental retardation (XLMR).11 This then led to the classification of families with XLMR where a family was given an individual MRX number if linkage was performed and a LOD score >2.0 was obtained. The term MRX25 or MRX11 therefore has a precise meaning. To date MRX1 to MRX81 are recorded as individual families with XLMR, some of whom now have precise mutations identified in the literature (http://xlmr.interfree.it/5_non.htm).
Diagnosis of XLMR
The clinical diagnosis of XLMR is usually a diagnosis of exclusion of other causes of developmental delay in a male (table 1).12 Only rarely does a new family present with sufficient affected males for a confident clinical diagnosis of XLMR to be made. All patients where XLMR is suspected should have the benefit of contemporary karyotype analysis at >550 banded resolution, as unbalanced autosomal translocations from balanced carriers can be misclassified as X linked if no male‐to‐male transmission is observed. Similarly, with the advent of subtelomeric analysis approximately 3–4% of familial mental retardation will be found to be due to submicroscopic telomeric deletions.13,14,15,16 Mutation analysis for Fragile X syndrome is also essential.
Table 1 Investigation of a male child with possible XLMR based on Shevell et al12.
| Obtain three generation pedigree and details of development of all |
| possibly affected individuals |
| Obtain a detailed clinical history of maternal health pre‐pregnancy |
| Pregnancy history |
| Birth history and birth height, weight and head circumference |
| Developmental milestones and growth rates |
| Neonatal PKU and hypothyroidism |
| Educational history and IQ |
| Examination for dysmorphic features and neurological signs |
| Karyotype analysis (550 banded resolution) |
| Fragile X |
| Telomere screen |
| Brain MRI if abnormal neurological findings or head circumference |
| indicates microcephaly or macrocephaly |
| EEG to assist definition of epilepsy phenotype |
| Metabolic screen if clinically indicated. Consider urine and plasma |
| screen of creatine/creatinine ratio where indicated and possible. |
| Consider free T3 thyroid function tests if spastic paraplegin is present. |
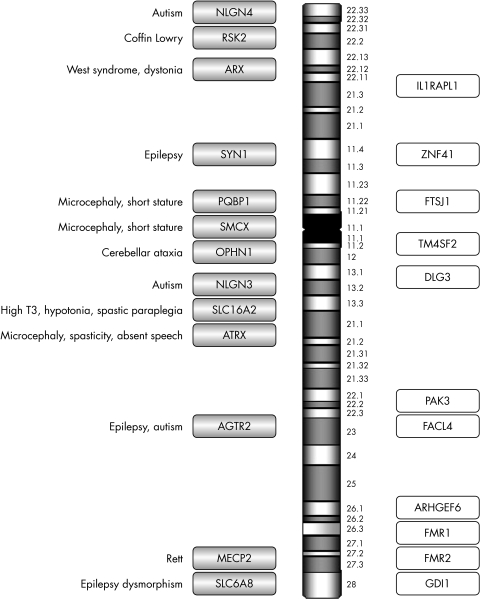
Having excluded a karyotype abnormality and Fragile X syndrome by seeking an expansion in FMR1, 23 X linked genes remain where mutations have been described that result in either syndromic or non‐syndromic mental retardation (fig 1). The decision as to which gene(s) to analyse depends on the identification of additional clinical features that could categorise the condition as syndromic (table 2) and the relative prevalence of the gene abnormality in the study population.
Figure 1 Summary of genes on the X chromosome reported to cause XLMR. Shaded bars are syndromic XLMR genes; open bars are non‐syndromic XLMR genes.
Table 2 Additional clinical features found in syndromic XLMR.
| Characteristic | Gene |
| Microcephaly | ATRX, MECP2, PQBP1, SMCX |
| Cleft lip and palate | PQBP1 |
| Congenital heart disease | PQBP1 |
| Spastic paraplegia | SLC16A2, ATRX, SMCX, MECP2 |
| Seizures | AGTR2, SYN1, ATRX, SLC6A8, ARX, SMCX |
| Absent speech | ATRX, SLC16A2, SLC6A8 |
| Cerebellar hypoplasia | OPHN1 |
| Short stature | PQBP1, SMCX |
| Autistic behaviour | NLGN3, NLGN4, AGTR2, SLC6A8 |
| Dystonia | ARX |
| Hypertelorism | RSK2 |
| Scoliosis | RSK2, ATRX |
| Abnormal thyroid function | SLC16A2 |
In this review a number of genes, the clinical features associated with the gene abnormality, and the prevalence of the disease gene will be discussed. Genes that are associated only with a syndrome are not discussed, for example L1CAM, PLP1, and DCX, as the review is limited to those genes where both non‐syndromic and syndromic phenotypes have been described, due to limitations of space. The original separation of genes causing syndromic disease from those causing non‐syndromic mental retardation was useful in the early days of identifying new genes that cause mental retardation. Increasingly, this divide is becoming blurred and somewhat arbitrary as the range of phenotypes associated with any one gene, for example ARX (see below), is becoming increasingly varied. Nevertheless, this distinction still has some limited merit when categorising genes associated with mental retardation.
Syndromic XLMR
Autism
A mutation was identified in NLGN3 (neuroligin 3; OMIM 300366) and NLGN4 (neuroligin 4, X linked; OMIM 300427) in two brother pairs with severe mental retardation and autism.17 Since then a further family has been described, but mutations have not been identified in any large cohort of autistic children to date, suggesting that abnormalities in this gene are a rare cause of autism. In addition, all cases have been associated with severe mental retardation.18,19,20 Routine testing is of unproven utility to date.
Coffin Lowry
Mutations in RPS6KA3 (ribosomal protein S6 kinase, 90 kDa polypeptide 3; OMIM 300075), previously known as RSK2, are associated with Coffin Lowry syndrome.21 Short stature, distinctive facies with a prominent forehead and coarse facies, hypertelorism, prominent lips, large soft hands with thickened tapering fingers, hypotonia, hyperextensibility, and skeletal changes are characteristic.22 A single family with non‐syndromic mental retardation has been reported with mutations in this gene, but recently mutations have been identified in three further families who did not meet the diagnostic criteria for Coffin Lowry syndrome (Raymond et al, unpublished data).23 This suggests that mutations in RPS6KA3 will prove to be a more common cause of XLMR and should be considered where the phenotype has some similarities. Testing therefore may yield further families.
Cerebellar ataxia
Families with mutations in OPHN1 (oligophrenin 1; OMIM 300127) were initially described as having a non‐syndromic mental retardation phenotype, but on re‐evaluation the affected males were found to have significant reduction in the size of the cerebellum. None of the families presented with significant ataxia or cerebellar signs clinically and the subtle cerebellar phenotype was only revealed on closer investigation. Obligate females also have reduced cerebellar size and this condition is now regarded as a syndrome.24,25 The prevalence of mutations in this gene is low, however systematic screening of this gene has not been performed in any large cohorts of patients with cerebellar hypoplasia or non‐syndromic mental retardation. To date mutations have been described in two families, a patient with a translocation and a singleton with a similar phenotype to that of the familial cases.24,25 Testing in non‐syndromic mental retardation alone is of unproven utility, but screening this gene in families with X linked cerebellar hypoplasia may be considered.
ATRX
X linked alpha thalassaemia was initially thought to be clinically homogenous, but mutation analysis of ATRX (alpha thalassaemia, mental retardation syndrome X linked; OMIM 300032) has found that the following conditions are all allelic: Juberg‐Marsidi, Chudley‐Lowry, Smith‐Fineman‐Myers, Carpenter‐Waziri, Holmes‐Gang, and Martinez. The phenotype is usually associated with severe mental retardation, commonly with absent speech, microcephaly, hypotonia, spasticity or seizures, and growth retardation with midface hypoplasia and skeletal abnormalities. A single large family has been reported with non‐syndromic mental retardation alone where the proband did not have the characteristic facial features and profound intellectual disability associated with ATRX syndrome. Other affected members of the family did have the characteristic phenotype, suggesting that abnormalities of this gene show some intra‐familial variation.26 Testing for this gene abnormality initially by screening for the presence of HbH bodies is certainly valuable where there is a syndromic phenotype, but routine screening of this gene in non‐syndromic mental retardation is not useful.
Epilepsy
Mental retardation in combination with epilepsy is relatively common, which means that the list of differential diagnoses remains long in cases that present with these two features. However, mutations in SLC6A8 (solute carrier family 6 (neurotransmitter transporter, creatine), member 8; OMIM 300036) are usually associated with epilepsy, severe mental retardation, and autistic spectrum behavioural problems with particular deficits in expressive speech and language often resulting in absent speech.27 Recently, a systematic screen of 288 families with mental retardation and either with proven X linked inheritance or having two or more affected male family members revealed mutations in 6/288 (2.1%) families, suggesting that mutations in this gene are a relatively common cause of mental retardation, although still 10 times less frequent than Fragile X syndrome in familial cases.28,29 The clinical features of these six families were not described in detail, so the presence or absence of epilepsy as a diagnostic criterion is not entirely clear. Patients with this condition have altered creatine/creatinine ratios and reduced creatine uptake. In future, the detection of this condition using biochemical assays of plasma and urine will be an invaluable screen and mutation analysis will then be used as confirmation of disease in affected individuals.30,31,32
The identification of ARX (aristaless related homeobox; OMIM 300382) as a cause of West syndrome, mental retardation and either hypsarrythmia, myoclonic epilepsy, dystonia (Partington syndrome), lissencephaly, and abnormal genitalia or mental retardation alone has altered the previous somewhat rigid delineation of conditions as syndromic or non‐syndromic, as the same mutation within this gene can lead to a wide variety of phenotypes.33,34 Intracerebral cysts have also now been reported.35 Problems associated with mood including aggression or depression were also a feature in some families. Within families where XLMR is highly suspected, the prevalence of mutations in this gene is relatively high at 9/136 (6.6%), but systematic screening of a larger cohort of smaller possible X linked families has revealed no mutations (0/151) and in further screening of affected singletons the prevalence is low (2/1501).36,37,38 Testing is useful in syndromic mental retardation and in familial cases of non‐syndromic mental retardation but not in singleton cases.
A truncating mutation in SYN1 (synapsin 1; OMIM 313440) has been reported in a single family with mental retardation and epilepsy.39 This is not a common cause of mental retardation as more than 300 families with X linked or possible XLMR have now been screened and no new mutations have been identified to date (Raymond et al, unpublished data).
Mutations in AGTR2 (angiotensin II receptor, type 2; OMIM 300034) have been described in 10 patients to date, but the same mutation p.G21V found in three patients appears to be a rare polymorphism and unlikely to be disease causing.40,41,42 Severe mental retardation associated with epilepsy was present in the other cases reported with likely pathological mutations and two families had autistic behaviour. Testing is of unproven value to date.
MECP2
The clinical spectrum seen in males with mutations in MECP2 (methyl CpG binding protein 2 (Rett syndrome); OMIM 300005) include neonatal encephalopathy, Angelman syndrome, Rett syndrome, severe mental retardation with or without progressive spasticity, and manic depression as in PPM‐X (mental retardation, psychosis, pyramidal signs, and macroorchidism X syndrome). Orrico et al reported a family where a mother had mild intellectual problems, a daughter had classic Rett syndrome, and four affected boys had severe mental retardation.43 All family members have a missense mutation A140V in MECP2, suggesting that mutations in MECP2 may be a common cause of mental retardation in males. Two further families were then reported, one with progressive spasticity and the other with PPM‐X syndrome, where Q406X and A140V, respectively, were found to be the causative mutations.44,45 This stimulated screening of male patients with severe mental retardation for mutations in MECP2. Many sequence changes have been identified, but few are disease causing as this gene is highly polymorphic. Recent cohort studies have identified one pathological mutation in almost 1000 samples (1/475 in a European consortium study, 0/300 in the Cambridge GOLD study cohort, and 0/>200 samples referred for testing of males with mental retardation to Wessex Clinical Genetics Laboratory, UK).46 Mutations in MECP2 are rare causes of non‐syndromic mental retardation and sequence analysis should not be routinely offered but remains invaluable in the diagnosis of Rett syndrome and related disorders with a high diagnostic yield.
Short stature and microcephaly
Mutations in PQBP1 (polyglutamine binding protein 1; OMIM 300463) have been published as a cause for XLMR, but all patients described to date have a range of syndromic features. Microcephaly, short stature, and mental retardation are common features, together with a variety of mid‐line defects including anal atresia, situs inversus, congenital heart disease, cleft palate, ocular coloboma, and small testes. One patient had spastic paraplegia. The phenotypes in Renpenning syndrome, cerebropalatocardiac (Hamel) syndrome, and Sutherland Hann syndrome are similar and mutations in PQBP1 have also been identified in these conditions, suggesting they are allelic.47,48,49 No non‐syndromic mental retardation patients have yet been described, but screening patients with the above clinical features would be useful.
Mutations in SMCX (Smcy homolog, X linked (mouse); OMIM 314690), previously known as JARID1C, are also associated with short stature and microcephaly.50 Of the seven families described, only one family has a normal head circumference. Other frequent clinical features of this syndrome are small testes, prognathism or micrognathia, strabismus, myopia, facial hypotonia, progressive spastic paraplegia, epilepsy, and aggressive behaviour. Only one family had non‐syndromic mental retardation and was relatively mildly affected compared to the others.50
High triiodothyronine concentrations (T3)
Mutations in SLC16A2 (solute carrier family 16 (monocarboxylic acid transporter), member 2; OMIM 300095), also known as MCT8, a thyroid hormone transporter gene, were first reported in five unrelated boys with severe mental retardation and high triiodothyronine T3 concentrations.51 Two of the boys had partial deletions of the gene with a 24 kb deletion that encompassed exon 1 and a 2.4 kb deletion which resulted in a deletion of exon 3 and exon 4. The other three boys had missense mutations and a nonsense mutation.51 Subsequently, two further families were reported with abnormal triiodothyronine (T3) levels, global developmental delay, central hypotonia, spastic paraplegia, dystonic movements, rotary nystagmus, and impaired hearing and gaze.52 Children with Allan‐Herndon‐Dudley syndrome have a similar phenotype to that of the families reported by Dumitrescu et al and six large families have all been found to carry mutations, five missense and one 3 bp pair deletion.53 These patients were all subsequently found to have associated abnormal T3 levels although the neonatal Guthrie screens for hypothyroidism were normal. Abnormalities in this gene appear to be relatively common and suggest that in the diagnosis of profound mental retardation with neurological features detailed and continued surveillance of thyroid function tests may be helpful. This will aid the early identification of families who have mutations in this gene.
In the differential diagnosis of X linked spastic paraplegia (SMCX), SCL16A2 should be considered together with L1CAM (OMIM 308840), PLP1 (OMIM 300401), MECP2, and ARX.
Non‐syndromic mental retardation genes
Nine genes have been identified where the clinical feature of the families is mental retardation alone: IL1RAPL1, TM4SF2, ZNF41, FTSJ1, DLG3, FACL4, PAK3, ARHGEF6, FMR2, and GDI. To date, no detailed comparative prevalence studies have been published for abnormalities in these genes. The prevalence of Fragile X syndrome in affected sib pairs and X linked families is approximately 12/45 (27%), although this figure predates molecular genetic analysis and is likely to be an overestimate.29,54 The prevalence of each of the non‐syndromic genes is 1–2% in selected research samples where at least two males are affected in the family pedigree.
IL1RAPL1 (interleukin 1 receptor accessory protein‐like 1; OMIM 300206) was first identified as a candidate gene for mental retardation after the finding of deletions in families with mental retardation, adrenal hypoplasia, Duchenne muscular dystrophy, and glycerol kinase deficiency. Initially, a mutation was identified in 1/20 small XLMR families screened and no mutations were found in five large X linked families.55 Since then a complex rearrangement of this gene has been described, but no new mutations have been found.56
A translocation disrupting TM4SF2 (OMIM 300096), now known as TSPAN7 (tetraspanin 7), identified this gene as a potential cause of mental retardation. Mutations in this gene were also identified in 2/33 small families and 0/3 large families, but no further mutations have been reported since.57 More recently, the significance of the missense mutation p.P172H in one of the families has been questioned, although it has been reported in another case of a singleton with mild to moderate mental retardation but without family follow‐up studies being carried out.58,59
Disruption of ZNF41 (zinc finger protein 41; OMIM 314995) in a child with mental retardation and a balanced X autosome translocation identified this as a candidate gene. Screening of a panel of 210 families with XLMR identified one missense and one splice site mutation which are likely to be pathological.60
Mutations in FTSJ1 (Fts J homolog (Escherichia coli); OMIM 300499) have been found in 2/219 small X linked families and 2/30 linked families. Three mutations affect splicing and one is a missense mutation.61,62
Four truncating mutations in DLG3 (discs, large homolog 3 (neuroendocrine‐dlg, Drosophila); OMIM 300189) have been identified in a cohort of 328 families with XLMR.63 All the affected males in the families had moderate to severe mental retardation while female carriers were usually of normal intellect. X inactivation studies showed no skewing of lymphocytes in obligate female carriers.63
Mutations in FACL4 (renamed ACSL4, acyl‐CoA synthetase long‐chain family member 4; OMIM 300157) have been reported. These are two missense and one splice site mutation that reduce the enzymatic activity.64,65 The gene was originally localised by characterising genomic deletions in patients with Alport's syndrome and mental retardation.66,67
Since the identification of a truncating mutation in PAK3 (p21(CDKN1A) activated kinase 3; OMIM 300142), two further missense mutations have been described.68,69,70 Only a few families have been screened to date. The initial study screened 18 families, all of whom had positive linkage data that mapped the gene abnormality in the family to Xq21, but no systematic prevalence data are available for this gene.
Disruption of ARHGEF6 (Rac/Cdc42 guanine nucleotide exchange factor (GEF) 6; OMIM 300267) in a balanced translocation patient and the identification of a single intronic IVS1‐11T>C mutation in 1/119 mentally retarded patients have been described to date.71
FMR2 was identified by characterising the genomic structure around the folate sensitive fragile site, FRAXE.72,73 The official name for this gene is AFF2 (AF4/FMR2 family, member 2; OMIM 309548). Two unrelated boys with mental retardation had submicroscopic deletions in this region and facilitated the localisation of the gene. Subsequently, two families were identified, one of whom was also found to have FRAXA. The penetrance of FMR2 is variable and the phenotype can be mild or borderline mental retardation. Currently most diagnostic laboratories offer a PCR based screen for expansions in this gene. Specific Southern blot analysis can then be performed if the diagnosis is suspected. The prevalence is rare compared to Fragile X syndrome and interpretation of results is sometimes difficult.74,75,76,77
Three mutations in GDI1 (GDP dissociation inhibitor 1; OMIM 300104) have been characterised. Two out of five X linked families with linkage data mapping to Xq28 have mutations and 1/164 males with non‐familial mental retardation have been screened and found to carry a mutation.78,79
Finally, Fragile X syndrome should be considered as a frequent cause of non‐syndromic mental retardation as the classic phenotype of mental retardation, macrocephaly, frontal bossing, large ears, prominent mandible with prognathism, and enlarged testes is rarely seen. The prevalence of an expansion in the 5′CGG repeat of FMR1 (fragile X mental retardation 1; OMIM 309550) in the population in an unselected sample of mainly singletons is 1/3500–1/9000 and many of the affected individuals have a non‐syndromic phenotype.80,81
Recurrence risks for mental retardation
Where a molecular genetic diagnosis has been made, accurate genetic advice can usually be given. Unfortunately, this is still rarely the case when a family presents in a clinic and recurrence risks need to be quantified. Much of the available data were published between 1971 and 198782,83,84,85,86 (table 3) and although the observations of recurrence risks of 2–14% are still valid, the quality of chromosome analysis, the advent of molecular genetic testing for Fragile X syndrome, and the improved clinical expertise in syndromic identification question the validity of some of these data.87 A recent population based study in Atlanta, USA discusses contemporary recurrence risks for developmental delay although the sample was relatively old by contemporary molecular and cytogenetic standards. The sample was based on children born to mothers between 1981 and 1991 and the total number of cases with a disability was 3685. Recurrence risks for isolated mental retardation were 8.4% if the first child had isolated mental retardation as compared with recurrence risks for cerebral palsy of 2.9–3.6%, hearing loss of 4.7–5.7%, and vision impairment of 5.3–6.9%. If the first child had mild mental retardation, recurrence risks for mild and severe mental retardation were 7.1% and 4.7%, respectively.88
Table 3 Summary of published recurrence risks for mental retardation by sex of proband.
| Reference | Affected male | Affected female | ||||
|---|---|---|---|---|---|---|
| Brother | Sister | All siblings | Brother | Sister | All siblings | |
| Turner et al82 | 2–9% | 3.5–4% | ||||
| Bundey et al83 | 6.7% | 3.2% | 5% | 4.4% | 6.3% | 5.4% |
| Herbst et al84 | 6% | 2.3% | 4.3% | 2.9% | 5.6% | 4.2% |
| Bundey et al85 | 10% | 5% | 7.5% | |||
| Costeff et al86 | 14% | 14% | 14% | 9.6% | 9.6% | 9.2% |
| Van Naarden Braun et al88 | 8.4% | 8.4% | ||||
Where detailed clinical assessment is possible and current molecular genetic and cytogenetic analysis is available, the calculations of Turner and Partington are an additional useful guide for calculating recurrence risks.89 These authors observed recurrence risks for mental retardation in the siblings of index cases referred to a genetics centre. Observed recurrences were 1:7.5 (11/83) for brother pairs and 1:20 (3/60) for sister pairs. These figures are comparable to those of Herbst and Miller from 1980 and those from the Colchester cohort collected by L Penrose and revisited by Morton et al.90,91 The calculated offspring risk to intellectually normal siblings of a single affected male were 1–2% for the offspring of a normal male sibling and 2–5% for the offspring of a sister. The figures include the risk of an undetected familial cryptic translocation and, for the sister, the estimated risk that disease in a singleton male brother is due to an X linked disease (∼25%). If there are two affected males in a family, the assumption is that ∼80% (the male excess) is due to X linked disease. The offspring risk to a normal brother remains the same as the risk of an undetected familial cryptic translocation, as above, whereas the offspring risk to an unaffected sister if there are two affected males is significantly higher at 10% to include the X linked disease risk. Although this guide is useful, the calculations are inevitably inaccurate as they are biased by ascertainment of families in a clinical genetics setting and assume that the majority of male sib pairs have X linked disease.
Mandel and Chelly have addressed the issue of whether the observed male excess of patients with mental retardation is due entirely to mutations in monogenic disease genes on the X chromosome or not.37 Observing the prevalence of a 24 bp expansion in ARX, they observed that 6.6% (9/136) of families with XLMR pedigrees but only 0.13% (2/1501) of singleton cases were found to carry this mutation. Based on this observation, they calculate that only ∼10% of the excess males observed are due to X linked genes. This observation does not alter the practical clinical recurrence risks we inform patients about, but suggests that the identification of the cause of mental retardation in some families, especially where a single generation is affected, will be even harder to elucidate. Accurate genetic counselling of those families where no mutation is identified will continue to be challenging in the future. The use and predictive value of predisposing alleles or polymorphisms in clinical practice is extremely limited and this situation is not likely to change. Furthermore, this suggests that genetic counselling should clearly distinguish families with an X linked pedigree from those where a single generation is affected and provide appropriate recurrence risks based on the probability of there being an X linked condition in the family.
To date more than 20 genes have been identified that cause XLMR. Estimates of the number of genes that remain to be identified vary considerably from 30 to 50.92,93,94 Until all the genes on the X chromosome have been scrutinised in a large sample cohort, the exact number of XLMR genes will remain unknown, as will the prevalence and importance of each gene as a cause of human mental retardation. The future challenge is to understand the molecular genetic basis of the observed excess of mentally retarded males, discover the autosomal causes of mental retardation, and determine the biological basis of this disease in each gene abnormality identified.
In summary, Fragile X syndrome remains the most common XLMR gene discovered so far. Syndromic features should always be sought in possible XLMR as this can lead to molecular diagnosis. The discovery of the plethora of genes that cause a small proportion of non‐syndromic XLMR has clinical value for those families where mutations are detected, but awaits the arrival of high throughput, cheap, and reliable sequence analysis methods that can be readily introduced to clinical service.
Acknowledgements
I would like to thank Professors G Turner, M Partington, and J Goodship for discussion of the clinical phenotype of the three families with novel mutations in RPS6KA3, and Dr G Woods for his helpful comments on the manuscript. I would like to thank the British Clinical Genetic Society for the invitation to present a review of X linked mental retardation in March 2005 which formed the basis of this article.
ELECTRONIC‐DATABASE INFORMATION
The names and symbols used in this review are those agreed by the HUGO nomenclature committee (http://www.gene.ucl.ac.uk/nomenclature). MRX families are listed at http://xlmr.interfree.it/5_non.htm.
Footnotes
The GOLD study project is supported by the Wellcome Trust and is being carried out in collaboration with the Wellcome Sanger Institute.
Competing interests: none declared
The names and symbols used in this review are those agreed by the HUGO nomenclature committee (http://www.gene.ucl.ac.uk/nomenclature). MRX families are listed at http://xlmr.interfree.it/5_non.htm.
References
- 1.Penrose L.A clinical and genetic study of 1280 cases of mental defect. vol 229. London: HMSO, 1938
- 2.Drillien C M. The incidence of mental and physical handicaps in school age children of very low birth weight. II. Pediatrics 196739(2)238–247. [PubMed] [Google Scholar]
- 3.McLaren J, Bryson S E. Review of recent epidemiological studies of mental retardation: prevalence, associated disorders, and etiology. Am J Ment Retard 198792(3)243–254. [PubMed] [Google Scholar]
- 4.Baird P A, Sadovnick A D. Mental retardation in over half‐a‐million consecutive livebirths: an epidemiological study. Am J Ment Defic 198589(4)323–330. [PubMed] [Google Scholar]
- 5.Stevenson R E, Schwartz C, Schroer R. Mental retardation in South Carolina. I. Characteristics of the study population. Proc Greenwood Genet Center 19961526 [Google Scholar]
- 6.Stoller A. Epidemiology of mental deficiency in Victoria. In: JD Van Zelt , ed. Proceedings of the Fourth Interstate Conference on Mental Deficiency. Melbourne, Australia: Australian Group for the Scientific Study of Mental Deficiency, 196518–28.
- 7.WHO The ICD‐10 classification of mental and behavioural disorders. Geneva: WHO, 1992
- 8.American Psychiatric Association Diagnostic and statistical manual of mental disorders DSM‐IV. Washington, DC: American Psychiatric Association, 1994
- 9.Martin J P, Bell J. A pedigree of mental defect showing sex‐linkage. J Neurol Psychiatry 19436154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oberle I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, Boue J, Bertheas M F, Mandel J L. Instability of a 550‐base pair DNA segment and abnormal methylation in fragile X syndrome. Science 1991252(5010)1097–1102. [DOI] [PubMed] [Google Scholar]
- 11.Kerr B, Turner G, Mulley J, Gedeon A, Partington M. Non‐specific X linked mental retardation. J Med Genet 199128(6)378–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shevell M, Ashwal S, Donley D, Flint J, Gingold M, Hirtz D, Majnemer A, Noetzel M, Sheth R D. Practice parameter: evaluation of the child with global developmental delay: report of the Quality Standards Subcommittee of the American Academy of Neurology and The Practice Committee of the Child Neurology Society. Neurology 200360(3)367–380. [DOI] [PubMed] [Google Scholar]
- 13.Knight S J, Regan R, Nicod A, Horsley S W, Kearney L, Homfray T, Winter R M, Bolton P, Flint J. Subtle chromosomal rearrangements in children with unexplained mental retardation. Lancet 1999354(9191)1676–1681. [DOI] [PubMed] [Google Scholar]
- 14.Slavotinek A, Rosenberg M, Knight S, Gaunt L, Fergusson W, Killoran C, Clayton‐Smith J, Kingston H, Campbell R H, Flint J, Donnai D, Biesecker L. Screening for submicroscopic chromosome rearrangements in children with idiopathic mental retardation using microsatellite markers for the chromosome telomeres. J Med Genet 199936(5)405–411. [PMC free article] [PubMed] [Google Scholar]
- 15.Koolen D A, Nillesen W M, Versteeg M H, Merkx G F, Knoers N V, Kets M, Vermeer S, van Ravenswaaij C M, de Kovel C G, Brunner H G, Smeets D, de Vries B B, Sistermans E A. Screening for subtelomeric rearrangements in 210 patients with unexplained mental retardation using multiplex ligation dependent probe amplification (MLPA). J Med Genet 200441(12)892–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flint J, Knight S. The use of telomere probes to investigate submicroscopic rearrangements associated with mental retardation. Curr Opin Genet Dev 200313(3)310–316. [DOI] [PubMed] [Google Scholar]
- 17.Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg I C, Soderstrom H, Giros B, Leboyer M, Gillberg C, Bourgeron T, Paris Autism Research International Sibpair Study Mutations of the X‐linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet 200334(1)27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laumonnier F, Bonnet‐Brilhault F, Gomot M, Blanc R, David A, Moizard M P, Raynaud M, Ronce N, Lemonnier E, Calvas P, Laudier B, Chelly J, Fryns J P, Ropers H H, Hamel B C, Andres C, Barthelemy C, Moraine C, Briault S. X‐linked mental retardation and autism are associated with a mutation in the NLGN4 gene, a member of the neuroligin family. Am J Hum Genet 200474(3)552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gauthier J, Bonnel A, St‐Onge J, Karemera L, Laurent S, Mottron L, Fombonne E, Joober R, Rouleau G A. NLGN3/NLGN4 gene mutations are not responsible for autism in the Quebec population. Am J Med Genet B Neuropsychiatr Genet 2005132(1)74–75. [DOI] [PubMed] [Google Scholar]
- 20.Vincent J B, Kolozsvari D, Roberts W S, Bolton P F, Gurling H M, Scherer S W. Mutation screening of X‐chromosomal neuroligin genes: no mutations in 196 autism probands. Am J Med Genet B Neuropsychiatr Genet 2004129(1)82–84. [DOI] [PubMed] [Google Scholar]
- 21.Trivier E, De Cesare D, Jacquot S, Pannetier S, Zackai E, Young I, Mandel J L, Sassone‐Corsi P, Hanauer A. Mutations in the kinase Rsk‐2 associated with Coffin‐Lowry syndrome. Nature 1996384(6609)567–570. [DOI] [PubMed] [Google Scholar]
- 22.Hanauer A, Young I D. Coffin‐Lowry syndrome: clinical and molecular features. J Med Genet 200239(10)705–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merienne K, Jacquot S, Pannetier S, Zeniou M, Bankier A, Gecz J, Mandel J L, Mulley J, Sassone‐Corsi P, Hanauer A. A missense mutation in RPS6KA3 (RSK2) responsible for non‐specific mental retardation. Nat Genet 199922(1)13–14. [DOI] [PubMed] [Google Scholar]
- 24.Billuart P, Bienvenu T, Ronce N, des Portes V, Vinet M C, Zemni R, Roest Crollius H, Carrie A, Fauchereau F, Cherry M, Briault S, Hamel B, Fryns J P, Beldjord C, Kahn A, Moraine C, Chelly J. Oligophrenin‐1 encodes a rhoGAP protein involved in X‐linked mental retardation. Nature 1998392(6679)923–926. [DOI] [PubMed] [Google Scholar]
- 25.Philip N, Chabrol B, Lossi A M, Cardoso C, Guerrini R, Dobyns W B, Raybaud C, Villard L. Mutations in the oligophrenin‐1 gene (OPHN1) cause X linked congenital cerebellar hypoplasia. J Med Genet 200340(6)441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerrini R, Shanahan J L, Carrozzo R, Bonanni P, Higgs D R, Gibbons R J. A nonsense mutation of the ATRX gene causing mild mental retardation and epilepsy. Ann Neurol 200047(1)117–121. [PubMed] [Google Scholar]
- 27.Salomons G S, van Dooren S J, Verhoeven N M, Cecil K M, Ball W S, Degrauw T J, Jakobs C. X‐linked creatine‐transporter gene (SLC6A8) defect: a new creatine‐deficiency syndrome. Am J Hum Genet 200168(6)1497–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg E H, Almeida L S, Kleefstra T, deGrauw R S, Yntema H G, Bahi N, Moraine C, Ropers H H, Fryns J P, deGrauw T J, Jakobs C, Salomons G S. High prevalence of SLC6A8 deficiency in X‐linked mental retardation. Am J Hum Genet 200475(1)97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mandel J L. Comparative frequency of fragile‐X (FMR1) and creatine transporter (SLC6A8) mutations in X‐linked mental retardation. Am J Hum Genet 200475(4)730–1 author reply, 7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carducci C, Birarelli M, Leuzzi V, Carducci C, Battini R, Cioni G, Antonozzi I. Guanidinoacetate and creatine plus creatinine assessment in physiologic fluids: an effective diagnostic tool for the biochemical diagnosis of arginine:glycine amidinotransferase and guanidinoacetate methyltransferase deficiencies. Clin Chem 200248(10)1772–1778. [PubMed] [Google Scholar]
- 31.Arias A, Garcia‐Villoria J, Ribes A. Guanidinoacetate and creatine/creatinine levels in controls and patients with urea cycle defects. Mol Genet Metab 200482(3)220–223. [DOI] [PubMed] [Google Scholar]
- 32.Cognat S, Cheillan D, Piraud M, Roos B, Jakobs C, Vianey‐Saban C. Determination of guanidinoacetate and creatine in urine and plasma by liquid chromatography‐tandem mass spectrometry. Clin Chem 200450(8)1459–1461. [DOI] [PubMed] [Google Scholar]
- 33.Stromme P, Mangelsdorf M E, Shaw M A, Lower K M, Lewis S M, Bruyere H, Lutcherath V, Gedeon A K, Wallace R H, Scheffer I E, Turner G, Partington M, Frints S G, Fryns J P, Sutherland G R, Mulley J C, Gecz J. Mutations in the human ortholog of Aristaless cause X‐linked mental retardation and epilepsy. Nat Genet 200230(4)441–445. [DOI] [PubMed] [Google Scholar]
- 34.Kitamura K, Yanazawa M, Sugiyama N, Miura H, Iizuka‐Kogo A, Kusaka M, Omichi K, Suzuki R, Kato‐Fukui Y, Kamiirisa K, Matsuo M, Kamijo S, Kasahara M, Yoshioka H, Ogata T, Fukuda T, Kondo I, Kato M, Dobyns W B, Yokoyama M, Morohashi K. Mutation of ARX causes abnormal development of forebrain and testes in mice and X‐linked lissencephaly with abnormal genitalia in humans. Nat Genet 200232(3)359–369. [DOI] [PubMed] [Google Scholar]
- 35.Stromme P, Bakke S J, Dahl A, Gecz J. Brain cysts associated with mutation in the Aristaless related homeobox gene, ARX. J Neurol Neurosurg Psychiatry 200374(4)536–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bienvenu T, Poirier K, Friocourt G, Bahi N, Beaumont D, Fauchereau F, Ben Jeema L, Zemni R, Vinet M C, Francis F, Couvert P, Gomot M, Moraine C, van Bokhoven H, Kalscheuer V, Frints S, Gecz J, Ohzaki K, Chaabouni H, Fryns J P, Desportes V, Beldjord C, Chelly J. ARX, a novel Prd‐class‐homeobox gene highly expressed in the telencephalon, is mutated in X‐linked mental retardation. Hum Mol Genet 200211(8)981–991. [DOI] [PubMed] [Google Scholar]
- 37.Mandel J L, Chelly J. Monogenic X‐linked mental retardation: is it as frequent as currently estimated? The paradox of the ARX (Aristaless X) mutations. Eur J Hum Genet 200412(9)689–693. [DOI] [PubMed] [Google Scholar]
- 38.Gronskov K, Hjalgrim H, Nielsen I M, Brondum‐Nielsen K. Screening of the ARX gene in 682 retarded males. Eur J Hum Genet 200412(9)701–705. [DOI] [PubMed] [Google Scholar]
- 39.Garcia C C, Blair H J, Seager M, Coulthard A, Tennant S, Buddles M, Curtis A, Goodship J A. Identification of a mutation in synapsin I, a synaptic vesicle protein, in a family with epilepsy. J Med Genet 200441(3)183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vervoort V S, Beachem M A, Edwards P S, Ladd S, Miller K E, de Mollerat X, Clarkson K, DuPont B, Schwartz C E, Stevenson R E, Boyd E, Srivastava A K. AGTR2 mutations in X‐linked mental retardation. Science 2002296(5577)2401–2403. [DOI] [PubMed] [Google Scholar]
- 41.Ylisaukko‐oja T, Rehnstrom K, Vanhala R, Tengstrom C, Lahdetie J, Jarvela I. Identification of two AGTR2 mutations in male patients with non‐syndromic mental retardation. Hum Genet 2004114(2)211–213. [DOI] [PubMed] [Google Scholar]
- 42.Erdmann J, Dahmlow S, Guse M, Hetzer R, Regitz‐Zagrosek V. The assertion that a G21V mutation in AGTR2 causes mental retardation is not supported by other studies. Hum Genet 2004114(4)369 author reply, 397. [DOI] [PubMed] [Google Scholar]
- 43.Orrico A, Lam C, Galli L, Dotti M T, Hayek G, Tong S F, Poon P M, Zappella M, Federico A, Sorrentino V. MECP2 mutation in male patients with non‐specific X‐linked mental retardation. FEBS Lett 2000481(3)285–288. [DOI] [PubMed] [Google Scholar]
- 44.Meloni I, Bruttini M, Longo I, Mari F, Rizzolio F, D'Adamo P, Denvriendt K, Fryns J P, Toniolo D, Renieri A. A mutation in the rett syndrome gene, MECP2, causes X‐linked mental retardation and progressive spasticity in males. Am J Hum Genet 200067(4)982–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klauck S M, Lindsay S, Beyer K S, Splitt M, Burn J, Poustka A. A mutation hot spot for nonspecific X‐linked mental retardation in the MECP2 gene causes the PPM‐X syndrome. Am J Hum Genet 200270(4)1034–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poirier K, Francis F, Hamel B, Moraine C, Fryns J P, Ropers H H, Chelly J, Bienvenu T. Mutations in exon 1 of MECP2B are not a common cause of X‐linked mental retardation in males. Eur J Hum Genet 200513(5)523–524. [DOI] [PubMed] [Google Scholar]
- 47.Kalscheuer V M, Freude K, Musante L, Jensen L R, Yntema H G, Gecz J, Sefiani A, Hoffmann K, Moser B, Haas S, Gurok U, Haesler S, Aranda B, Nshedjan A, Tzschach A, Hartmann N, Roloff T C, Shoichet S, Hagens O, Tao J, Van Bokhoven H, Turner G, Chelly J, Moraine C, Fryns J P, Nuber U, Hoeltzenbein M, Scharff C, Scherthan H, Lenzner S, Hamel B C, Schweiger S, Ropers H H. Mutations in the polyglutamine binding protein 1 gene cause X‐linked mental retardation. Nat Genet 200335(4)313–315. [DOI] [PubMed] [Google Scholar]
- 48.Lenski C, Abidi F, Meindl A, Gibson A, Platzer M, Frank Kooy R, Lubs H A, Stevenson R E, Ramser J, Schwartz C E. Novel truncating mutations in the polyglutamine tract binding protein 1 gene (PQBP1) cause Renpenning syndrome and X‐linked mental retardation in another family with microcephaly. Am J Hum Genet 200474(4)777–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleefstra T, Franken C E, Arens Y H, Ramakers G J, Yntema H G, Sistermans E A, Hulsmans C F, Nillesen W N, van Bokhoven H, de Vries B B, Hamel B C. Genotype‐phenotype studies in three families with mutations in the polyglutamine‐binding protein 1 gene (PQBP1). Clin Genet 200466(4)318–326. [DOI] [PubMed] [Google Scholar]
- 50.Jensen L R, Amende M, Gurok U, Moser B, Gimmel V, Tzschach A, Janecke A R, Tariverdian G, Chelly J, Fryns J P, Van Esch H, Kleefstra T, Hamel B, Moraine C, Gecz J, Turner G, Reinhardt R, Kalscheuer V M, Ropers H H, Lenzner S. Mutations in the JARID1C gene, which is involved in transcriptional regulation and chromatin remodeling, cause X‐linked mental retardation. Am J Hum Genet 200576(2)227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friesema E C, Grueters A, Biebermann H, Krude H, von Moers A, Reeser M, Barrett T G, Mancilla E E, Svensson J, Kester M H, Kuiper G G, Balkassmi S, Uitterlinden A G, Koehrle J, Rodien P, Halestrap A P, Visser T J. Association between mutations in a thyroid hormone transporter and severe X‐linked psychomotor retardation. Lancet 2004364(9443)1435–1437. [DOI] [PubMed] [Google Scholar]
- 52.Dumitrescu A M, Liao X H, Best T B, Brockmann K, Refetoff S. A novel syndrome combining thyroid and neurological abnormalities is associated with mutations in a monocarboxylate transporter gene. Am J Hum Genet 200474(1)168–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz C E, May M M, Carpenter N J, Rogers R C, Martin J, Bialer M G, Ward J, Sanabria J, Marsa S, Lewis J A, Echeverri R, Lubs H A, Voeller K, Simensen R J, Stevenson R E. Allan‐Herndon‐Dudley syndrome and the monocarboxylate transporter 8 (MCT8) gene. Am J Hum Genet 200577(1)41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fishburn J, Turner G, Daniel A, Brookwell R. The diagnosis and frequency of X‐linked conditions in a cohort of moderately retarded males with affected brothers. Am J Med Genet 198314(4)713–724. [DOI] [PubMed] [Google Scholar]
- 55.Carrie A, Jun L, Bienvenu T, Vinet M C, McDonell N, Couvert P, Zemni R, Cardona A, Van Buggenhout G, Frints S, Hamel B, Moraine C, Ropers H H, Strom T, Howell G R, Whittaker A, Ross M T, Kahn A, Fryns J P, Beldjord C, Marynen P, Chelly J. A new member of the IL‐1 receptor family highly expressed in hippocampus and involved in X‐linked mental retardation. Nat Genet 199923(1)25–31. [DOI] [PubMed] [Google Scholar]
- 56.Wheway J M, Yau S C, Nihalani V, Ellis D, Irving M, Splitt M, Roberts R G. A complex deletion‐inversion‐deletion event results in a chimeric IL1RAPL1‐dystrophin transcript and a contiguous gene deletion syndrome. J Med Genet 200340(2)127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zemni R, Bienvenu T, Vinet M C, Sefiani A, Carrie A, Billuart P, McDonell N, Couvert P, Francis F, Chafey P, Fauchereau F, Friocourt G, des Portes V, Cardona A, Frints S, Meindl A, Brandau O, Ronce N, Moraine C, van Bokhoven H, Ropers H H, Sudbrak R, Kahn A, Fryns J P, Beldjord C, Chelly J. A new gene involved in X‐linked mental retardation identified by analysis of an X;2 balanced translocation. Nat Genet 200024(2)167–170. [DOI] [PubMed] [Google Scholar]
- 58.Gomot M, Ronce N, Dessay S, Zemni R, Ayrault A D, Moizard M P, Nivelon A, Gilgenkrantz S, Dourlens J, Des Portes V, Chelly J, Moraine C. TM4SF2 gene involvement reconsidered in an XLMR family after neuropsychological assessment. Am J Med Genet 2002112(4)400–404. [DOI] [PubMed] [Google Scholar]
- 59.Maranduba C M, Sa Moreira E, Muller Orabona G, Pavanello R C, Vianna‐Morgante A M, Passos‐Bueno M R. Does the P172H mutation at the TM4SF2 gene cause X‐linked mental retardation? Am J Med Genet A 2004124(4)413–415. [DOI] [PubMed] [Google Scholar]
- 60.Shoichet S A, Hoffmann K, Menzel C, Trautmann U, Moser B, Hoeltzenbein M, Echenne B, Partington M, Van Bokhoven H, Moraine C, Fryns J P, Chelly J, Rott H D, Ropers H H, Kalscheuer V M. Mutations in the ZNF41 gene are associated with cognitive deficits: identification of a new candidate for X‐linked mental retardation. Am J Hum Genet 200373(6)1341–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Freude K, Hoffmann K, Jensen L R, Delatycki M B, des Portes V, Moser B, Hamel B, van Bokhoven H, Moraine C, Fryns J P, Chelly J, Gecz J, Lenzner S, Kalscheuer V M, Ropers H H. Mutations in the FTSJ1 gene coding for a novel S‐adenosylmethionine‐binding protein cause nonsyndromic X‐linked mental retardation. Am J Hum Genet 200475(2)305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramser J, Winnepenninckx B, Lenski C, Errijgers V, Platzer M, Schwartz C E, Meindl A, Kooy R F. A splice site mutation in the methyltransferase gene FTSJ1 in Xp11.23 is associated with non‐syndromic mental retardation in a large Belgian family (MRX9). J Med Genet 200441(9)679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tarpey P, Parnau J, Blow M, Woffendin H, Bignell G, Cox C, Cox J, Davies H, Edkins S, Holden S, Korny A, Mallya U, Moon J, O'Meara S, Parker A, Stephens P, Stevens C, Teague J, Donnelly A, Mangelsdorf M, Mulley J, Partington M, Turner G, Stevenson R, Schwartz C, Young I, Easton D, Bobrow M, Futreal P A, Stratton M R, Gecz J, Wooster R, Raymond F L. Mutations in the DLG3 gene cause nonsyndromic X‐linked mental retardation. Am J Hum Genet 200475(2)218–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meloni I, Muscettola M, Raynaud M, Longo I, Bruttini M, Moizard M P, Gomot M, Chelly J, des Portes V, Fryns J P, Ropers H H, Magi B, Bellan C, Volpi N, Yntema H G, Lewis S E, Schaffer J E, Renieri A. FACL4, encoding fatty acid‐CoA ligase 4, is mutated in nonspecific X‐linked mental retardation. Nat Genet 200230(4)436–440. [DOI] [PubMed] [Google Scholar]
- 65.Longo I, Frints S G, Fryns J P, Meloni I, Pescucci C, Ariani F, Borghgraef M, Raynaud M, Marynen P, Schwartz C, Renieri A, Froyen G. A third MRX family (MRX68) is the result of mutation in the long chain fatty acid‐CoA ligase 4 (FACL4) gene: proposal of a rapid enzymatic assay for screening mentally retarded patients. J Med Genet 200340(1)11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jonsson J J, Renieri A, Gallagher P G, Kashtan C E, Cherniske E M, Bruttini M, Piccini M, Vitelli F, Ballabio A, Pober B R. Alport syndrome, mental retardation, midface hypoplasia, and elliptocytosis: a new X linked contiguous gene deletion syndrome? J Med Genet 199835(4)273–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Piccini M, Vitelli F, Bruttini M, Pober B R, Jonsson J J, Villanova M, Zollo M, Borsani G, Ballabio A, Renieri A. FACL4, a new gene encoding long‐chain acyl‐CoA synthetase 4, is deleted in a family with Alport syndrome, elliptocytosis, and mental retardation. Genomics 199847(3)350–358. [DOI] [PubMed] [Google Scholar]
- 68.Allen K M, Gleeson J G, Bagrodia S, Partington M W, MacMillan J C, Cerione R A, Mulley J C, Walsh C A. PAK3 mutation in nonsyndromic X‐linked mental retardation. Nat Genet 199820(1)25–30. [DOI] [PubMed] [Google Scholar]
- 69.Bienvenu T, des Portes V, McDonell N, Carrie A, Zemni R, Couvert P, Ropers H H, Moraine C, van Bokhoven H, Fryns J P, Allen K, Walsh C A, Boue J, Kahn A, Chelly J, Beldjord C. Missense mutation in PAK3, R67C, causes X‐linked nonspecific mental retardation. Am J Med Genet 200093(4)294–298. [DOI] [PubMed] [Google Scholar]
- 70.Gedeon A K, Nelson J, Gecz J, Mulley J C. X‐linked mild non‐syndromic mental retardation with neuropsychiatric problems and the missense mutation A365E in PAK3. Am J Med Genet 2003120A(4)509–517. [DOI] [PubMed] [Google Scholar]
- 71.Kutsche K, Yntema H, Brandt A, Jantke I, Nothwang H G, Orth U, Boavida M G, David D, Chelly J, Fryns J P, Moraine C, Ropers H H, Hamel B C, van Bokhoven H, Gal A. Mutations in ARHGEF6, encoding a guanine nucleotide exchange factor for rho GTPases, in patients with X‐linked mental retardation. Nat Genet 200026(2)247–250. [DOI] [PubMed] [Google Scholar]
- 72.Gecz J, Gedeon A K, Sutherland G R, Mulley J C. Identification of the gene FMR2, associated with FRAXE mental retardation. Nat Genet 199613(1)105–108. [DOI] [PubMed] [Google Scholar]
- 73.Gu Y, Shen Y, Gibbs R A, Nelson D L. Identification of FMR2, a novel gene associated with the FRAXE CCG repeat and CpG island. Nat Genet 199613(1)109–113. [DOI] [PubMed] [Google Scholar]
- 74.Mazzocco M M, Myers G F, Hamner J L, Panoscha R, Shapiro B K, Reiss A L. The prevalence of the FMR1 and FMR2 mutations among preschool children with language delay. J Pediatr 1998132(5)795–801. [DOI] [PubMed] [Google Scholar]
- 75.Faradz S M, Leggo J, Murray A, Lam‐Po‐Tang P R, Buckley M F, Holden J J. Distribution of FMR1 and FMR2 alleles in Javanese individuals with developmental disability and confirmation of a specific AGG‐interruption pattern in Asian populations. Ann Hum Genet 200165(Pt 2)127–135. [DOI] [PubMed] [Google Scholar]
- 76.Patsalis P C, Sismani C, Hettinger J A, Boumba I, Georgiou I, Stylianidou G, Anastasiadou V, Koukoulli R, Pagoulatos G, Syrrou M. Molecular screening of fragile X (FRAXA) and FRAXE mental retardation syndromes in the Hellenic population of Greece and Cyprus: incidence, genetic variation, and stability. Am J Med Genet 199984(3)184–190. [PubMed] [Google Scholar]
- 77.Sharma D, Gupta M, Thelma B K. Expansion mutation frequency and CGG/GCC repeat polymorphism in FMR1 and FMR2 genes in an Indian population. Genet Epidemiol 200120(1)129–144. [DOI] [PubMed] [Google Scholar]
- 78.D'Adamo P, Menegon A, Lo Nigro C, Grasso M, Gulisano M, Tamanini F, Bienvenu T, Gedeon A K, Oostra B, Wu S K, Tandon A, Valtorta F, Balch W E, Chelly J, Toniolo D. Mutations in GDI1 are responsible for X‐linked non‐specific mental retardation. Nat Genet 199819(2)134–139. [DOI] [PubMed] [Google Scholar]
- 79.Bienvenu T, des Portes V, Saint Martin A, McDonell N, Billuart P, Carrie A, Vinet M C, Couvert P, Toniolo D, Ropers H H, Moraine C, van Bokhoven H, Fryns J P, Kahn A, Beldjord C, Chelly J. Non‐specific X‐linked semidominant mental retardation by mutations in a Rab GDP‐dissociation inhibitor. Hum Mol Genet 19987(8)1311–1315. [DOI] [PubMed] [Google Scholar]
- 80.de Vries B B, van den Ouweland A M, Mohkamsing S, Duivenvoorden H J, Mol E, Gelsema K, van Rijn M, Halley D J, Sandkuijl L A, Oostra B A, Tibben A, Niermeijer M F. Screening and diagnosis for the fragile X syndrome among the mentally retarded: an epidemiological and psychological survey. Collaborative Fragile X Study Group. Am J Hum Genet 199761(3)660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Crawford D C, Acuna J M, Sherman S L. FMR1 and the fragile X syndrome: human genome epidemiology review. Genet Med 20013(5)359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turner G, Collins E, Turner B. Recurrence risk of mental retardation in sibs. Med J Aust 19711(22)1165–1167. [PubMed] [Google Scholar]
- 83.Bundey S, Carter C O. Recurrence risks in severe undiagnosed mental deficiency. J Ment Defic Res 197418(2)115–134. [DOI] [PubMed] [Google Scholar]
- 84.Herbst D S, Baird P A. Sib risks for nonspecific mental retardation in British Columbia. Am J Med Genet 198213(2)197–208. [DOI] [PubMed] [Google Scholar]
- 85.Bundey S, Webb T P, Thake A, Todd J. A community study of severe mental retardation in the West Midlands and the importance of the fragile X chromosome in its aetiology. J Med Genet 198522(4)258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Costeff H, Weller L. The risk of having a second retarded child. Am J Med Genet 198727(4)753–766. [DOI] [PubMed] [Google Scholar]
- 87.Crow Y J, Tolmie J L. Recurrence risks in mental retardation. J Med Genet 199835(3)177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Naarden Braun K, Autry A, Boyle C. A population‐based study of the recurrence of developmental disabilities‐‐Metropolitan Atlanta Developmental Disabilities Surveillance Program, 1991–94. Paediatr Perinat Epidemiol 200519(1)69–79. [DOI] [PubMed] [Google Scholar]
- 89.Turner G, Partington M. Recurrence risks in undiagnosed mental retardation. J Med Genet 200037(12)E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herbst D S, Miller J R. Nonspecific X‐linked mental retardation II: the frequency in British Columbia. Am J Med Genet 19807(4)461–469. [DOI] [PubMed] [Google Scholar]
- 91.Morton N E, Rao D C, Lang‐Brown H, Maclean C J, Bart R D, Lew R. Colchester revisited: a genetic study of mental defect. J Med Genet 197714(1)1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chelly J. MRX review. Am J Med Genet 200094(5)364–366. [DOI] [PubMed] [Google Scholar]
- 93.Gecz J, Mulley J. Genes for cognitive function: developments on the X. Genome Res 200010(2)157–163. [DOI] [PubMed] [Google Scholar]
- 94.Ropers H H, Hoeltzenbein M, Kalscheuer V, Yntema H, Hamel B, Fryns J P, Chelly J, Partington M, Gecz J, Moraine C. Nonsyndromic X‐linked mental retardation: where are the missing mutations? Trends Genet 200319(6)316–320. [DOI] [PubMed] [Google Scholar]