Abstract
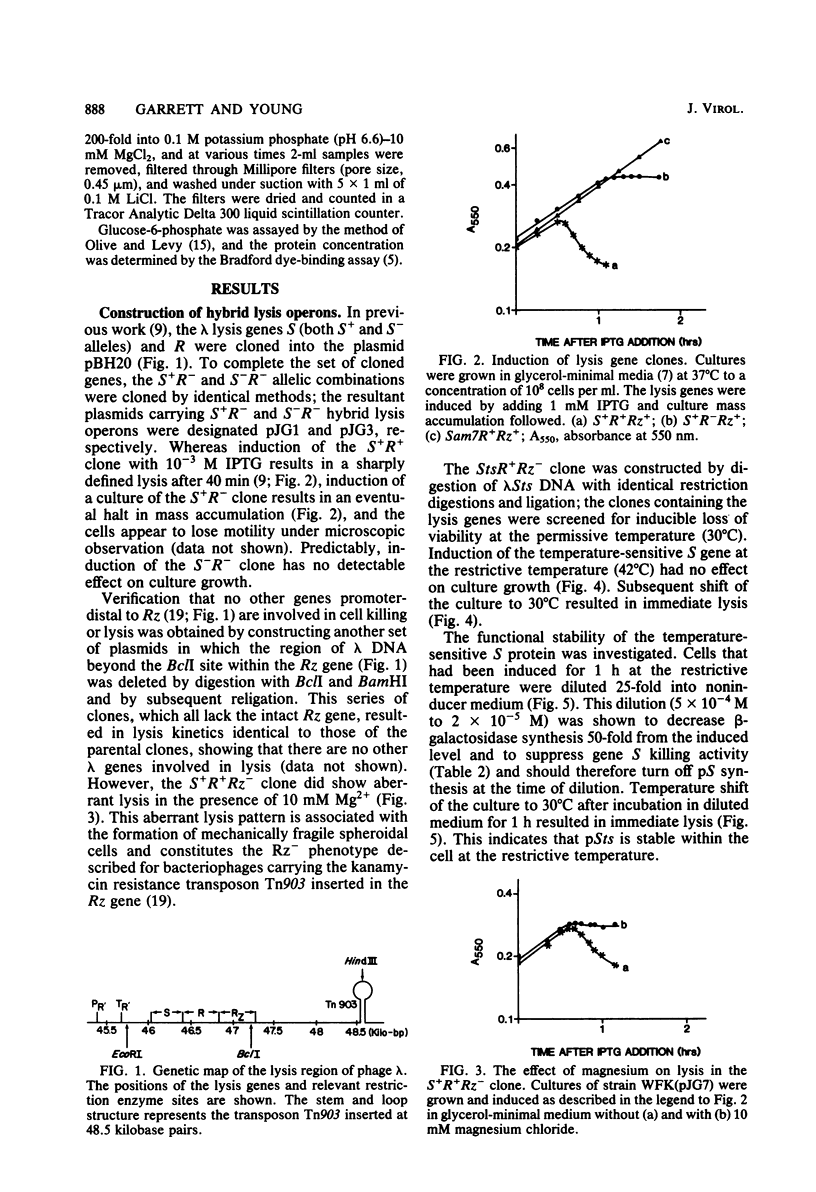
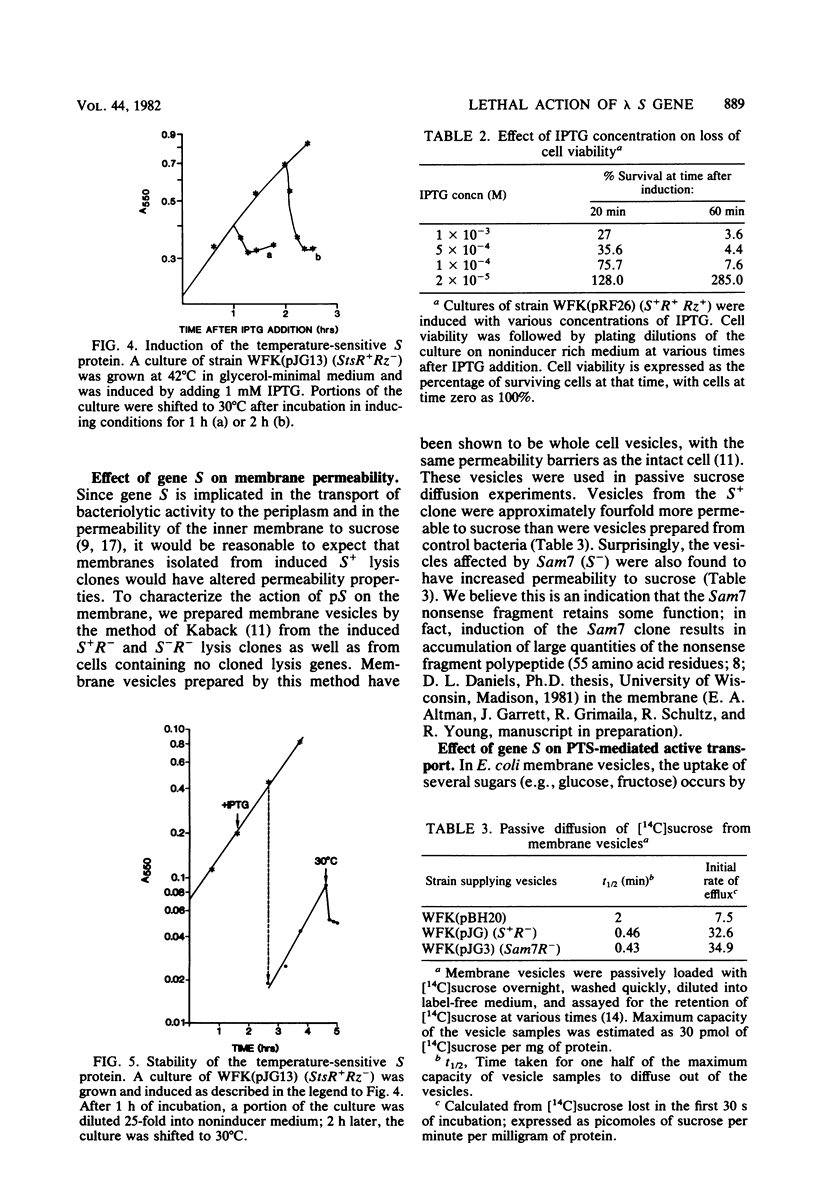
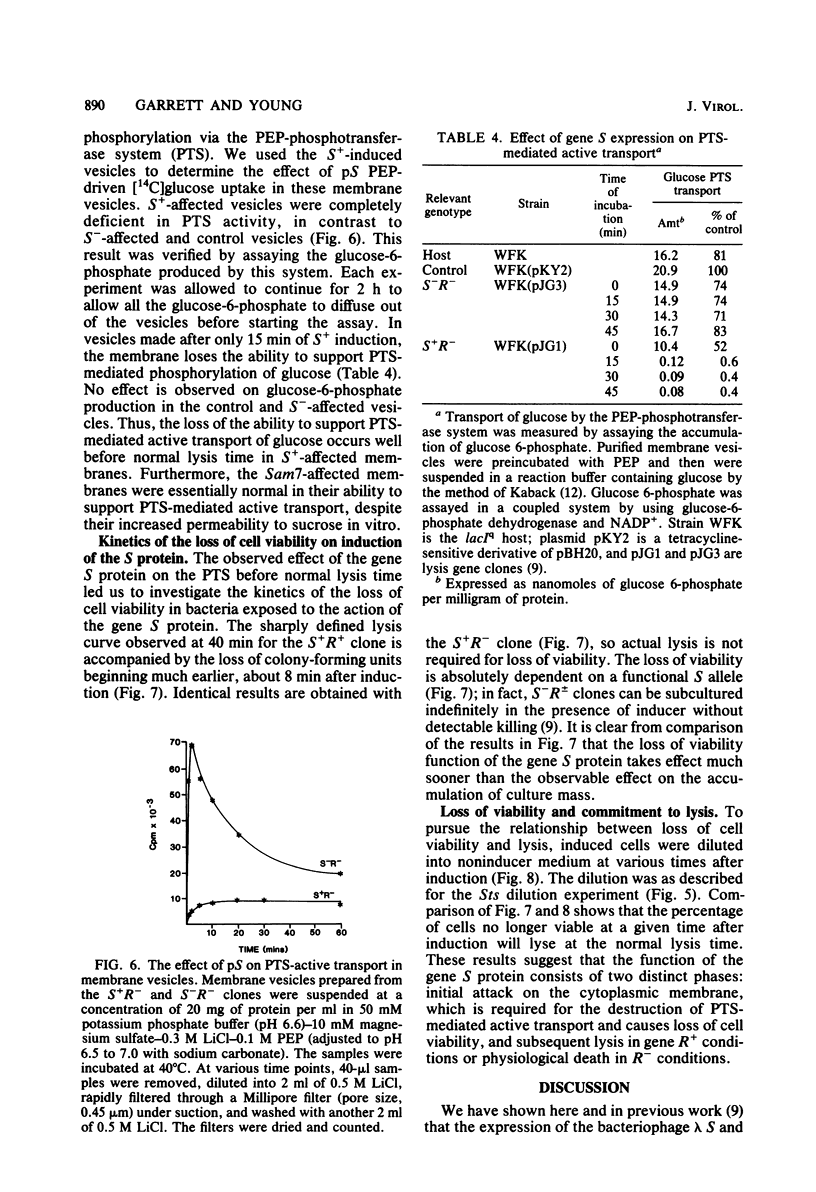
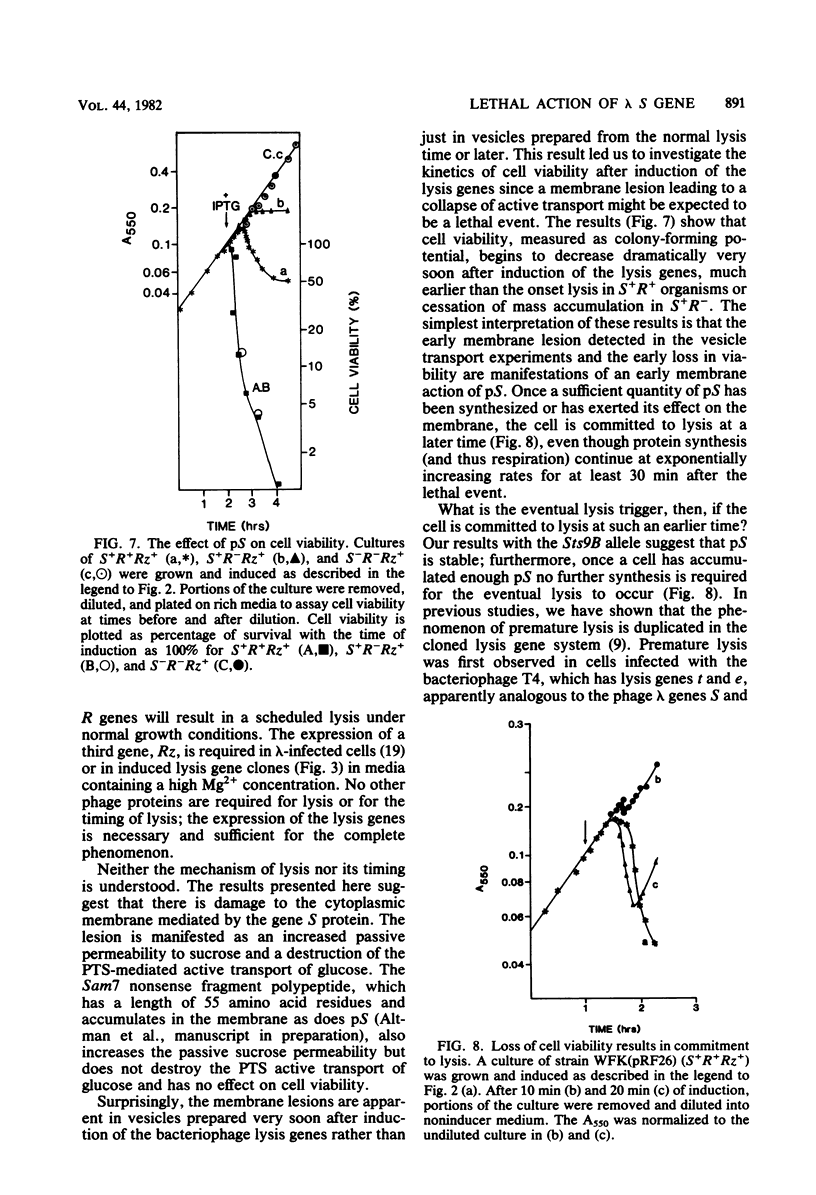
The functions of the bacteriophage lambda lysis genes S, R, and Rz were investigated. Different combinations of wild-type and inactive alleles of all three lysis genes were cloned into the plasmid pBH20 and were expressed under the control of a lac operator-promoter. The involvement of the Rz gene in lysis was proposed in our previous work and was confirmed by the Mg2+-dependent lysis defect of clones in which part of the Rz gene is deleted. Membrane vesicles prepared from induced S+ cells were shown to have a severely reduced capacity for active transport of glucose; this defect was detectable at least 20 min before lysis. Cell viability was also shown to decrease very soon after induction, long before physiological death and lysis; this decrease in viability is absolutely dependent on S expression and independent of R and Rz. The nonviable fraction of cells at any time after induction was demonstrated to be equal to the fraction committed to eventual lysis. Induction of an Sts clone showed that the S gene product is stable and capable of inducing lysis long after the cessation of synthesis of S gene product. A model for S action is proposed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienkowska-Szewczyk K., Lipinska B., Taylor A. The R gene product of bacteriophage lambda is the murein transglycosylase. Mol Gen Genet. 1981;184(1):111–114. doi: 10.1007/BF00271205. [DOI] [PubMed] [Google Scholar]
- Bieńkowska-Szewczyk K., Taylor A. Murein transglycosylase from phage lambda lysate. Purification and properties. Biochim Biophys Acta. 1980 Oct;615(2):489–496. doi: 10.1016/0005-2744(80)90515-x. [DOI] [PubMed] [Google Scholar]
- Black L. W., Hogness D. S. The lysozyme of bacteriophage lambda. I. Purification and molecular weight. J Biol Chem. 1969 Apr 25;244(8):1968–1975. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campbell J. H., Rolfe B. G. Evidence for a dual control of the initiation of host-cell lysis caused by phage lambda. Mol Gen Genet. 1975 Aug 5;139(1):1–8. doi: 10.1007/BF00267990. [DOI] [PubMed] [Google Scholar]
- Daniels D. L., Blattner F. R. Nucleotide sequence of the Q gene and the Q to S intergenic region of bacteriophage lambda. Virology. 1982 Feb;117(1):81–92. doi: 10.1016/0042-6822(82)90509-8. [DOI] [PubMed] [Google Scholar]
- Garrett J., Fusselman R., Hise J., Chiou L., Smith-Grillo D., Schulz J., Young R. Cell lysis by induction of cloned lambda lysis genes. Mol Gen Genet. 1981;182(2):326–331. doi: 10.1007/BF00269678. [DOI] [PubMed] [Google Scholar]
- Itakura K., Hirose T., Crea R., Riggs A. D., Heyneker H. L., Bolivar F., Boyer H. W. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science. 1977 Dec 9;198(4321):1056–1063. doi: 10.1126/science.412251. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport in isolated bacterial membrane vesicles. Methods Enzymol. 1974;31:698–709. doi: 10.1016/0076-6879(74)31075-0. [DOI] [PubMed] [Google Scholar]
- Morrison D. A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- Mukherjee P. K., Mandal R. K. Role of S gene of bacteriophage lambda in host lysis. Biochem Biophys Res Commun. 1976 May 3;70(1):302–309. doi: 10.1016/0006-291x(76)91142-6. [DOI] [PubMed] [Google Scholar]
- Olive C., Levy H. R. The preparation and some properties of crystalline glucose 6-phosphate dehydrogenase from Leuconostoc mesenteroides. Biochemistry. 1967 Mar;6(3):730–736. doi: 10.1021/bi00855a012. [DOI] [PubMed] [Google Scholar]
- Reader R. W., Siminovitch L. Lysis defective mutants of bacteriophage lambda: genetics and physiology of S cistron mutants. Virology. 1971 Mar;43(3):607–622. doi: 10.1016/0042-6822(71)90286-8. [DOI] [PubMed] [Google Scholar]
- Reader R. W., Siminovitch L. Lysis defective mutants of bacteriophage lambda: on the role of the S function in lysis. Virology. 1971 Mar;43(3):623–637. doi: 10.1016/0042-6822(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Taylor A. Endopeptidase activity of phage lamba-endolysin. Nat New Biol. 1971 Dec 1;234(48):144–145. doi: 10.1038/newbio234144a0. [DOI] [PubMed] [Google Scholar]
- Young R., Way J., Way S., Yin J., Syvanen M. Transposition mutagenesis of bacteriophage lambda: a new gene affecting cell lysis. J Mol Biol. 1979 Aug 15;132(3):307–322. doi: 10.1016/0022-2836(79)90262-6. [DOI] [PubMed] [Google Scholar]



