Abstract
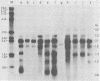
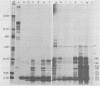
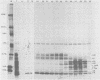
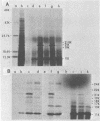
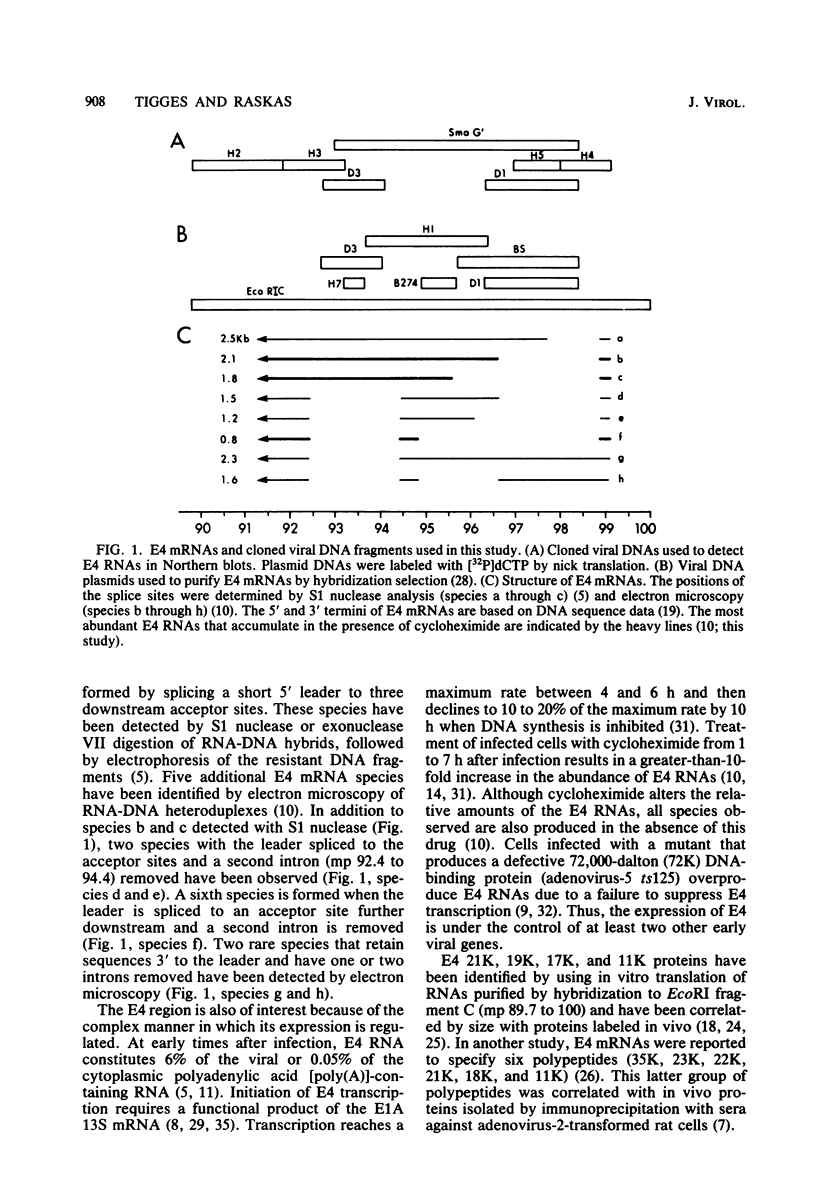
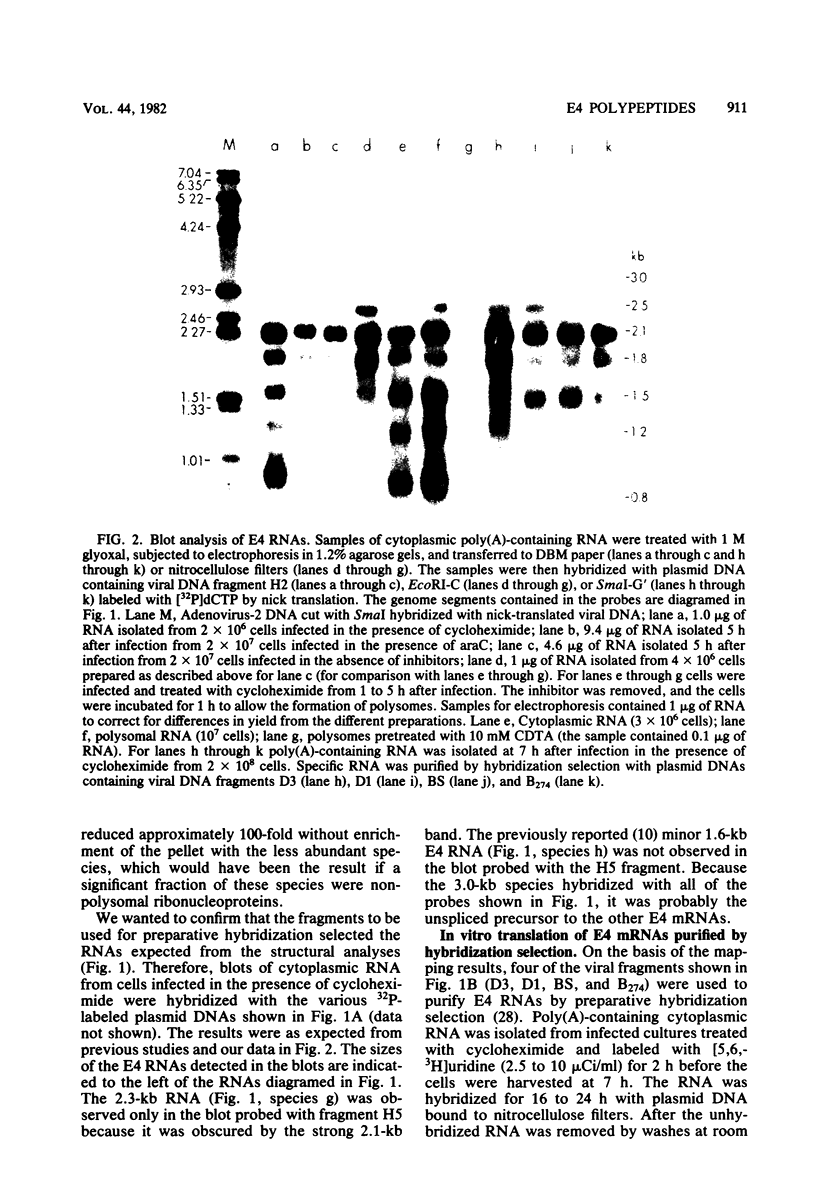
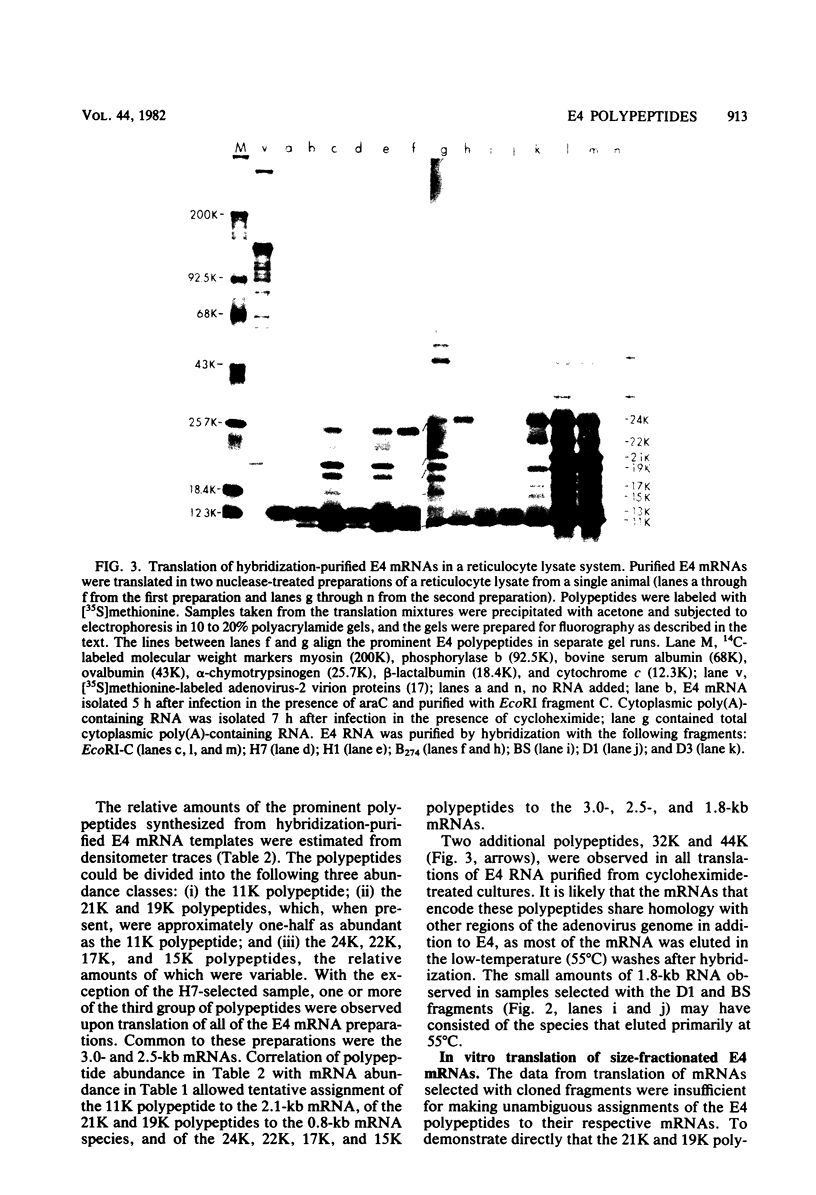
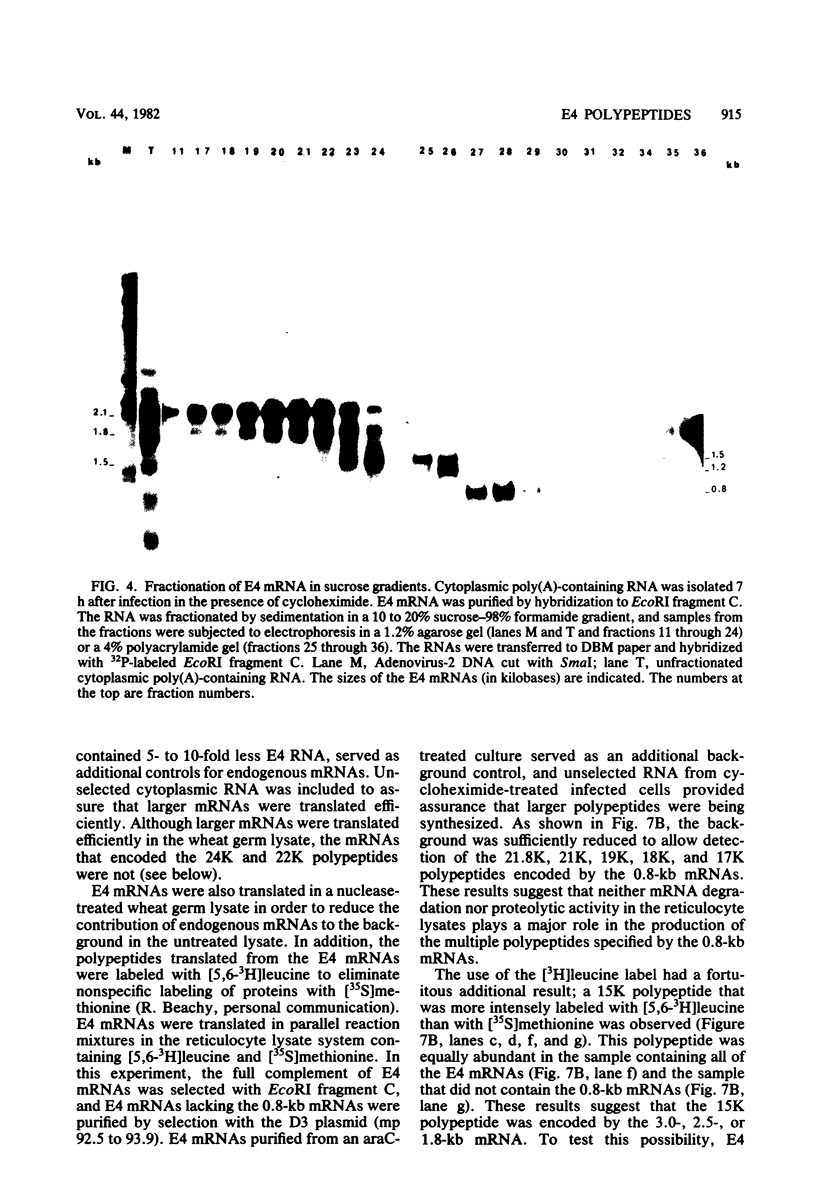
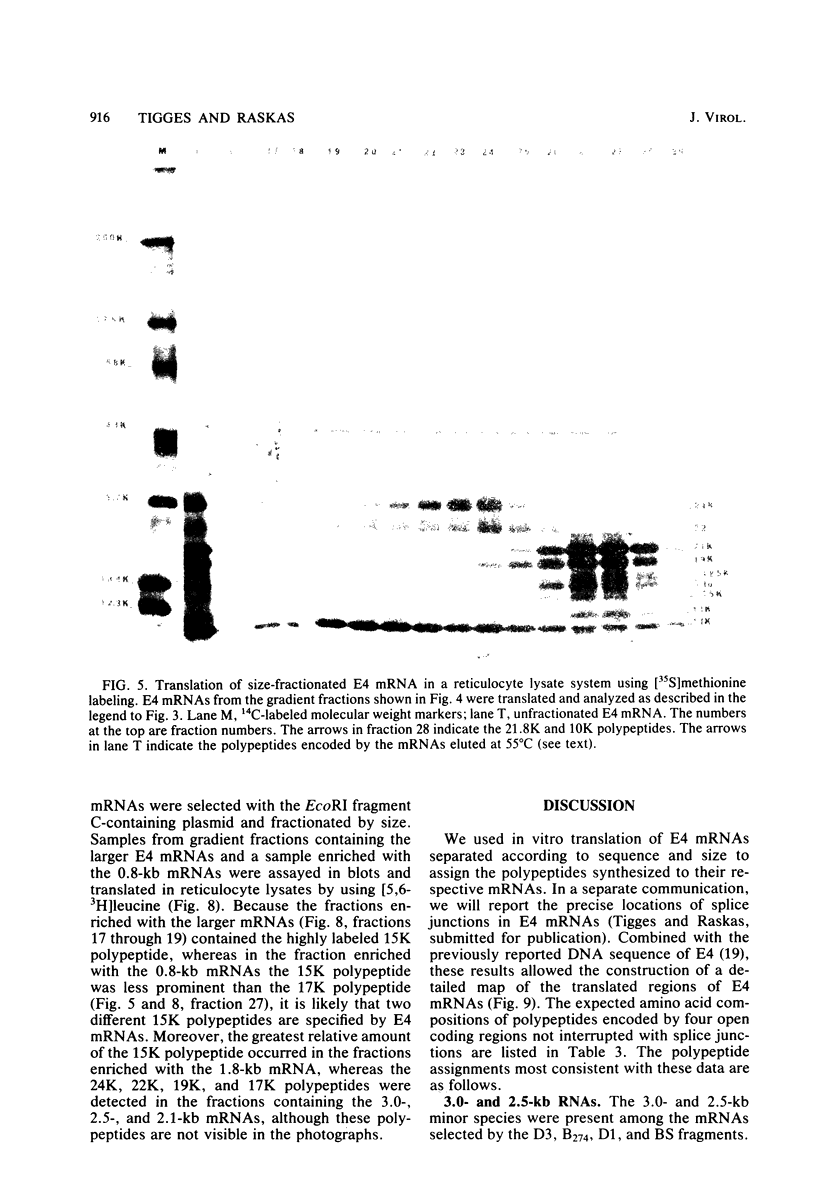
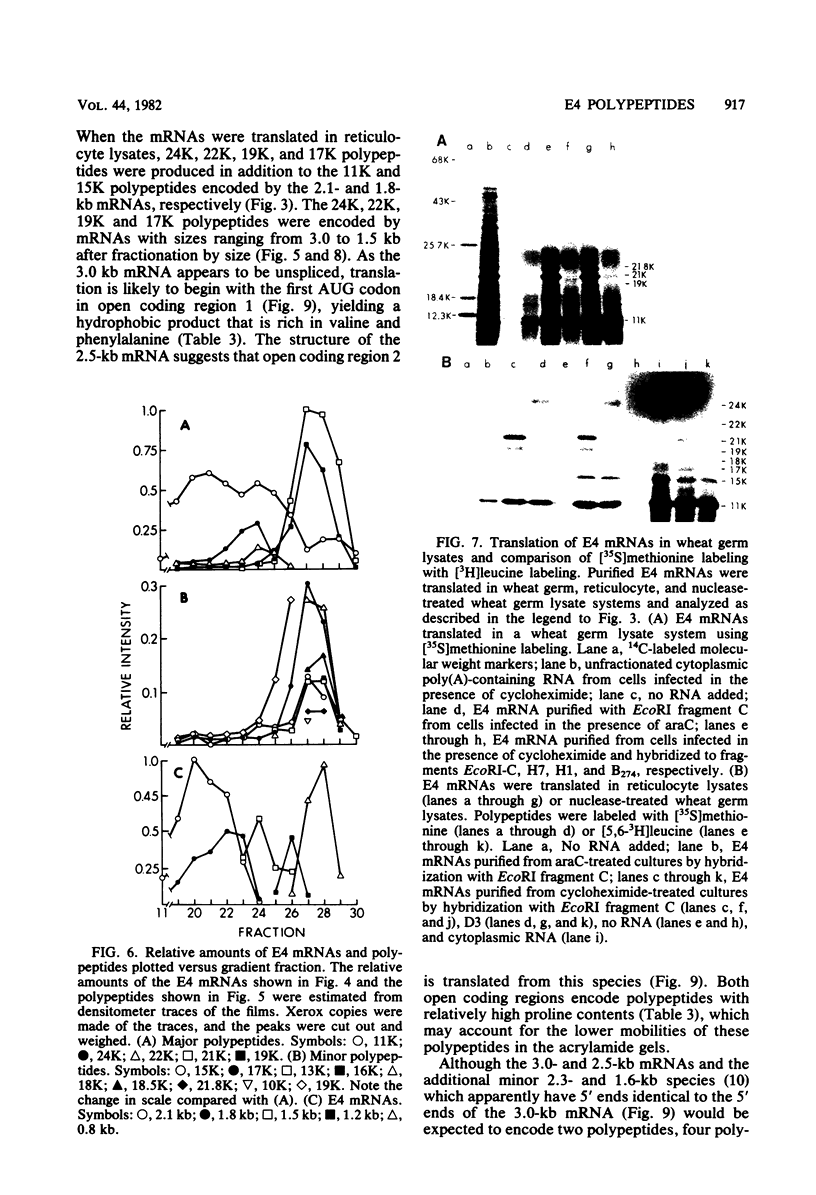
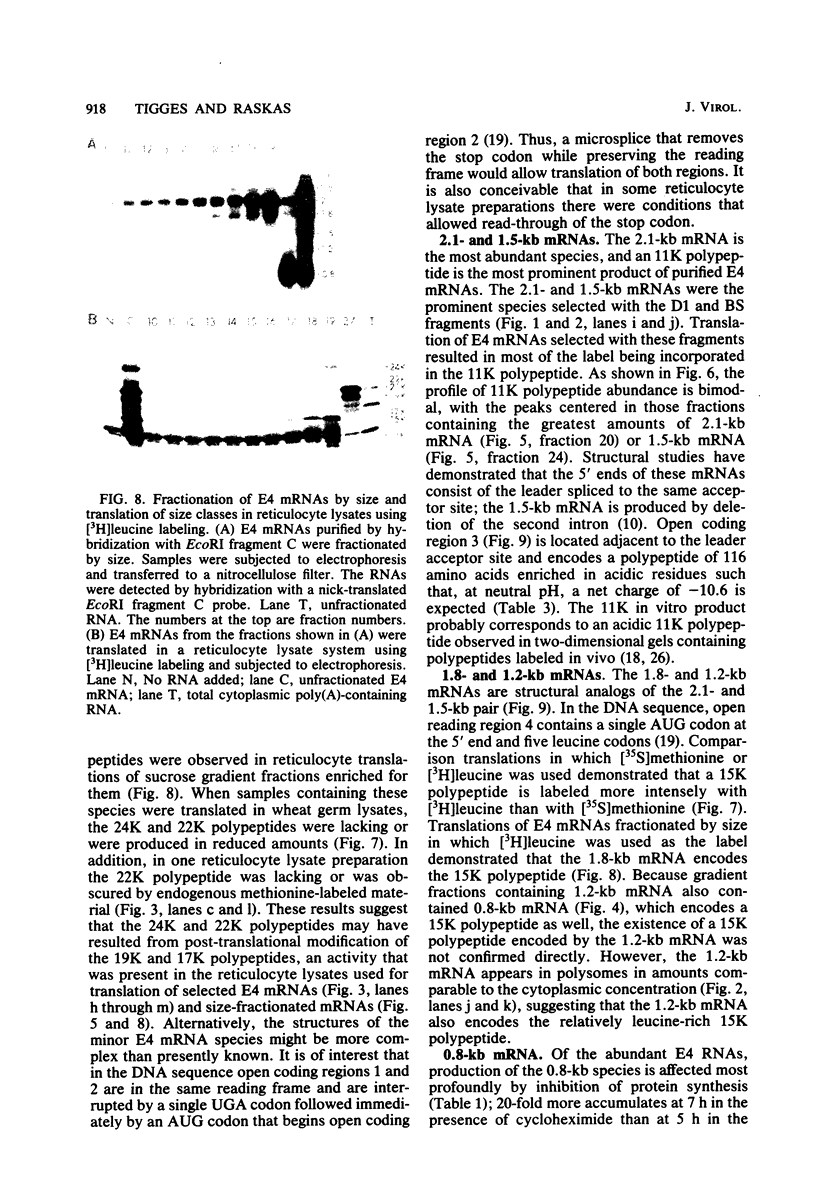
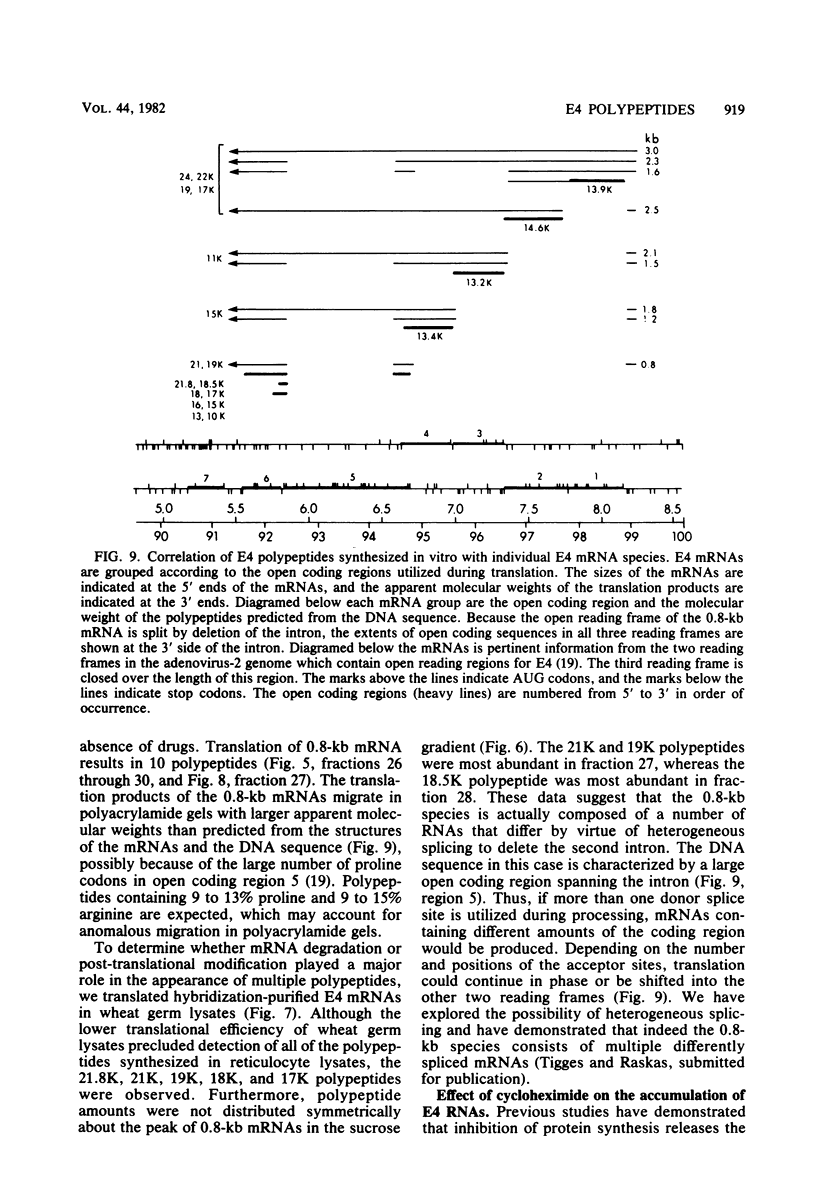
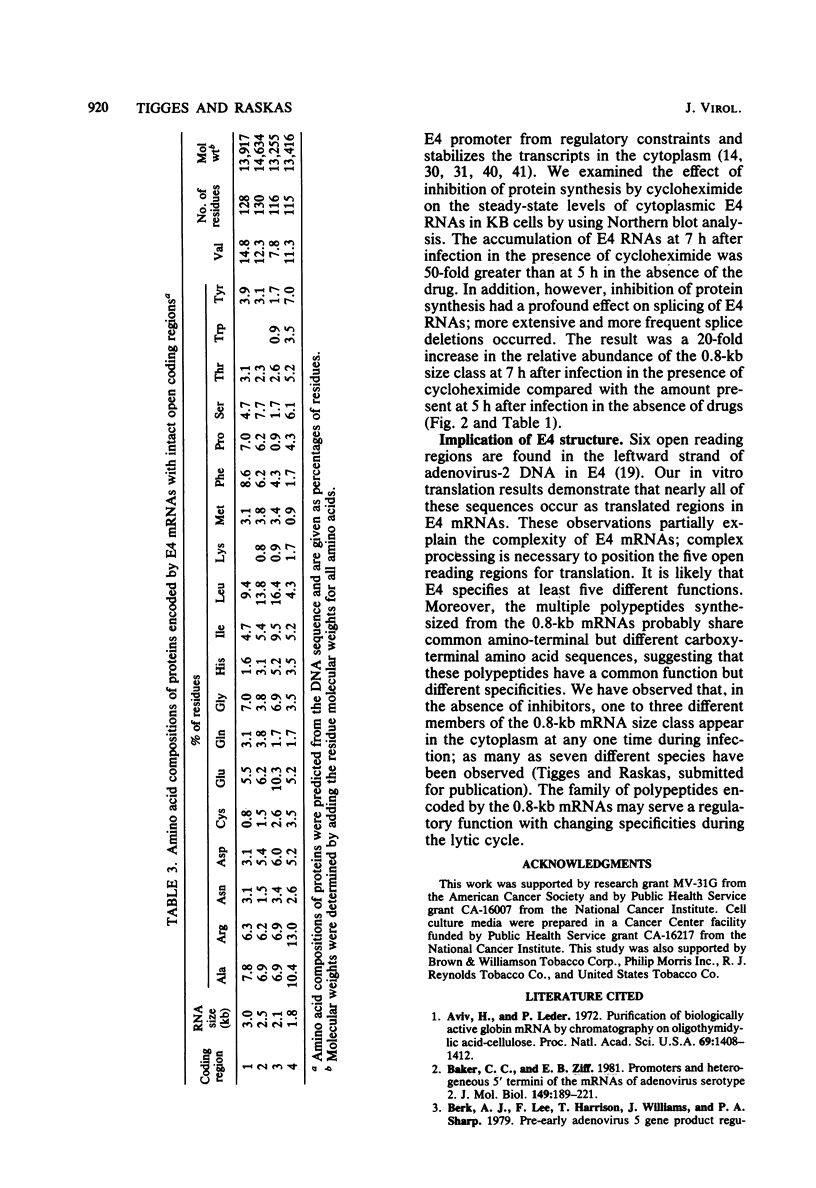
Adenovirus-2 early region 4 (E4; map positions 91.3 to 99.1) encodes six 5' and 3' coterminal, differently spliced mRNAs, which are 2.5, 2.1, 1.8, 1.5, 1.2, and 0.8 kilobases (kb) long. Hybridization selection with five cloned viral DNA fragments that hybridize with subsets of E4 mRNAs was used to purify these six mRNAs and a previously unreported 3.0-kb mRNA from virus-infected cells. E4 mRNAs which were purified by hybridization selection with cloned EcoRI fragment C (map positions 89.7 to 100) were also fractionated by size. The purified mRNAs were then translated in rabbit reticulocyte or wheat germ lysate systems. The full complement of E4 mRNAs specified as many as 16 different polypeptides, with molecular weights ranging from 24,000 (24K) to 10K. The most abundant E4 mRNA, which was 2.1 kb long, specified an 11K polypeptide. The 1.5-kb mRNA, which differed from the 2.1-kb mRNA only by deletion of a second intron from the 3' untranslated region, also specified an 11K polypeptide. The second most abundant mRNA, which was 1.8 kb long, and the 1.2-kb mRNA, which had an intron deleted from the 3' untranslated region, specified a 15K polypeptide. This polypeptide was labeled more intensely with [5,6-(3)H]leucine than with [35S]methionine. The 3.0- and 2.5-kb mRNAs specified four polypeptides (24K, 22K, 19K, and 17K). Translation of E4 mRNAs with a mean size of 0.8 kb, which accumulated preferentially in the presence of cycloheximide, yielded at least 10 polypeptides that migrated in polyacrylamide gels with apparent molecular weights ranging from 21,800 to 10,000. On the basis of translation in wheat germ lysates and the distribution of polypeptides encoded by size-fractionated mRNAs, we concluded that the 0.8-kb mRNA size class includes a heterogeneous mixture of mRNAs which are probably formed as the result of utilization of alternate splice acceptor and donor sites during removal of the second intron. Our polypeptide assignments for the 2.1-, 1.8-, 1.5-, and 1.2-kb mRNAs are compatible with locations of two open coding regions in the DNA sequence (Herisse et al., Nucleic Acids Res. 9:4023-4042, 1981). The relationship between the four polypeptides encoded by the 3.0- and 2.5-kb mRNAs and the two open coding regions is discussed. The production of multiple polypeptides from a heterogenous mixture of mRNAs in the 0.8-kb size class is compatible with two large open coding regions in that part of the sequence. Thus, nearly all of the potential coding information in the leftward strand of E4 is expressed in translatable form during infection. Moreover, alternate splicing of the 0.8-kb mRNA size class can produce multiple polypeptides with common amino-terminal and different carboxy-terminal amino acid sequences, which may have the same function but different specificities.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. C., Ziff E. B. Promoters and heterogeneous 5' termini of the messenger RNAs of adenovirus serotype 2. J Mol Biol. 1981 Jun 25;149(2):189–221. doi: 10.1016/0022-2836(81)90298-9. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Lee F., Harrison T., Williams J., Sharp P. A. Pre-early adenovirus 5 gene product regulates synthesis of early viral messenger RNAs. Cell. 1979 Aug;17(4):935–944. doi: 10.1016/0092-8674(79)90333-7. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Structure of the adenovirus 2 early mRNAs. Cell. 1978 Jul;14(3):695–711. doi: 10.1016/0092-8674(78)90252-0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Ultraviolet mapping of the adenovirus 2 early promoters. Cell. 1977 Sep;12(1):45–55. doi: 10.1016/0092-8674(77)90184-2. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Brackmann K. H., Green M., Wold W. S., Cartas M., Matsuo T., Hashimoto S. Identification and peptide mapping of human adenovirus type 2-induced early polypeptides isolated by two-dimensional gel electrophoresis and immunoprecipitation. J Biol Chem. 1980 Jul 25;255(14):6772–6779. [PubMed] [Google Scholar]
- Carlock L. R., Jones N. C. Transformation-defective mutant of adenovirus type 5 containing a single altered E1a mRNA species. J Virol. 1981 Dec;40(3):657–664. doi: 10.1128/jvi.40.3.657-664.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. H., Blanton R. A. Possible role of the 72,000 dalton DNA-binding protein in regulation of adenovirus type 5 early gene expression. J Virol. 1978 Feb;25(2):664–674. doi: 10.1128/jvi.25.2.664-674.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Broker T. R., Lewis J. B. Complex splicing patterns of RNAs from the early regions of adenovirus-2. J Mol Biol. 1979 Oct 25;134(2):265–303. doi: 10.1016/0022-2836(79)90036-6. [DOI] [PubMed] [Google Scholar]
- Craig E. A. Analysis of early adenovirus 2 RNA using Eco R-R1 viral DNA fragments. J Virol. 1975 May;15(5):1202–1213. doi: 10.1128/jvi.15.5.1202-1213.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Effect of cycloheximide on RNA metabolism early in productive infection with adenovirus 2. J Virol. 1974 Jul;14(1):26–32. doi: 10.1128/jvi.14.1.26-32.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A., Raskas H. J. Nuclear transcripts larger than the cytoplasmic mRNAs are specified by segments of the adenovirus genome coding for early functions. Cell. 1976 Jun;8(2):205–213. doi: 10.1016/0092-8674(76)90004-0. [DOI] [PubMed] [Google Scholar]
- Eggerding F., Raskas H. J. Effect of protein synthesis inhibitors on viral mRNA's synthesized early in adenovirus type 2 infection. J Virol. 1978 Jan;25(1):453–458. doi: 10.1128/jvi.25.1.453-458.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R. C. Biochemical studies on adenovirus multiplication. XII. Plaquing efficiencies of purified human adenoviruses. Virology. 1967 Mar;31(3):562–565. doi: 10.1016/0042-6822(67)90241-3. [DOI] [PubMed] [Google Scholar]
- Halbert D. N., Spector D. J., Raskas H. J. In vitro translation products specified by the transforming region of adenovirus type 2. J Virol. 1979 Sep;31(3):621–629. doi: 10.1128/jvi.31.3.621-629.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter M. L., Lewis J. B. Adenovirus type 2 early proteins synthesized in vitro and in vivo: identification in infected cells of the 38,000- to 50,000- molecular-weight protein encoded by the left end of the adenovirus type 2 genome. J Virol. 1978 Jun;26(3):736–749. doi: 10.1128/jvi.26.3.736-749.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Bonner J. Preparation, molecular weight, base composition, and secondary structure of giant nuclear ribonucleic acid. Biochemistry. 1973 Jun 5;12(12):2330–2338. doi: 10.1021/bi00736a023. [DOI] [PubMed] [Google Scholar]
- Hérissé J., Rigolet M., de Dinechin S. D., Galibert F. Nucleotide sequence of adenovirus 2 DNA fragment encoding for the carboxylic region of the fiber protein and the entire E4 region. Nucleic Acids Res. 1981 Aug 25;9(16):4023–4042. doi: 10.1093/nar/9.16.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. G. Separation and isolation of DNA fragments using linear polyacrylamide gradient gel electrophoresis. Methods Enzymol. 1980;65(1):305–319. doi: 10.1016/s0076-6879(80)65041-1. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Baum P. R., Solem R., Gesteland R. F., Anderson C. W. Location and identification of the genes for adenovirus type 2 early polypeptides. Cell. 1976 Jan;7(1):141–151. doi: 10.1016/0092-8674(76)90264-6. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Esche H., Smart J. E., Stillman B. W., Harter M. L., Mathews M. B. Organization and expression of the left third of the genome of adenovirus. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):493–508. doi: 10.1101/sqb.1980.044.01.052. [DOI] [PubMed] [Google Scholar]
- Matsuo T., Hashimoto S., Wold W. S., Symington J., Rankin A., Green M. Identification of adenovirus 2 early region 4 polypeptides by in vitro translation and tryptic peptide map analysis. J Virol. 1982 Jan;41(1):334–339. doi: 10.1128/jvi.41.1.334-339.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrogan M., Raskas H. J. Species identification and genome mapping of cytoplasmic adenovirus type 2 RNAs synthesized late in infection. J Virol. 1977 Aug;23(2):240–249. doi: 10.1128/jvi.23.2.240-249.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrogan M., Spector D. J., Goldenberg C. J., Halbert D., Raskas H. J. Purification of specific adenovirus 2 RNAs by preparative hybridization and selective thermal elution. Nucleic Acids Res. 1979 Feb;6(2):593–607. doi: 10.1093/nar/6.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Resolving the functions of overlapping viral genes by site-specific mutagenesis at a mRNA splice site. Nature. 1982 Feb 4;295(5848):380–384. doi: 10.1038/295380a0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Ginsberg H. S., Blanchard J. M., Wilson M. C., Darnell J. E., Jr Regulation of the primary expression of the early adenovirus transcription units. J Virol. 1979 Dec;32(3):727–733. doi: 10.1128/jvi.32.3.727-733.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Winkler J. J. Regulation of early adenovirus transcription: a protein product of early region 2 specifically represses region 4 transcription. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1893–1897. doi: 10.1073/pnas.77.4.1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Jones R. L., Cepko C. L., Sharp P. A., Roberts B. E. Expression of early adenovirus genes requires a viral encoded acidic polypeptide. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6121–6125. doi: 10.1073/pnas.78.10.6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier M. H., Staehelin T. Initiation of mammalian protein synthesis: the importance of ribosome and initiation factor quality for the efficiency of in vitro systems. J Mol Biol. 1973 Feb 19;73(3):329–349. doi: 10.1016/0022-2836(73)90346-x. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Gallimore P. H., Flint S. J. Mapping of adenovirus 2 RNA sequences in lytically infected cells and transformed cell lines. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):457–474. doi: 10.1101/sqb.1974.039.01.058. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. C., Darnell J. E., Jr Control of messenger RNA concentration by differential cytoplasmic half-life. Adenovirus messenger RNAs from transcription units 1A and 1B. J Mol Biol. 1981 May 25;148(3):231–251. doi: 10.1016/0022-2836(81)90537-4. [DOI] [PubMed] [Google Scholar]
- Wilson M. C., Nevins J. R., Blanchard J. M., Ginsberg H. S., Darnell J. E., Jr Metabolism of mRNA from the transforming region of adenovirus 2. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):447–455. doi: 10.1101/sqb.1980.044.01.048. [DOI] [PubMed] [Google Scholar]
- Yang V. W., Flint S. J. Synthesis and processing of adenoviral RNA in isolated nuclei. J Virol. 1979 Nov;32(2):394–403. doi: 10.1128/jvi.32.2.394-403.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]