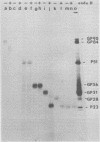
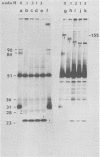
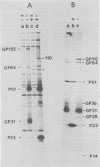
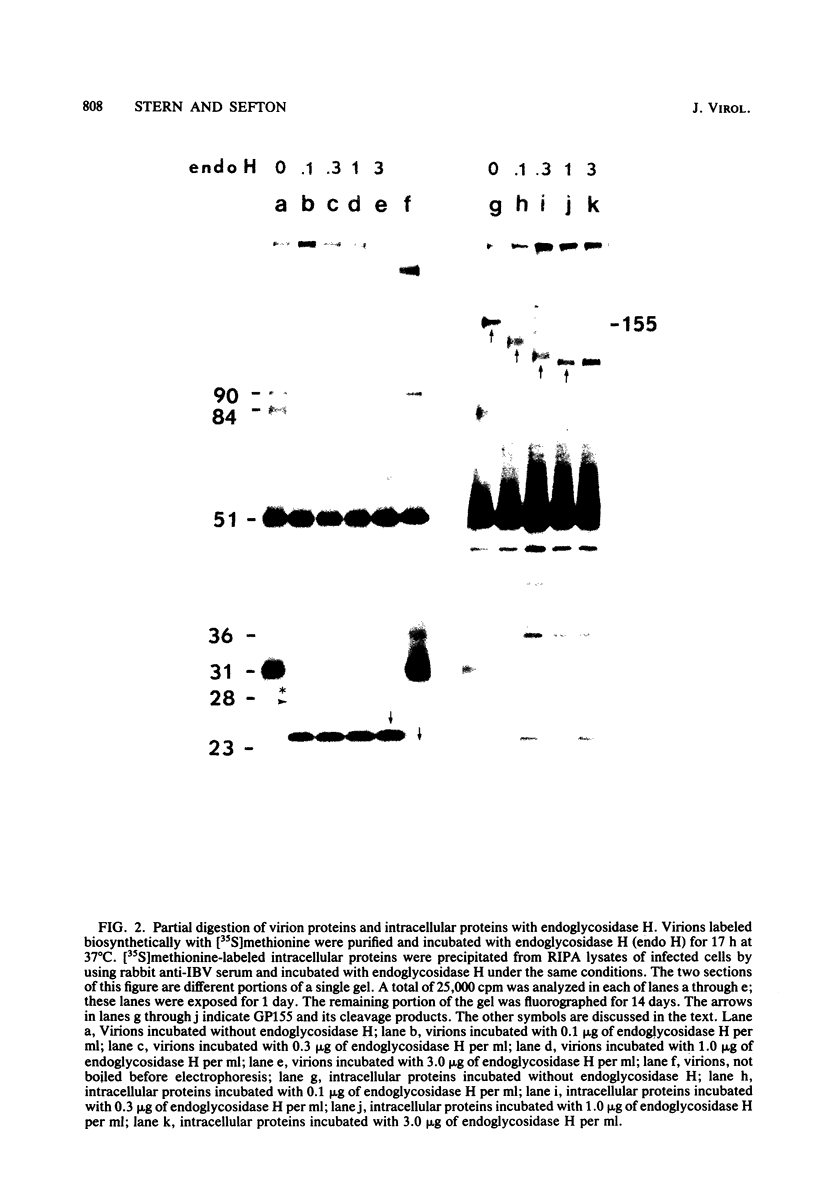
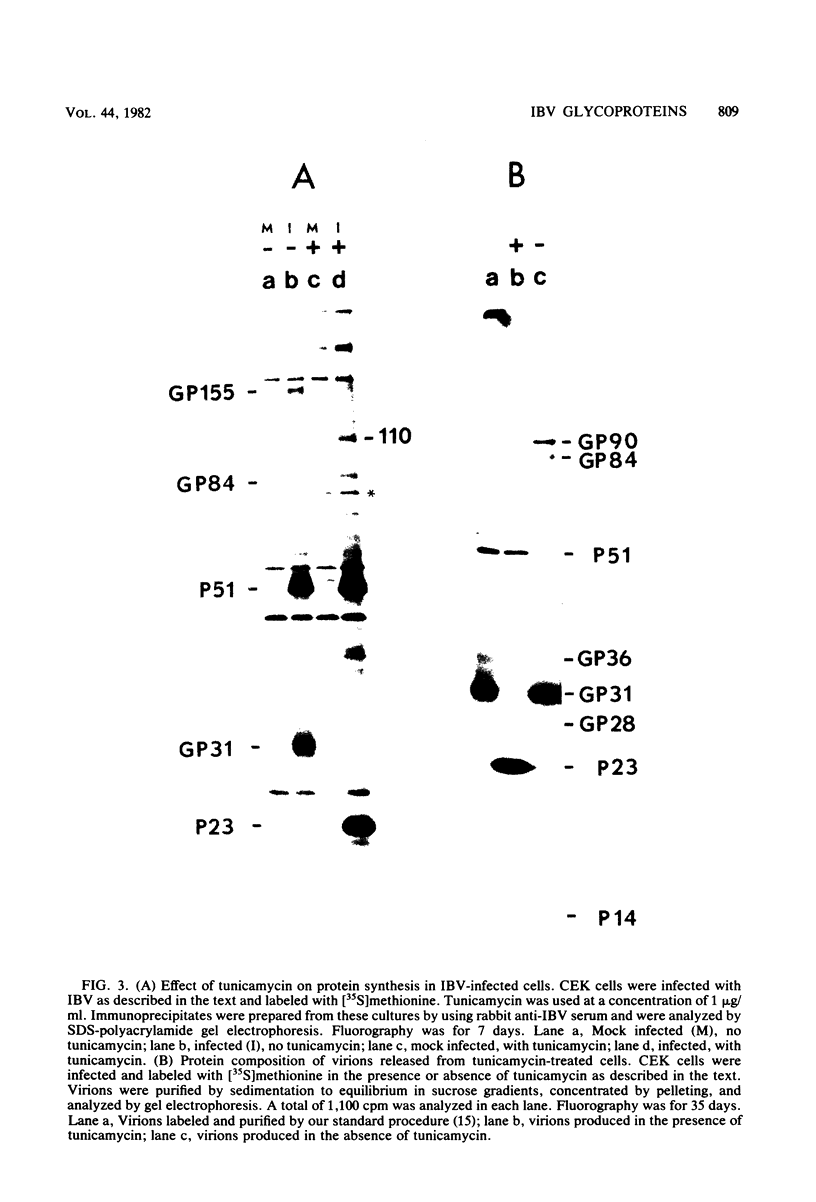
Abstract
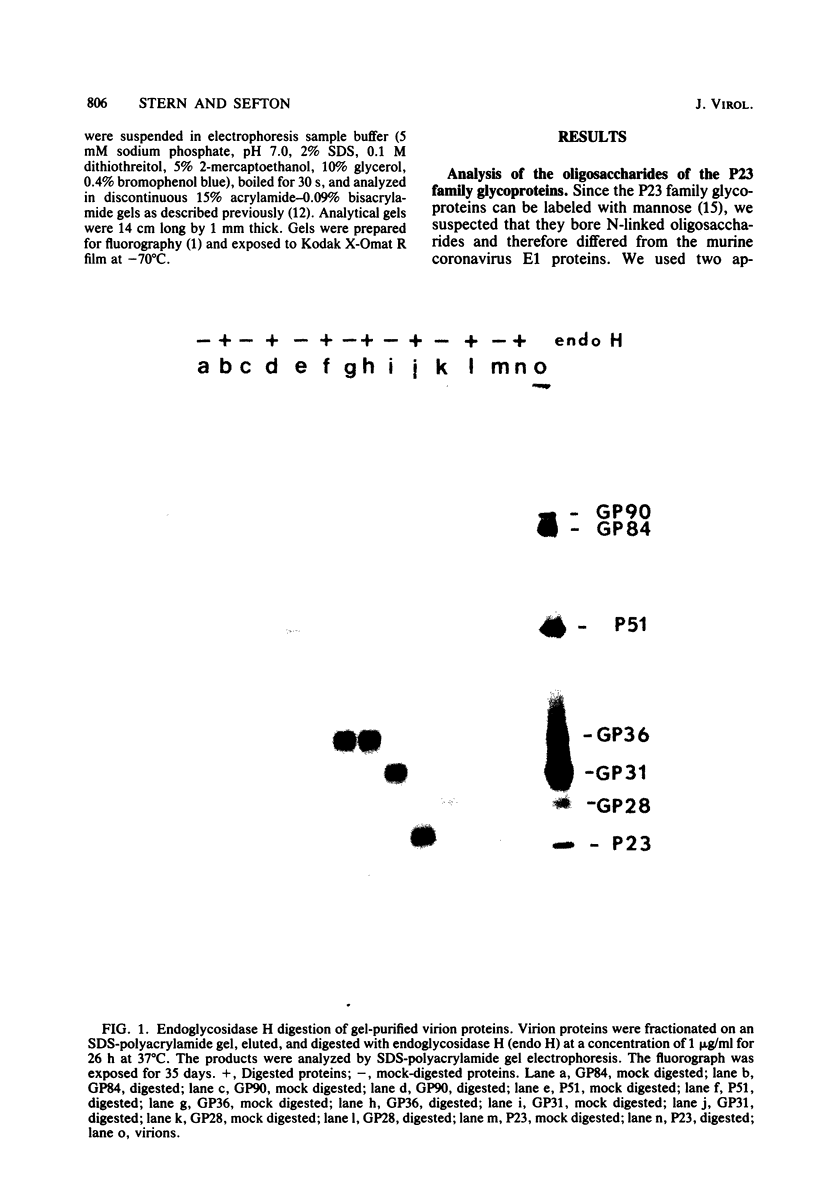
The recent finding that the E1 glycoproteins of murine coronaviruses contain only O-linked oligosaccharides suggested that this unusual modification might be a distinguishing feature of coronaviruses and might play an essential role in the life cycle of this family of viruses. To examine these possibilities, we analyzed the oligosaccharide moieties of the membrane proteins of the avian coronavirus infectious bronchitis virus. In addition, we determined the effect of inhibiting the glycosylation of these proteins on viral maturation and infectivity. Infectious bronchitis virus virions contain nine proteins. Four of these proteins, GP36, GP31, GP28, and P23, are closely related structurally and appear to be homologous to the E1 proteins of murine coronaviruses. We found that the oligosaccharides of GP31 and GP28 could be removed with endoglycosidase H and that neither of these glycoproteins was detectable in tunicamycin-treated cells. These two results indicated that GP31 and GP28 contain N-linked oligosaccharides. Therefore, O-linked oligosaccharides are not a universal feature of the small coronavirus membrane glycoproteins. Tunicamycin inhibited glycosylation of all of the viral glycoproteins but did not inhibit production of virions by infectious bronchitis virus-infected cells. The virions released by these cells contained only the three non-glycosylated viral proteins P51, P23, and P14. These particles were not infectious. Therefore, it appears that glycosylated infectious bronchitis virus polypeptides are not required for particle formation. However, the viral glycoproteins are apparently indispensible for viral infectivity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Structural polypeptides of coronavirus IBV. J Gen Virol. 1981 Mar;53(Pt 1):93–103. doi: 10.1099/0022-1317-53-1-93. [DOI] [PubMed] [Google Scholar]
- Cheley S., Anderson R. Cellular synthesis and modification of murine hepatitis virus polypeptides. J Gen Virol. 1981 Jun;54(Pt 2):301–311. doi: 10.1099/0022-1317-54-2-301. [DOI] [PubMed] [Google Scholar]
- Holmes K. V., Doller E. W., Sturman L. S. Tunicamycin resistant glycosylation of coronavirus glycoprotein: demonstration of a novel type of viral glycoprotein. Virology. 1981 Dec;115(2):334–344. doi: 10.1016/0042-6822(81)90115-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenk H. D., Rott R. Cotranslational and posttranslational processing of viral glycoproteins. Curr Top Microbiol Immunol. 1980;90:19–48. doi: 10.1007/978-3-642-67717-5_2. [DOI] [PubMed] [Google Scholar]
- Niemann H., Klenk H. D. Coronavirus glycoprotein E1, a new type of viral glycoprotein. J Mol Biol. 1981 Dec 25;153(4):993–1010. doi: 10.1016/0022-2836(81)90463-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olden K., Bernard B. A., White S. L., Parent J. B. Function of the carbohydrate moieties of glycoproteins. J Cell Biochem. 1982;18(3):313–335. doi: 10.1002/jcb.1982.240180306. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Rottier P. J., Horzinek M. C., van der Zeijst B. A. Viral protein synthesis in mouse hepatitis virus strain A59-infected cells: effect of tunicamycin. J Virol. 1981 Nov;40(2):350–357. doi: 10.1128/jvi.40.2.350-357.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Beemon K., Hunter T. Comparison of the expression of the src gene of Rous sarcoma virus in vitro and in vivo. J Virol. 1978 Dec;28(3):957–971. doi: 10.1128/jvi.28.3.957-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: intracellular protein synthesis. J Gen Virol. 1981 Mar;53(Pt 1):145–155. doi: 10.1099/0022-1317-53-1-145. [DOI] [PubMed] [Google Scholar]
- Siddell S., Wege H., ter Meulen V. The structure and replication of coronaviruses. Curr Top Microbiol Immunol. 1982;99:131–163. doi: 10.1007/978-3-642-68528-6_4. [DOI] [PubMed] [Google Scholar]
- Stern D. F., Burgess L., Sefton B. M. Structural analysis of virion proteins of the avian coronavirus infectious bronchitis virus. J Virol. 1982 Apr;42(1):208–219. doi: 10.1128/jvi.42.1.208-219.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Kennedy S. I. Coronavirus multiplication strategy. I. Identification and characterization of virus-specified RNA. J Virol. 1980 Jun;34(3):665–674. doi: 10.1128/jvi.34.3.665-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Sefton B. M. Coronavirus proteins: biogenesis of avian infectious bronchitis virus virion proteins. J Virol. 1982 Dec;44(3):794–803. doi: 10.1128/jvi.44.3.794-803.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck D. K., Lennarz W. J. Evidence for the participation of saccharide-lipids in the synthesis of the oligosaccharide chain of ovalbumin. J Biol Chem. 1977 Feb 10;252(3):1007–1013. [PubMed] [Google Scholar]
- Sturman L. S., Holmes K. V. Characterization of coronavirus II. Glycoproteins of the viral envelope: tryptic peptide analysis. Virology. 1977 Apr;77(2):650–660. doi: 10.1016/0042-6822(77)90489-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarentino A. L., Plummer T. H., Jr, Maley F. The release of intact oligosaccharides from specific glycoproteins by endo-beta-N-acetylglucosaminidase H. J Biol Chem. 1974 Feb 10;249(3):818–824. [PubMed] [Google Scholar]
- Tkacz J. S., Lampen O. Tunicamycin inhibition of polyisoprenyl N-acetylglucosaminyl pyrophosphate formation in calf-liver microsomes. Biochem Biophys Res Commun. 1975 Jul 8;65(1):248–257. doi: 10.1016/s0006-291x(75)80086-6. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Wirth D. F. Structure of the murine leukemia virus envelope glycoprotein precursor. J Virol. 1979 Feb;29(2):735–743. doi: 10.1128/jvi.29.2.735-743.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]