Abstract
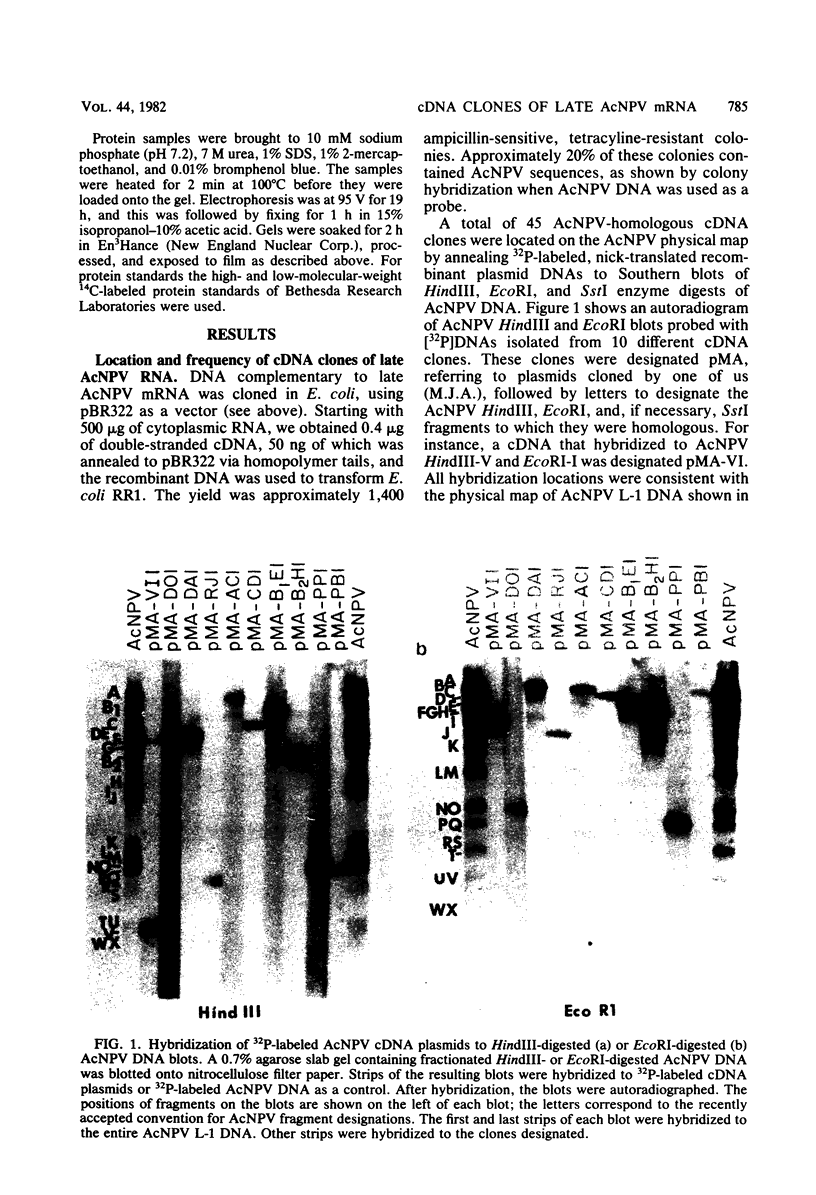
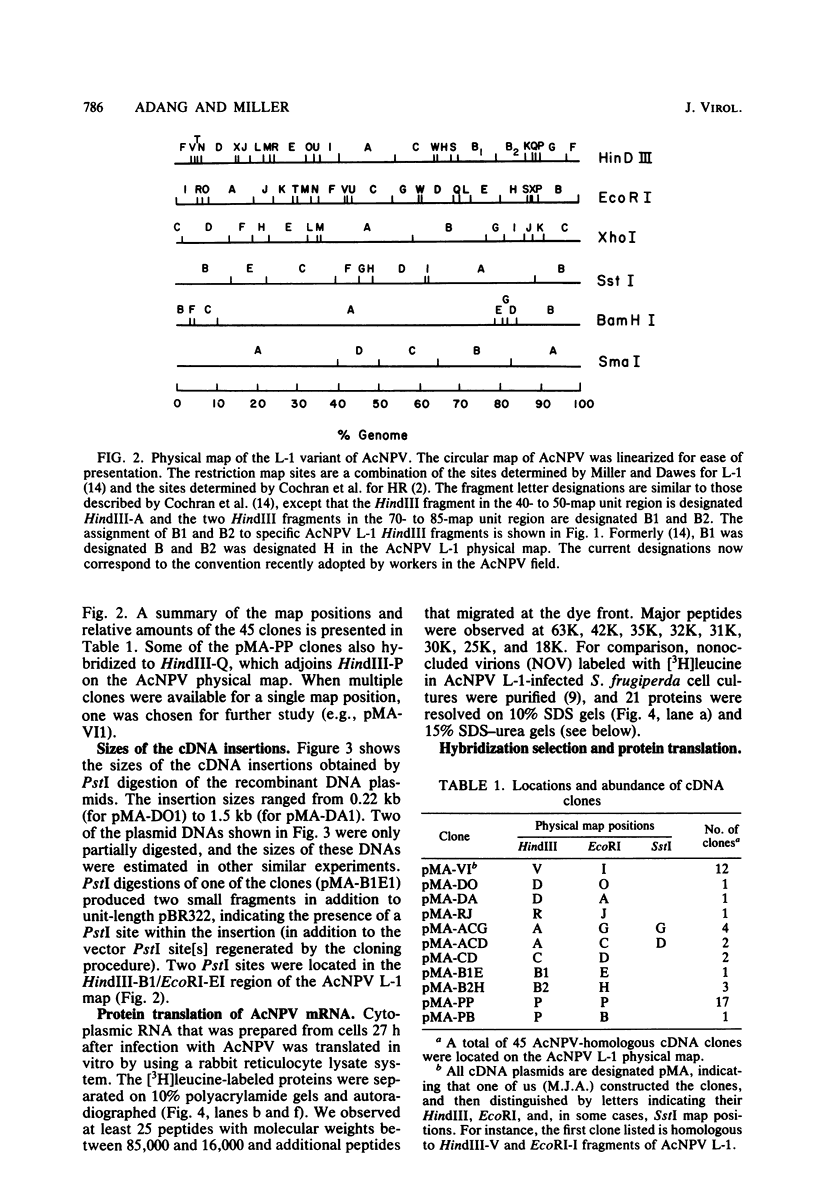
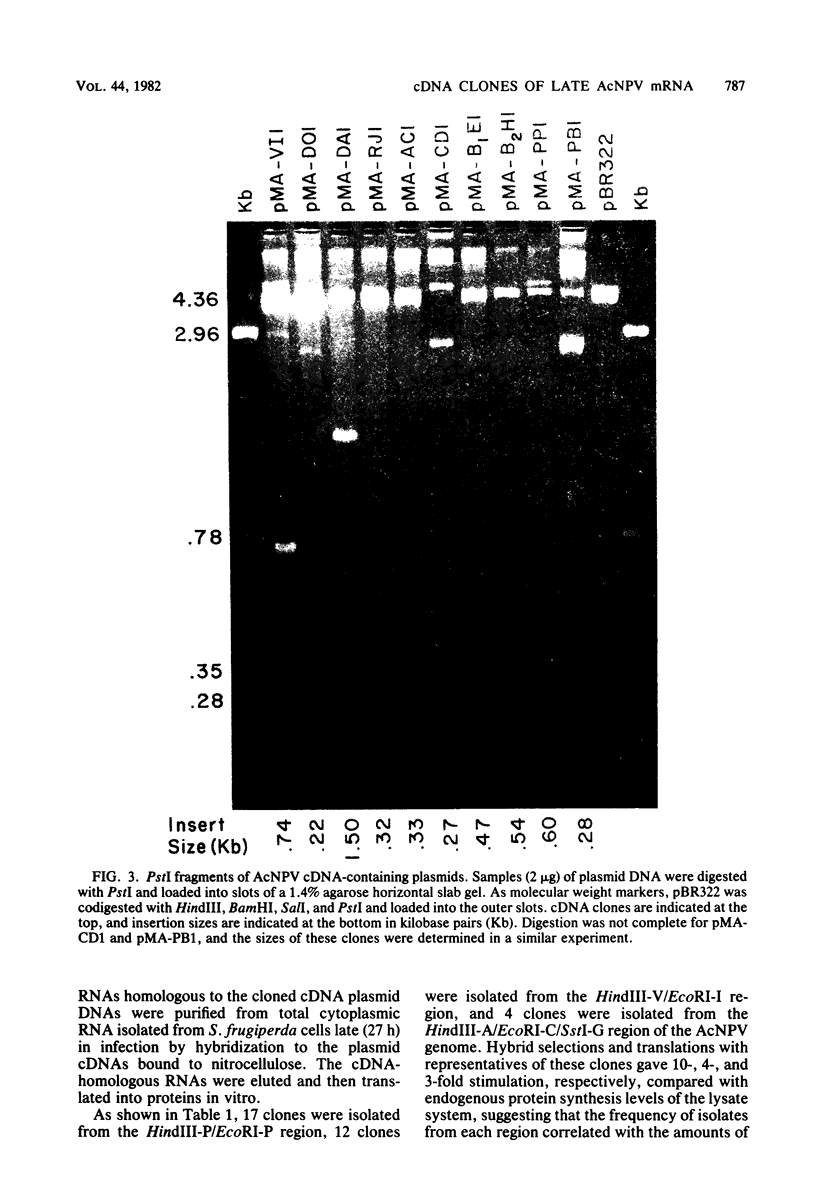
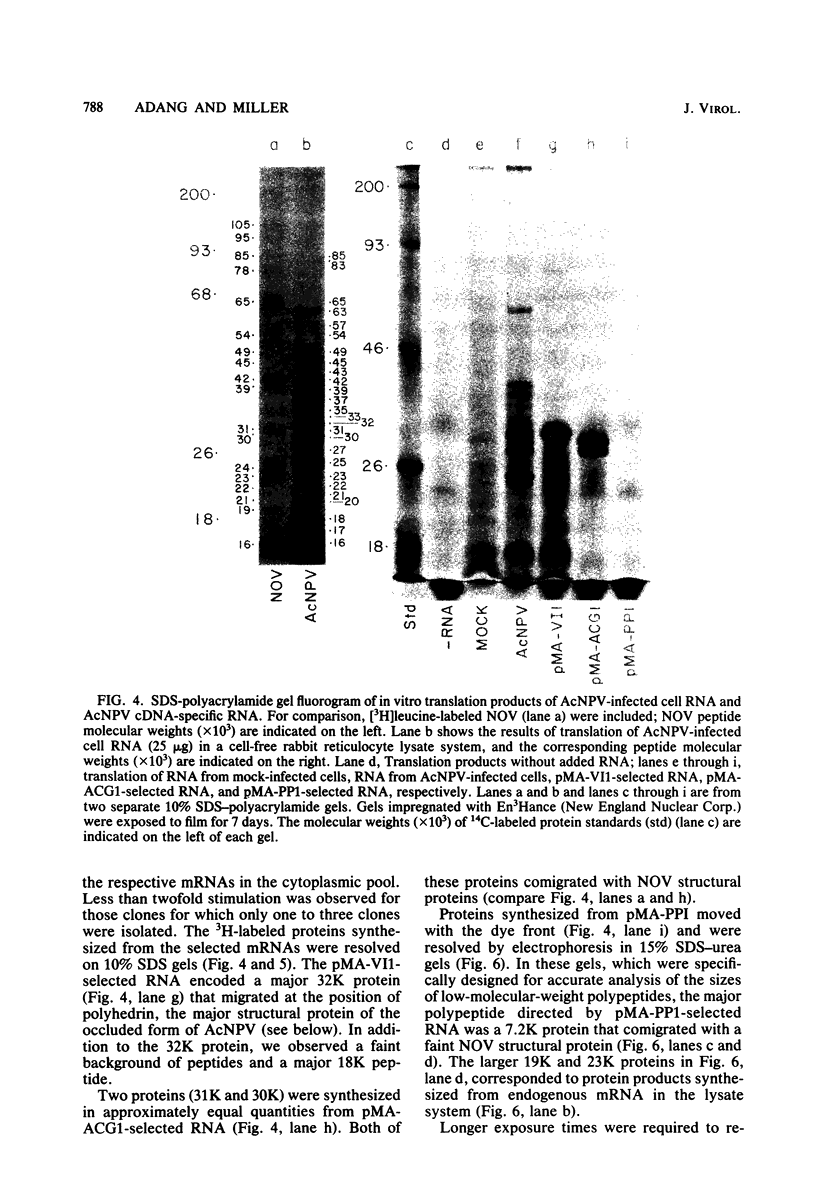
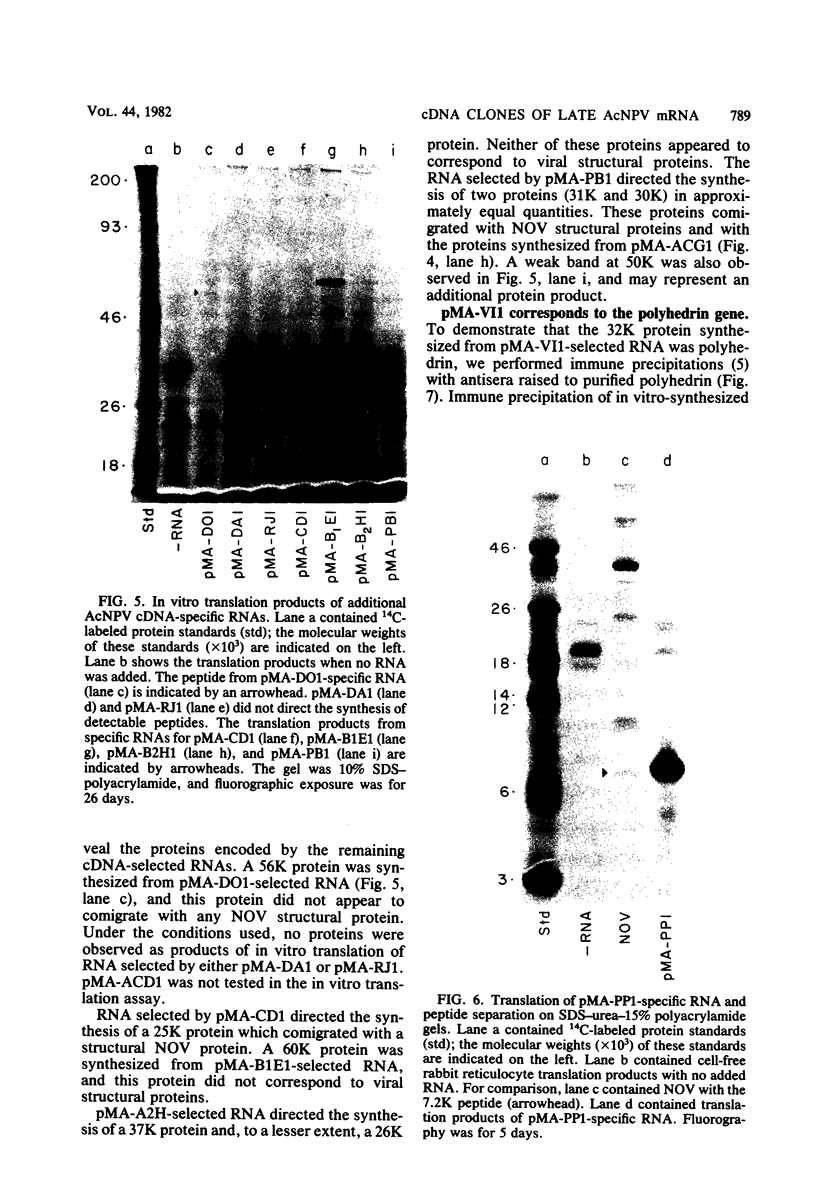
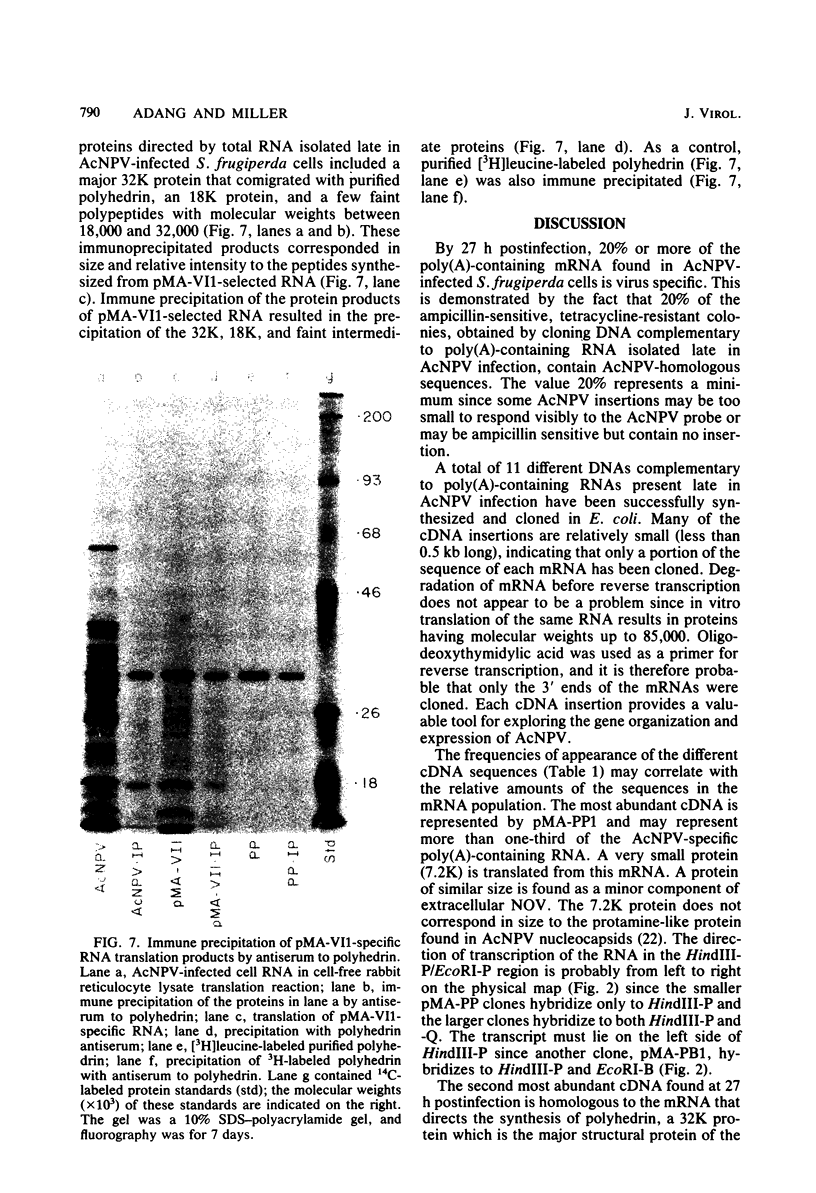
DNAs complementary to late Autographa californica nuclear polyhedrosis virus (AcNPV) mRNA were synthesized by reverse transcription and cloned in Escherichia coli by using pBR322 as a vector. Eleven different cDNAs were distinguished in our screening of 45 AcNPV-homologous clones. Location of the regions of cDNA homology with respect to the AcNPV physical map showed that the 11 cDNAs were dispersed throughout the genome. The most abundant cDNA insertion, representing approximately one-third of the late viral mRNAs, was homologous to the AcNPV HindIII-P,Q and EcoRI-P fragments. The direction of transcription in this region was from left to right on a linearized AcNPV physical map. Hybridization selection followed by in vitro translation showed that this region encoded a 7,200-dalton (7.2K) protein which comigrated with a minor protein found in the extracellular nonoccluded form of the virus (NOV). Similarly, the gene for polyhedrin, the major structural protein of the occluded virus form, was located, at least in part, in the HindIII-V/EcoRI-I region of the AcNPV map. The polyhedrin transcript represented approximately one-quarter of the viral polyadenylic acid-containing RNAs at 27 h postinfection. Another relatively abundant cDNA was homologous to the HindIII-A/EcoRI-C/SstI-G region, and RNA selected by this cDNA directed the synthesis of two proteins (31K and 30K). The protein products of five other cDNA-selected RNAs were identified. The HindIII-D/EcoRI-O, HindIII-C/EcoRI-D, HindIII-B1/EcoRI-E, and HindIII-B2/EcoRI-H regions of the AcNPV L-1 genome were homologous to RNAs which directed the synthesis of a 57K protein, a 25K protein, a 61K protein, and a 37K protein (plus a minor 26K protein), respectively. Late mRNA selected by a cDNA homologous to the HindIII-P/EcoRI-B region of the AcNPV map directed the synthesis of 31K and 30K proteins which comigrated with the 31K and 30K proteins translated from RNA selected by the HindIII-A/EcoRI-C/SstI-G cDNA. Three other cDNAs have not been correlated yet with specific protein products.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carstens E. B., Tjia S. T., Doerfler W. Infection of Spodoptera frugiperda cells with Autographa californica nuclear polyhedrosis virus I. Synthesis of intracellular proteins after virus infection. Virology. 1979 Dec;99(2):386–398. doi: 10.1016/0042-6822(79)90017-5. [DOI] [PubMed] [Google Scholar]
- Cochran M. A., Carstens E. B., Eaton B. T., Faulkner P. Molecular Cloning and Physical Mapping of Restriction Endonuclease Fragments of Autographa californica Nuclear Polyhedrosis Virus DNA. J Virol. 1982 Mar;41(3):940–946. doi: 10.1128/jvi.41.3.940-946.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., McCarthy B. J., Wadsworth S. C. Sequence organization of two recombinant plasmids containing genes for the major heat shock-induced protein of D. melanogaster. Cell. 1979 Mar;16(3):575–588. doi: 10.1016/0092-8674(79)90031-x. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Bonner J. Preparation, molecular weight, base composition, and secondary structure of giant nuclear ribonucleic acid. Biochemistry. 1973 Jun 5;12(12):2330–2338. doi: 10.1021/bi00736a023. [DOI] [PubMed] [Google Scholar]
- Kahn M., Kolter R., Thomas C., Figurski D., Meyer R., Remaut E., Helinski D. R. Plasmid cloning vehicles derived from plasmids ColE1, F, R6K, and RK2. Methods Enzymol. 1979;68:268–280. doi: 10.1016/0076-6879(79)68019-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee H. H., Miller L. K. Isolation of genotypic variants of Autographa californica nuclear polyhedrosis virus. J Virol. 1978 Sep;27(3):754–767. doi: 10.1128/jvi.27.3.754-767.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley J. L., Sharp P. A., Gefter M. L. RNA synthesis in isolated nuclei: identification and comparison of adenovirus 2 encoded transcripts synthesized in vitro and vivo. J Mol Biol. 1979 Nov 25;135(1):171–197. doi: 10.1016/0022-2836(79)90346-2. [DOI] [PubMed] [Google Scholar]
- McGrogan M., Spector D. J., Goldenberg C. J., Halbert D., Raskas H. J. Purification of specific adenovirus 2 RNAs by preparative hybridization and selective thermal elution. Nucleic Acids Res. 1979 Feb;6(2):593–607. doi: 10.1093/nar/6.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. K. Construction of a Genetic Map of the Baculovirus Autographa californica Nuclear Polyhedrosis Virus by Marker Rescue of Temperature-Sensitive Mutants. J Virol. 1981 Sep;39(3):973–976. doi: 10.1128/jvi.39.3.973-976.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L. K., Dawes K. P. Physical Map of the DNA Genome of Autographa californica Nuclear Polyhedrosis Virus. J Virol. 1979 Mar;29(3):1044–1055. doi: 10.1128/jvi.29.3.1044-1055.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Wu R. Terminal transferase-catalyzed addition of nucleotides to the 3' termini of DNA. Methods Enzymol. 1980;65(1):43–62. doi: 10.1016/s0076-6879(80)65009-5. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Tweeten K. A., Bulla L. A., Consigli R. A. Characterization of an extremely basic protein derived from granulosis virus nucleocapsids. J Virol. 1980 Feb;33(2):866–876. doi: 10.1128/jvi.33.2.866-876.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]









