Abstract
Caused by a microdeletion at the q11.2 locus of chromosome 22, velo-cardio-facial syndrome (also known as VCFS, 22q11 deletion syndrome, DiGeorge sequence, and conotruncal anomalies face syndrome) is associated with a distinctive physical, neurocognitive and psychiatric phenotype. Increasing interest has centered on identifying the candidate genes within the deleted region that may contribute to this phenotype. One attractive candidate gene is catechol-O-methyltransferase (COMT) because it encodes for a protein that degrades dopamine. COMT activity is variable related to a Val158Met polymorphism that has been implicated in prefrontal lobe cognitive and neuropsychiatric function. We examined the effect of this polymorphism on prefrontal anatomy and frontally-mediated neuropsychological function in 58 children with VCFS, 26 who were hemizygous for the Met allele and 32 for the Val allele. We found an allele by gender interaction effect on the volumes of the dorsal prefrontal and orbital prefrontal cortices. We did not find significant allele or gender by allele effects on neuropsychological tasks, although girls with the Met allele tended to perform better on the Wisconsin Card Sorting Task. These data suggest that this functional COMT polymorphism may play a gender-moderated role in determining the neuroanatomic phenotype of individuals with VCFS. Longitudinal evaluation of these children is essential in order to identify potential clinical implications of this allele by gender interaction.
Keywords: velocardiofacial syndrome (VCFS), 22q11 deletion, COMT polymorphism, prefrontal cortex, executive function
Introduction
Velocardiofacial syndrome, caused by an interstitial microdeletion of more than 40 genes on one copy of chromosome 22, is associated with heart malformations, palate anomalies, learning disabilities and behavioral disorders (Goldberg et al., 1993; Shprintzen et al., 1978). Individuals with VCFS exhibit a distinct neuropsychological and psychiatric phenotype, and frequently display deficits in executive function, working memory, visual-spatial abilities and abstract thinking (Bish et al., 2005; Henry et al., 2002; Simon et al., 2005; Sobin et al., 2004; Van Amelsvoort et al., 2004). Relative to other children with learning / developmental disorders, children with VCFS have significantly higher rates of attention deficit hyperactivity disorder, specific phobias, and in some samples, major depressive disorder (Arnold et al., 2001; Feinstein et al., 2002). Schizophrenia and other psychoses have been reported to be clinical features of VCFS and the rate of psychosis may be as high as 20% - 25%. (Bassett and Chow, 1999; Murphy et al., 1999; Pulver et al., 1994; Shprintzen et al., 1992). Although we do not fully understand the mechanisms that produce this phenotype in VCFS, several of the genes that are located within the 22q11.2 deleted region have been associated with working memory, executive function, and the occurrence of schizophrenia in the general population (Egan et al., 2001; Goldberg et al., 2003; Li et al., 2004; Liu et al., 2002a; Liu et al., 2002b; Meyer-Lindenberg et al., 2005; Shiffman et al., 2004; Shifman et al., 2002).
One gene that has been implicated in neurocognition and psychiatric status is the catechol-O-methyltransferase gene (COMT). This gene encodes for a protein that degrades catecholamines, including dopamine. COMT is located within the 22q11.2 region that is deleted in children with VCFS. One of the polymorphisms of this gene is the Val158Met polymorphism. The activity of the Met allele in the prefrontal cortex is about 40% lower than the activity of the Val allele (Chen et al., 2004), resulting in decreased dopamine degradation and increased availability of dopamine at the synaptic clefts. Presumably due to its expression in the prefrontal cortex (Matsumoto et al., 2003), variation on the Val158Met polymorphism is associated with performance on neuropsychological tasks of working memory and executive function (Diamond et al., 2004; Egan et al., 2001). In addition, several studies have linked COMT haplotypes containing this polymorphism to risk for schizophrenia and bipolar disorder in the general population (Shiffman et al., 2004; Shifman et al., 2002), although these findings are not consistent (Funke et al., 2005) and have been challenged by recent reports (see review, Fan et al., 2005).
Because individuals with VCFS only have one COMT gene, the availability of synaptic dopamine is already compromised. We do not know what effect hemizygosity of the COMT gene has on catecholamine neurotransmission and consequent brain function in individuals with VCFS. There are relatively few published studies of the effect of hemizygosity for the Met or the Val allele on the psychiatric or neuropsychological phenotype in VCFS. Papolous and colleagues (1996) found an association between the Met allele and the presence of bipolar disorder in adolescents and adults with VCFS. Bearden and coworkers, in evaluations of the behavioral and neuropsychological functioning of children and youth with VCFS, found that individuals with the Met allele exhibited fewer behavioral problems and better performance on tasks of executive function (Bearden et al., 2004; Bearden et al., 2005). Based on a longitudinal study of 24 adolescents with VCFS, Gothelf and colleagues (2005) recently identified the Met allele as a risk factor for decline in prefrontal volumes and verbal IQ, and increase in psychotic symptoms. The apparent discrepancy in these findings is probably not surprising given the relatively small impact that a single gene might have on behavior, particularly in a disorder characterized by the microdeletion of multiple genes, several of which may be affecting (in conjunction with environmental factors) cognition and / or psychiatric symptoms. Accordingly, it may be fruitful to investigate endophenotypes (e.g., biologically-driven features that may be more closely associated with genetic variation) (Durston et al., 2005). Neuroanatomy is one such endophenotype. We have previously shown gender differences in volumes of the dorsolateral prefrontal cortex in a relatively small sample of children with VCFS (Kates et al., 2005). However, we did not have COMT genotyping data available for that report.
Here, we compare the effect of variation in the COMT polymorphism on volumes of the dorsal and orbital aspects of the prefrontal cortex in children with VCFS. Based on our previous findings, we explore the effect of gender on the association between COMT and prefrontal volumes. In addition, we explore the effect of this polymorphism on performance on tasks of executive function and working memory, both associated with the dorsolateral prefrontal cortex, and a task of response inhibition, thought to be associated with the orbital prefrontal cortex. We hypothesized that in VCFS, variation in the Val158Met polymorphism will be associated with prefrontal volumes. In light of previously inconclusive findings of an association between genotype and behavior in VCFS, and evidence that behavior may not be as closely associated with genetic variation as neuroanatomy (Hariri & Weinberger, 2003), we hypothesized that variation in the Val158Met polymorphism is not necessarily associated with performance on neurocognitive tasks.
Methods
Participants
The sample of children on whom we currently have genotype data is a subsample of children involved in a longitudinal study of biomarkers for psychosis in VCFS (Antshel et al., 2005b; Kates et al., 2005; Kates et al., in press). The sample for the current report consisted of 26 girls (Mean Age = 11.0, SD = 2.5; range 6 – 15) and 32 boys (Mean Age = 11.1, SD = 2.9; range 6 – 15) with VCFS. Eleven (42%) girls and fifteen (47%) boys were hemizygous for the Met allele. With the exception of two boys who were Asian (one hemizygous for Met, the other for Val) and two boys who were African-American (one hemizygous for Met, the other for Val), all children were Caucasian. Sixteen (28%) of the children were taking psychotropic medications at the time of their participation, primarily stimulants or mood stabilizers (See Table 1 for gender / allele breakdown). Children were recruited from the Center for the Diagnosis, Treatment, and Study of VCFS at SUNY Upstate Medical University. Only children with a Fluorescent In Situ Hybridization (FISH) confirmed deletion in the 22q11.2 region of chromosome 22 were included in the sample. Children with intractable seizure disorder, traumatic brain injury or pre-term birth were excluded from participation. All participants provided informed consent based on guidelines of the SUNY Upstate Institutional Review Board.
Table I.
Background Information by allele and gender
| A (Met Allele) | G (Val Allele) | |||
|---|---|---|---|---|
| Females
(N=11) |
Males
(N=15) |
Females
(N=15) |
Males
(N= 17) |
|
| Age (Mean, S.D.) | 10.7 (3.3) | 10.8 (2.2) | 11.0 (1.8) | 11.2 (3.3) |
| Age Range | 6.4 – 15.1 | 7.4 – 15.8 | 8.9 – 13.7 | 6.2 – 15.9 |
| Full Scale IQ (Mean, S.D.) | 78.8 (11.7) | 67.7 (12.6) | 76.4 (11.1) | 71.2 (12.7) |
| Frequency (%) medication use | 1 (9) | 5 (33) | 3 (20) | 6 (35) |
| Frequency (%) DSM-IV diagnoses | ||||
| Major Depressive Disorder | 0 | 1 ( 7) | 1 (7) | 1 (6) |
| Bipolar Disorder | 1 (9) | 0 | 0 | 1 (6) |
| Generalized Anxiety | 0 | 0 | 1 (7) | 1 (6) |
| ADHD | 2 (18) | 5 (35) | 6 (42) | 4 (24) |
| Simple Phobias | 4 (36) | 1 ( 7) | 2 (14) | 0 |
COMT Genotyping
Samples were genotyped in duplicate by sequencing or the ABI PRISM 5′nuclease assay (TaqMan®). For sequencing, PCR products spanning the Val158Met variant were generated using the primers ValMet-F (5′ ctcatcaccatcgagatcaa) and ValMet-R (5′ gatgaccctggtgatagtgg) as previously described (Lachman et al., 1996). For the ABI 5′ nuclease assay, TaqMan® primers and probes were designed using the Primer Express® Oligo Design software v2.0 (ABI PRISM) or using the ABI Assays-By-Design service. PCR amplification was carried out on 5-20 ng DNA using 1 X TaqMan® universal PCR master mix (No Amp-erase UNG), 900nM forward and reverse primers, 200nM of the FAM labeled probe and 200nM of the VIC labeled probe in a 5ul reaction volume. Amplification conditions on an AB 9700 dual plate thermal cycle (Applied Biosystems, Foster City, CA) were as follows: 1 cycle of 95°C for 10min, followed by 50 cycles of 92°C for 15s and 58°C for 1 min.
MRI Imaging Acquisition and Measurement
Magnetic resonance imaging scans were acquired in the axial plane on a 1.5 T Philips Gyroscan scanner (Philips Medical Systems, Best, The Netherlands), utilizing the following T-1 weighted inversion recovery 3-D pulse sequence: TE = 4.6; TR = 20; 2 repetitions; matrix size 256 X 154; FOV 24; multishot = 32; TFE pre IR shortest (394 ms), 1.5 mm slice thickness. We were unable to acquire scans on 7 of the boys (3 with the Met allele; 4 with the Val allele) due to their relatively young age (all were between the ages of 7 and 9 years) and their inability to lie still in the scanner. (The gender breakdown of children on whom we acquired MRI data is presented in Table 2.)
Table II.
Mean (s.d.) whole brain, total prefrontal, dorsal prefrontal and orbital prefrontal volumes (cc.) by gender and allele
| Allele / Gender | ||||
|---|---|---|---|---|
| A (Met Allele) | G (Val Allele) | |||
| Females
(N=11) |
Males
(N= 12) |
Females
(N= 15) |
Males
(N=13) |
|
| Whole Brain Volume | 1296.24 (120.8) | 1357.81 (124.85) | 1284.01 (142.3) | 1582.38 (134.6) |
| Total Prefrontal | 162.88 (17.7) | 173.02 (27.0) | 159.17 (22.0) | 171.00 (19.1) |
| Dorsal Prefrontal | 132.30 (16.6) | 124.89 (19.1) | 117.11 (18.1) | 137.17 (15.6) |
| Orbital Prefrontal | 30.60 (6.3) | 48.13 (18.8) | 42.05 (12.8) | 33.83 (7.3) |
Raw, formatted image data were transferred from the MRI scanner to Apple Macintosh Power PC workstations via existing network connections. The image data were imported into the program BrainImage (A.L. Reiss, Stanford University, 2002) for removal of non-brain tissue and measurement of whole brain volume. The isolated brain tissue was then transferred to the software program Measure (Barta et al., 1997) for regional volumetric measurement. After aligning the 3D images along the anterior-posterior commissures and interhemispheric fissure, the amygdala, hippocampus, and subregions of the prefrontal cortex were isolated.
The protocol for measuring subregions of the prefrontal cortex, including its dorsal and orbital subregions, was adapted from Gur and coworkers (Gur et al., 2000) and has been described previously (Kates et al., 2005). Interrater reliability, assessed with the intraclass correlation coefficient, exceeded .90. Neuroanatomic raters were blind to COMT status and gender for all volumetric measures.
Cognitive, neuropsychological, and psychiatric assessment
Overall intellectual functioning was assessed with the Wechsler Intelligence Scale for Children, 3rd Edition (Wechsler, 1991). Working memory was assessed with the Visual Span Test (Davis and Keller, 1998). This test is similar to the Corsi Block Tapping Test (Milner, 1971), and consists of an irregular array of squares displayed on the screen; a subset of the blocks are illuminated briefly and the child must reproduce these sequences of increasing length. Scores for forward and backward memory span were obtained. Executive function was assessed with the Wisconsin Card Sorting Test (WCST) (Heaton, 1983). We used the standard scores for total trials to first category and for perseverative errors for the current set of analyses. Sustained attention was assessed with a standardized continuous performance task, the Gordon Diagnostic System (Gordon, 1983; McClure and Gordon, 1985). This task requires the child to respond (press a button) each time a “9” follows a “1” on a computer screen. Our primary outcome variables from the continuous performance task were errors of omission (the child failed to respond when he/she needed to respond), errors of commission (the child responded when he / she should not have responded), and total reaction time (which Stefanis and colleagues [2005] have recently reported vary as a function of COMT status). Psychiatric function was assessed with the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 1997). Data on the WISC-III and the K-SADS-PL for the entire longitudinal study sample (including the children on whom we have genotype data) are reported elsewhere (Antshel et al., in press; Antshel et al., 2005a).
Planned Analyses
Two-factor analyses of variance and covariance were conducted in order to investigate allele and gender effects on brain volumes and on neuropsychological performance. Whole brain volume was entered as a covariate in analyses of brain volumes. Full-scale IQ scores were entered as a covariate in all analyses of neurocognitive performance.
Results
We have examined a total of 58 subjects in this study. Data on age, intellectual function, medication use and psychiatric status are presented in Table 1. Age did not vary as a function of gender or COMT allele. Children who were taking medication did not differ from non-medicated children by either gender or COMT allele (Fisher's Exact Test, p = .14,.77, respectively). Similarly, children with a DSM-IV diagnosis did not differ from those without a DSM-IV diagnosis by either gender or COMT allele (Fisher's Exact Test, p = .58, .19, respectively). The FSIQ of girls with the Met allele was significantly higher than boys with the Met allele (F [1,24] = 5.24; p = .03). No IQ differences were found between girls and boys who were hemizygous for the Val allele.
Effect of gender and allele on brain morphology
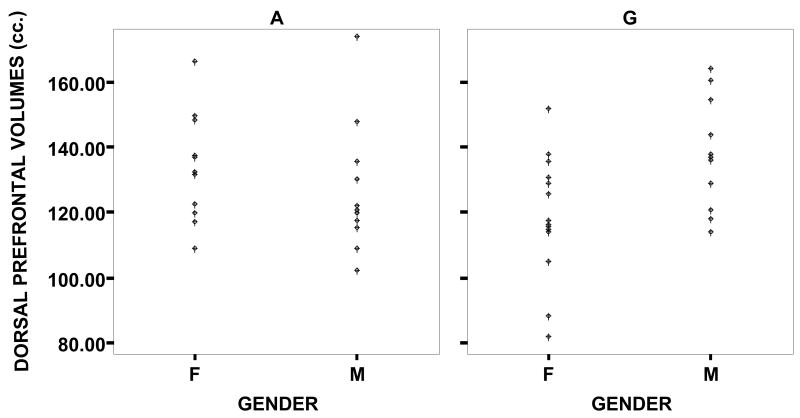
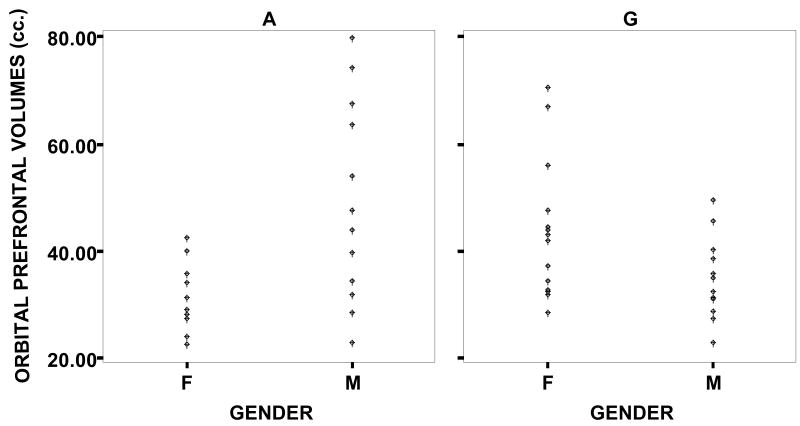
Data on brain morphology from this cohort of patients are presented in Table 2. Whole brain volume was entered as a covariate in all analyses. Analyses of covariance revealed that whole brain volume accounted for a significant portion of the variance, and that neither dorsal nor orbital prefrontal volumes varied as a function of COMT status or gender alone. However, a gender by allele interaction effect was found for both dorsal [F (4,46) = 11.69, p < .001, η2 = .20] and orbital [F (4,46) = 17.26, p < .000, η2 = .27] prefrontal volumes. As shown in Table 2, girls who were hemizygous for the Met allele and boys who were hemizygous for the Val allele exhibited larger dorsal prefrontal volumes, and smaller orbital frontal volumes than girls hemizygous for the Val allele and boys hemizygous for the Met allele. (See Figures 1 and 2.)
Figure 1.
Datapoints of the volumes of the dorsal prefrontal cortex, by allele (A = Met allele / G = Val allele) and gender.
Figure 2.
Datapoints of the volumes of the orbital prefrontal cortex, by allele (A = Met allele / G = Val allele) and gender.
Neither allele nor allele by gender effects were found for volumes of the total prefrontal cortex (derived from the sum of the dorsal and orbital prefrontal volumes) or whole brain volume.
Effect of gender and allele on prefrontal-based neuropsychological tasks
Data on performance on tests of neuropsychological function are presented in Table 3. Since FSIQ is lower in boys than girls in our overall sample (Antshel et al., 2005a), and is lower in boys with the Met allele in this subsample, FSIQ was entered as a covariate in all analyses of neuropsychological function. Neither allele nor allele by gender effects were found for performance on tasks of visual working memory or sustained attention. A gender effect was found for the continuous performance task, with girls making fewer errors of commission than boys [F (3,51) = 4.8; p = .034; η2 = .08], and tending to make less errors of omission as well [F (3,51) = 3.1; p = .08; η2 = .06]. No effects were found for reaction time.
Table III.
Neuropsychological test scores [mean (s.d.)] by gender and allele
| Allele / Gender | ||||
|---|---|---|---|---|
| A (Met Allele) | G (Val Allele) | |||
| Females
(N=11) |
Males
(N= 15) |
Females
(N= 15) |
Males
(N=17) |
|
| Visual Span Test | ||||
| Forward (z-score) | - .54 (.76) | - .73 (1.08) | - .58 ( .84) | -1.21 ( .86) |
| Backward (z-score) | -1.21 (.81) | -1.25 (1.11) | -1.04 (1.12) | -1.29 (1.00) |
| Gordon Diagnostic System | ||||
| Total Correct (z-score) | - .96 (1.81) | -2.44 (3.01) | -.55 (2.02) | -2.91 (4.50) |
| Commissions (z-score) | -1.00 (2.60) | -4.62 (4.24) | -.97 (1.59) | -5.22 (8.36) |
| Latency (z-score) | - .46 ( .96) | - .26 (1.05) | -.17 (1.39) | - .23 ( .79) |
| Wisconsin Card Sorting Task | ||||
| Total Trials to 1st Category | 106.00 (22) | 125.14 (10.69) | 120.80 (14.76) | 125.13 (11.10) |
| Perseverative Errors | 85.82 (23) | 66.14 (10.17) | 76.20 (15.41) | 62.33 (12.33) |
On the Wisconsin Card Sorting Task, an allele by gender interaction effect reached trend level on the total trials to first category measure [F (3,51) = 2.9; p = .09; η2 = .06]. Although boys did not differ in performance on this measure, girls with the Met allele tended to achieve better scores. On the measure of perseverative errors, individuals with the Met allele tended to perform better that those with the Val allele [F (3,51) = 2.9; p = .097; η2 = .05], and girls performed better than boys [F (3,51) = 10.8; p = .002; η2 = .18]. (FSIQ accounted for almost 10% of the variance in this model as well: [F (3,51) = 5.4; p = .02; η2 = .097]). However an allele by gender interaction was not found.
Discussion
We have demonstrated that the COMT Val158Met polymorphism affects prefrontal tissue volumes in a gender-specific manner in children with VCFS. We did not find a genotype effect or a genotype by gender effect on total frontal lobe or whole brain volume, suggesting that this polymorphism affects prefrontal tissue differentially. This is consistent with previous studies that have demonstrated that the COMT gene is expressed preferentially in the prefrontal cortex (Chen et al., 2004; Matsumoto et al., 2003).
Since we did not find allele by gender variation in age, psychiatric comorbidity or medication use, it is unlikely that these factors account for our findings. Nonetheless, the mechanism by which this polymorphism affects brain morphology in VCFS is not clear. Due to the role that COMT plays in neurotransmission, most previous studies of non-VCFS individuals have focused on the effect of the Val158Met polymorphism on prefrontal function rather than anatomy (Diamond et al., 2004; Egan et al., 2001; Goldberg et al., 2003). These studies have found that individuals who are homozygous for the Met allele perform better on functional MRI – mediated tasks of working memory and executive function, which depend primarily on prefrontal function, suggesting that higher levels of available dopamine in the prefrontal cortex permit more efficient neurocognitive function.
A recent study focusing on the effect of this polymorphism on prefrontal anatomy in individuals with schizophrenia did not find an effect of genotype on anatomy (Ho et al., 2005). However, we do not know the effect of hemizygosity in individuals with VCFS. Interestingly, in their longitudinal study of children with VCFS, Gothelf and colleagues (2005) did not find COMT-related differences in prefrontal volume at their Time 1 assessment, but did find reduced prefrontal volumes in individuals hemizygous for the Met allele at Time 2. This supports the notion that the dopaminergic environment in the brains of patients with VCFS influences prefrontal morphology as well as function; however this influence may be moderated by a complex interplay between other genes (Karayiorgou & Gogos, 2004) and environmental factors (Grossman et al., 2003).
Our finding of differential effects of this polymorphism on subregions of the prefrontal cortex was unexpected. Since dorsal PFC and orbital PFC differ in function and in connectivity (Kaufer & Lewis, 1999), it is possible that each subregion requires different levels of dopamine for optimal development. Insofar as the evidence for the expression of COMT in the orbital PFC is less robust than that of the dorsal PFC, alterations in the orbitofrontal cortex may also be due to an interaction between COMT and other genes that are expressed in that subregion, or to a compensatory mechanism in response to COMT-related changes in the dorsal PFC.
The precise mechanism that underlies our findings of region-specific sexual dimorphism is also not clear. Women have been shown to have lower COMT activity than men (Boudikova et al., 1990; Floderus et al., 1981), presumably due to the downregulation of COMT by estradiol (Jiang et al., 2003). Moreover, the COMT Val158Met polymorphism has been reported to differentially affect gender in individuals with schizophrenia (Shifman et al., 2002), anxiety (Enoch et al., 2002) and obsessive compulsive disorder (Alsobrook et al., 2002; Karayiorgou et al., 1999). Gogos and colleagues (1998) further demonstrated sexually dimorphic effects on dopamine levels and behaviors in COMT-deficient mice. Interestingly, sexual dimorphism has also been reported in the volumes of the orbitofrontal cortex in typical individuals and those with schizophrenia (Gur et al., 2002; Gur et al., 2004). In general, these findings, taken together, support but do not explain our findings of gender by allele differences in prefrontal volumes. It is likely that the COMT gene is either in interaction or linkage disequilibrium with other genes to produce this effect. In addition, gender differences in compensatory capacity (Gogos et al., 1998) may be contributing to our findings as well.
Although these findings suggest that COMT and gender affect the morphology of the network of brain regions that are involved in executive function, working memory and response inhibition, we did not find that either allelic variation alone, or an allele by gender interaction, affected performance on our tasks of working memory or sustained attention. We did find that individuals with the Met allele, particularly girls, tended to achieve higher scores on a test of executive function. This finding is somewhat consistent with those of Bearden and colleagues (Bearden et al., 2004), who found that individuals with VCFS who were hemizygous for the Val allele exhibited poorer performance on tests of prefrontal function, although Bearden et al.'s findings were more robust than ours. However, Bearden and coworkers did not find a gender effect. The partial discrepancy in findings could be explained by the differences in study instruments, as well as potential differences in sample homogeneity and demographics (e.g. age). To the extent that our sample was more heterogeneous than Bearden's sample, either according to age or cognitive function, it may have been more difficult to detect a more robust effect of the COMT polymorphism on neuropsychological function.
It is possible (but unlikely) that the absence of a gender by allele effect on most tests of neuropsychological function could have been due to the fact that these tests of executive function have not been normed specifically on children with intellectual disabilities. However, they have been used meaningfully and successfully in studies of individuals with a wide range of disabilities, including fragile X syndrome (Loesch et al., 2003) and autism (Joseph et al., 2005). Moreover, previous studies indicate that IQ accounts for a very modest proportion of the variance in executive function skills (Crinella & Yu, 2000). It is more likely that our findings result from the indirect association between genes and behavior. Whereas brain morphology is an endophenotype (Almasy and Blangero, 2001) that may be affected relatively directly by genotype, it is likely that specific behavioral phenotypes result, instead, from a confluence of multiple genetic and environmental factors. Accordingly, the effect of a specific gene on behavior is likely to be smaller and less direct than its effect on brain morphology.
Despite the indirect effect of genotype on behavioral phenotype, a relationship between COMT and gender has been demonstrated in several disorders, as noted above. COMT continues to be expressed throughout adolescence and adulthood (Maynard et al., 2003), suggesting that this gene may mediate an abnormal neurodevelopmental process that unfolds with age. Since our sample was relatively young (ranging in age from 6 to 15 years), it is possible that the full effect of variation in the COMT gene may not have manifested itself. Accordingly, we may not be able to discern potential clinical implications of this gender by allele interaction until the children in our sample move into late adolescence or early adulthood. This notion is consistent with the study cited above by Gothelf and colleagues (2005), who found that COMT-related differences in cognition and psychiatric symptoms as well as prefrontal volumes changed with age. Accordingly, we will be reassessing these children in three years and may be able to identify at that time whether this interaction confers susceptibility to psychiatric disorder.
Acknowledgments
Grant sponsor: NIMH: MH64824 and MH65481 to Wendy Kates
References
- Almasy L, Blangero J. Endophenotypes as Quantitative Risk Factors for Psychiatric Disease: Rationale and Study Design. Am J of Med Genet (Neuropsychiatr Genet) 2001;105:42–44. [PubMed] [Google Scholar]
- Alsobrook JP, Zohar AH, Leboyer M, Chabane N, Ebstein RP, Pauls DL. Association between the COMT locus and obsessive-compulsive disorder in females but not males. Am J Med Genet. 2002;114:116–120. doi: 10.1002/ajmg.10040. [DOI] [PubMed] [Google Scholar]
- Antshel K, AbdulSabur N, Fremont W, Roizen N, Kates WR. Sex differences in cognitive functioning in velocardiofacial syndrome. Dev Neuropsychol. 2005a;28:849–870. doi: 10.1207/s15326942dn2803_6. [DOI] [PubMed] [Google Scholar]
- Antshel K, Conchelos J, Lanzetta G, Fremont W, Kates WR. Behavior and corpus callosum morphology relationships in velocardiofacial syndrome (22q11.2 deletion syndrome) Psychiatry Res. 2005b;138:235–245. doi: 10.1016/j.pscychresns.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Antshel K, Fremont W, Roizen N, Shprintzen RJ, Higgins AM, Dhamoon A, Kates WR. ADHD and simple phobias are prevalent psychiatric conditions in youth with velocardiofacial syndrome (VCFS) J Am Acad Ch Adol Psych. doi: 10.1097/01.chi.0000205703.25453.5a. in press. [DOI] [PubMed] [Google Scholar]
- Arnold PD, Siegel-Bartelt J, Cytrynbaum C, Teshima I, Schachar R. Velo-Cardio-Facial Syndrome: Implications of Microdeletion 22q11 for Schizophrenia and Mood Disorders. Am J of MedGenet (NeuropsychiatrGenet) 2001;105:354–362. doi: 10.1002/ajmg.1359. [DOI] [PubMed] [Google Scholar]
- Barta P, Dhingra L, Royall R, Schwartz E. Improving stereological estimates for the volume of structures identified in three-dimensional arrays of spatial data. J Neurosci Methods. 1997;75:111–118. doi: 10.1016/s0165-0270(97)00049-6. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Chow EW. 22q11 deletion syndrome: a genetic subtype of schizophrenia. Biol Psychiatry. 1999;46:882–91. doi: 10.1016/s0006-3223(99)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Jawad AF, Lynch DR, Sokol S, Kanes SJ, M M-MD, Saita SC, Harris SE, Moss E, et al. Effects of a functional COMT polymorphism on prefrontal cognitive function in the 22q11.2 deletion syndrome. Am J Psychiatry. 2004;161:1700–1702. doi: 10.1176/appi.ajp.161.9.1700. [DOI] [PubMed] [Google Scholar]
- Bish JP, Ferrante SM, McDonald-McGinn D, Zackai E, Simon T. Maladaptive conflict monitoring as evidence for executive dysfunction in children with chromosome 22q11.2 deletion syndrome. Dev Sci. 2005;8:36–43. doi: 10.1111/j.1467-7687.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- Boudikova B, Szumlanski C, Maidak B, Weinshilboum R. Human liver catechol-O-methyltransferase pharmacogenetics. Clin Pharmacol Ther. 1990;48:381–389. doi: 10.1038/clpt.1990.166. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75:807–821. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinella FM, Yu J. Brain mechanisms and intelligence: Psychometric g and executive function. Intelligence. 2000;27:299–327. [Google Scholar]
- Davis HP, Keller FR. Colorado Assessments Tests. Vol. 1.1. Author; Colorado Springs: 1998. [Google Scholar]
- Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. Am J Psychiatry. 2004;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- Durston S, Fossella JA, Casey BJ, Hulshoff Pol HE, Galvan A, Schnack HG, Steenhuis MP, Minderaa RB, Buitelaar JK, et al. Differential effects of DRD4 and DAT1 genotype on fronto-striatal gray matter volumes in a sample of subjects with attention deficit hyperactivity disorder, their unaffected siblings, and controls. Mol Psychiatry. 2005;10:678–685. doi: 10.1038/sj.mp.4001649. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, Goldman D, Weinberger DR. Effect of COMT Val108/158Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci USA. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch M, Xu K, Ferro E, Harris CR, Goldman D. Genetic origins of anxiety in women: a role for a functional catechol-o-methytransferase polymorphism. Psychiatr Genet. 2002;13:33–41. doi: 10.1097/00041444-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Fan JB, Zhang CS, Gu NF, Li XW, Sun WW, Wang HY, Feng GY, St Clair D, He L. Catechol-O-methyltransferase gene Val/Met functional polymorphism and risk of schizophrenia: a large-scale association study plus meta-analysis. Biol Psych. 2005;57:139–144. doi: 10.1016/j.biopsych.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Feinstein C, Eliez S, Blasey C, Reiss AL. Psychiatric disorders and behavioral problems in children with velocardiofacial syndrome: usefulness as phenotypic indicators of schizophrenia risk. Biological Psychiatry. 2002;15:312–8. doi: 10.1016/s0006-3223(01)01231-8. [DOI] [PubMed] [Google Scholar]
- Floderus Y, Ross SB, Wetterberg L. Erythrocyte catechol-O-methyltransferase activity inn a Swedish population. Clin Genet. 1981;19:389–392. doi: 10.1111/j.1399-0004.1981.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Funke B, Malhotra AK, Finn CT, Plocik Am, Lake SL, Lencz T, DeRosse P, Kane JM, Kucherlapati R. COMT genetic variation confers risk for psychotic and affective disorders: a case control study. Behav Brain Funct. 2005;1:19. doi: 10.1186/1744-9081-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg R, Motzkin B, Marion R, Scambler PJ, Shprintzen RJ. Velo-cardio-facial syndrome: a review of 120 patients. Am J Med Genet. 1993;45:313–319. doi: 10.1002/ajmg.1320450307. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS, Goldman D, Weinberger DR. Executive subprocesses in working memory. Arch Gen Psychiatry. 2003;60:889–896. doi: 10.1001/archpsyc.60.9.889. [DOI] [PubMed] [Google Scholar]
- Gordon M. The Gordon Diagnostic System. Gordon Systems; Dewitt, NY: 1983. [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C, Kwon H, Jin S, Jo B, Antonarakis SE, Morris MA, Reiss AL. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat Neurosci. 2005;8:1500–1502. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- Grossman AW, Churchill JD, McKinney BC, Kodish IM, Otte SL, Greenough WT. Experience effects on brain development: possible contributions to psychopathology. J Child Psychol Psychiatry. 2003;44:33–63. doi: 10.1111/1469-7610.t01-1-00102. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gunning-Dixon F, Bilker WB, Gur RE. Sex differences in temporo-limbic and frontal brain volumes of healthy adults. Cereb Cortex. 2002;12:998–1003. doi: 10.1093/cercor/12.9.998. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell PE, Latshaw A, Turetsky BL, Grossman RI, Arnold SE, Bilker WB, Gur RC. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Genl Psychiatry. 2000;57:761–768. doi: 10.1001/archpsyc.57.8.761. [DOI] [PubMed] [Google Scholar]
- Gur RE, Kohler C, Turetsky BI, Siegel SJ, Kanes SJ, Bilker WB, Brennan AR, Gur RC. A sexually dimorphic ratio of orbitofrontal to amygdala volume is altered in schizophrenia. Biol Psychiatry. 2004;55:512–517. doi: 10.1016/j.biopsych.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Weinberger DR. Imaging Genomics. Br Med Bull. 2003;65:259–270. doi: 10.1093/bmb/65.1.259. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test manual: Revised and expanded. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- Henry JC, van Amelsvoort T, Morris RG, Owen MJ, Murphy DGM, Murphy KC. An investigation of the neuropsychological profile in adults with velo-cardio-facial syndrome (VCFS) Neuropsychologia. 2002;40:471–478. doi: 10.1016/s0028-3932(01)00136-1. [DOI] [PubMed] [Google Scholar]
- Ho BC, Wassink TH, O'Leary DS, Sheffield VC, Andreasen NC. Catechol-O-methyl transferase Val158Met gene polymorphism in schizophrenia: working memory, frontal lobe MRI morphology and frontal cerebral blood flow. Mol Psychiatry. 2005;10:287–98. doi: 10.1038/sj.mp.4001616. [DOI] [PubMed] [Google Scholar]
- Jiang H, Xie T, Ramsden DB, Ho SL. Human catechol-O-methyltransferase down-regulation by estradiol. Neuropharmacology. 2003;45:1011–1019. doi: 10.1016/s0028-3908(03)00286-7. [DOI] [PubMed] [Google Scholar]
- Joseph RM, McGrath LM, Tager-Flusberg H. Executive dysfunction and its relation to language ability in verbal school-age children with autism. Dev Neuropsychol. 27:361–378. doi: 10.1207/s15326942dn2703_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayiorgou M, Gogos JA. The molecular genetics of the 22q11-associated schizopphrenia. Brain Res Mol Brain Res. 2004;132:95–104. doi: 10.1016/j.molbrainres.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Sobin C, Blundell ML, Galke BL, Malinova L, Goldberg P, Ott J, Gogos JA. Family-based association studies support a sexually dimorphic effect of COMT and MAOA on genetic susceptibility to obsessive-compulsive disorder. Biol Psychiatry. 1999;45:1178–1189. doi: 10.1016/s0006-3223(98)00319-9. [DOI] [PubMed] [Google Scholar]
- Kates WR, Antshel K, Willhite R, Bessette BA, AbdulSabur N, Higgins AM. Gender moderated dorsolateral prefrontal reductions in 22q11.2 deletion syndrome: Implications for risk for schizophrenia. Neuropsychol Dev Cogn C Child Neuropsychol. 2005;11:73–85. doi: 10.1080/09297040590911211. [DOI] [PubMed] [Google Scholar]
- Kates WR, Miller AM, AbdulSabur N, Antshel K, Conchelos J, Fremont W, Roizen N. Temporal lobe anatomy and psychiatric symptoms in velocardiofacial syndrome (22q11.2 deletion syndrome) J Am Acad Child Adolesc Psychiatry. doi: 10.1097/01.chi.0000205704.33077.4a. in press. [DOI] [PubMed] [Google Scholar]
- Kaufer DI, Lewis DA. Frontal lobe anatomy and cortical connectivity. In: Miller BL, Cummings JL, editors. The Human Frontal Lobes. New York: Guilford Press; 1999. pp. 27–44. [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for affective disorders and schizophrenia for school-age children present and lifetimeversion (K_SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Morrow B, Shprintzen R, Veit S, Parsia SS, Faedda G, Goldberg R, Kucherlapati R, Papolos DF. Association of Codon 108/158 Catechol-O-Methyltransferase gene polymorphism with the psychiatric manifestations of velo-cardio-facial syndrome. Am J Med Genet. 1996;67:468–472. doi: 10.1002/(SICI)1096-8628(19960920)67:5<468::AID-AJMG5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Li T, Ma X, Sham PC, Sun X, Hu X, Wang Q, Meng H, Deng W, Liu X, et al. Evidence for association between novel polymorphisms in the PRODH gene and schizophrenia in a Chinese population. Am J Med Genet B Neuropsychiatr Genet. 2004;129:13–15. doi: 10.1002/ajmg.b.30049. [DOI] [PubMed] [Google Scholar]
- Liu H, Abecasis G, Heath S, Knowles A, Demars S, Chen Y, Roos J, Rapoport J, Gogs J, et al. Genetic variation in the 2211 locus and susceptibility to schizophrenia. Proc Natl Acad Sci USA. 2002a;99:16859–16864. doi: 10.1073/pnas.232186099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Heath S, Sobin C, Roos J, Galke B, Blundell M, Lenane M, Robertson B, Wijsman E, et al. Genetic variation at the 22q11 PRODH2/DGCR6 locus presents an unusual pattern and increases susceptibility to schizophrenia. Proc Natl Acad Sci USA. 2002b;99:3717–3722. doi: 10.1073/pnas.042700699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Bui QM, Grigsby J, Butler E, Epstein J, Huggins RM, Taylor AK, Hagerman RJ. Effect of the fragile X status categories and the fragile X mental retardation protein levels on executive functioning in males and females with fragile X. Neuropsychology. 2003;17:646–657. doi: 10.1037/0894-4105.17.4.646. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Weickert CS, Beltaifa S, Kolachana B, Chen J, Hyde TM, Herman MM, Weinberger DR, Kleinman JE. Catechol O-methyltransferase (COMT) mRNA expression in the dorsolateral prefrontal cortex of patients with schizophrenia. Neuropsychopharmacology. 2003;28:1521–1530. doi: 10.1038/sj.npp.1300218. [DOI] [PubMed] [Google Scholar]
- Maynard T, Haskell G, Peters A, Sikich L, Lieberman J, LaMantia A. A comprehensive analysis of 22q11 gene expression in the developing and adult brain. Proc Natl Acad Sci USA. 2003;100:14433–14438. doi: 10.1073/pnas.2235651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure FD, Gordon M. Performance of disturbed hyperactive and nonhyperactive children on an objective measure of hyperactivity. J Abnorm Child Psychol. 1985;12:561–572. doi: 10.1007/BF00916850. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn PD, Kolachana B, Kippenhan S, McInerney-Leo A, Nussbaum R, Weinberger DR, Berman KF. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nat Neurosci. 2005;8:594–596. doi: 10.1038/nn1438. [DOI] [PubMed] [Google Scholar]
- Milner B. Interhemispheric differences in the localization of psychological processes in man. British Medical Bulletin. 1971;27:272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- Murphy K, Jones L, Owen M. High rates of schizophrenia in adults with belo-cardio-facial syndrome. Arch Gen Psychiatry. 1999;56:940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Pulver A, Nestadt G, Goldberg R, Shprintzen R, Lamacz M, Wolyniec P, Morrow B, Karayiorgou M, Antonarakis S, et al. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J Nerv Ment Dis. 1994;182:476–478. doi: 10.1097/00005053-199408000-00010. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Bronstein M, Sternfeld M, Pisante A, Weizman A, Reznik I, Spivak B, Grisaru N, Karp L, et al. COMT: a common susceptibility gene in bipolar disorder and schizophrenia. Am J Med Genet. 2004;128B:61–65. doi: 10.1002/ajmg.b.30032. [DOI] [PubMed] [Google Scholar]
- Shifman S, Bronstein M, Sternfeld M, Risante-Shalom A, Lev-Lehman E, Weizman A, Reznik I, Spivak B, Grisaru, et al. A highly significant association between a COMT Haplotype and schizophrenia. Am J Hum Genet. 2002;71:1296–1302. doi: 10.1086/344514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprintzen R, Goldberg R, Golding-Kushner K, Marion R. Late-onset psychosis in the velo-cardio-facial syndrome. Am J Med Genet. 1992;42:141–142. doi: 10.1002/ajmg.1320420131. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ, Goldberg RB, Lewin ML, Sidoti EJ, Berkman MD, Argamaso RV, Young DA. A new syndrome involving cleft palate, cardiac, anomalies, typical facies, and learning disabilities: velo-cardio-facial syndrome. Cleft Palate. 1978;15:56–62. [PubMed] [Google Scholar]
- Simon TJ, Bearden CE, Mc-Ginn DM, Zackai E. Visuospatial and numerical cognitive deficits in children with chromosome 22q11.2 deletion syndrome. Cortex. 2005;41:145–155. doi: 10.1016/s0010-9452(08)70889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Daniels S, Blundell M, Anyane-Yeboa K, Karayiorgou M. Networks of attention in children with the 22q11 deletion syndrome. Dev Neuropsychol. 2004;26:611–626. doi: 10.1207/s15326942dn2602_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanis NC, van Os J, Avramopoulos D, Smyrnis N, Evdokimidis I, Stefanis CN. Effect of COMT Val158Met polymorphism on the Continuous Performance Test, Identical Pairs Version: tuning rather than improving performance. Am J Psych. 2005;162:1752–1754. doi: 10.1176/appi.ajp.162.9.1752. [DOI] [PubMed] [Google Scholar]
- Van Amelsvoort T, Henry J, Morris R, Owen M, Linszen D, Murphy K, Murphy D. Cognitive deficits associated with schizophrenia in velo-cardio-facial syndrome. Schizophr Res. 2004;70:223–232. doi: 10.1016/j.schres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children-Third Edition. The Psychological Corporation; San Antonio: 1991. [Google Scholar]